Glucagon-like Peptide-2 Acts Partially Through Central GLP-2R and MC4R in Mobilizing Stored Lipids from the Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides
2.2. Animals
2.3. Intracerebroventricular (icv) Cannulation
2.4. Mesenteric Lymph Duct (MLD), Intraduodenal (id), and Intraperitoneal (ip) Cannulations
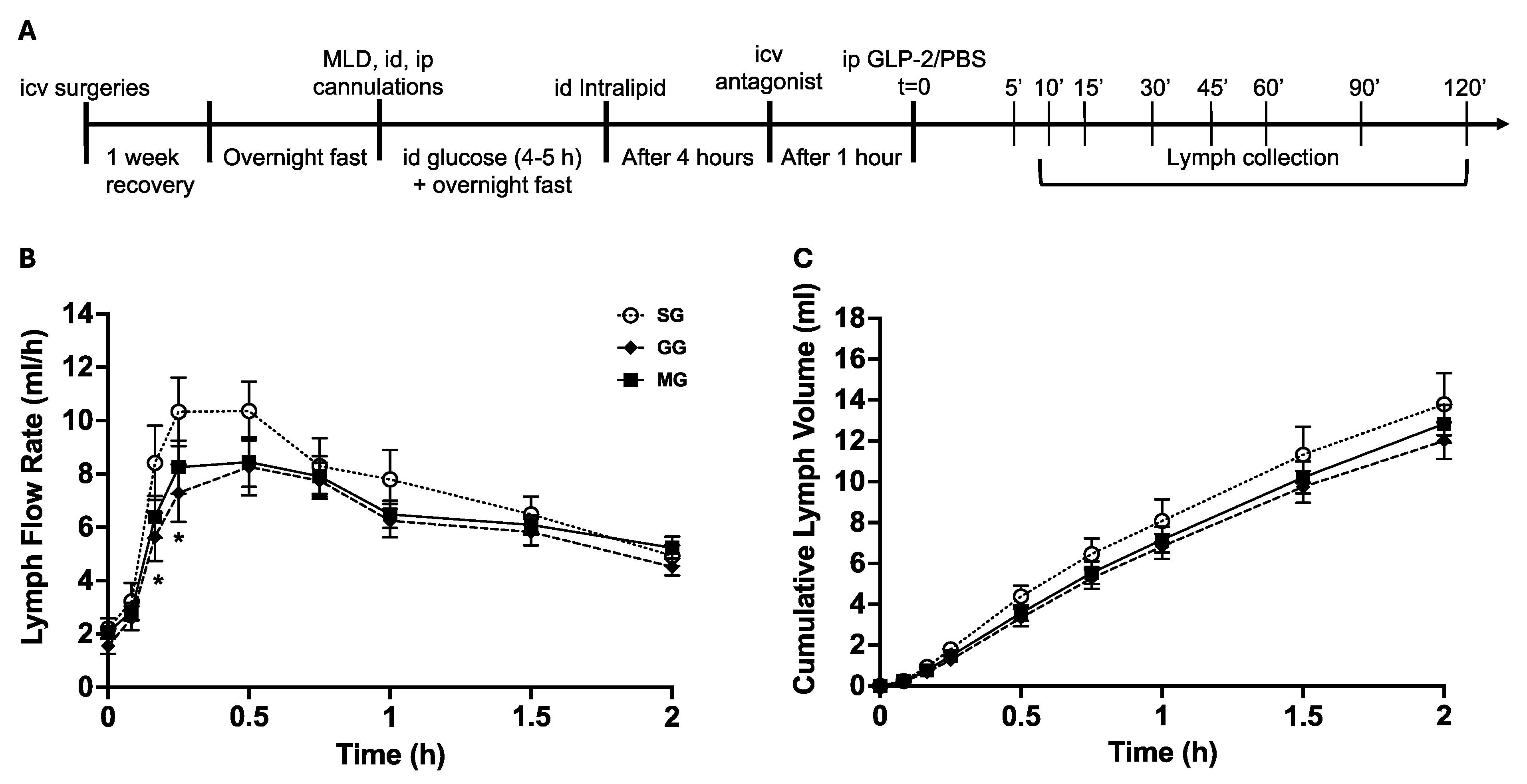
2.5. Study Design
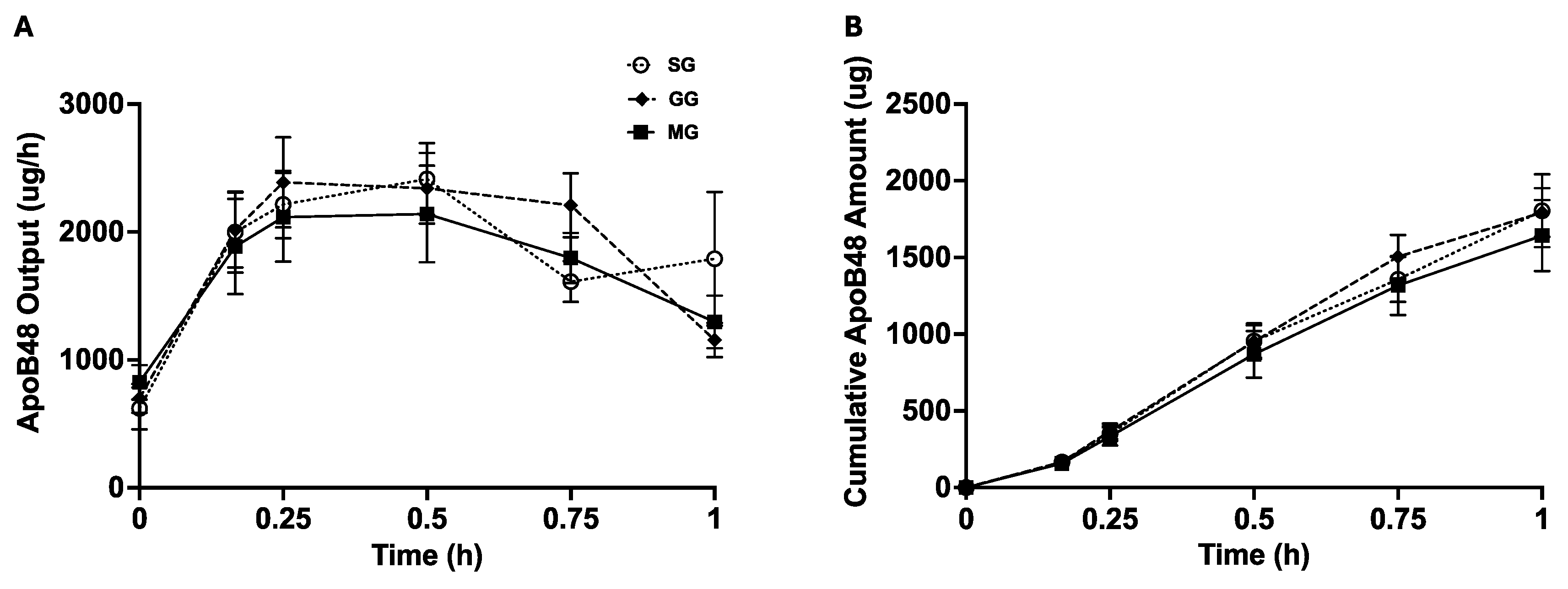
2.6. Lymph Flow Rate, Triglyceride and ApoB48 Assays, and Calculations of Gut Lipid Output
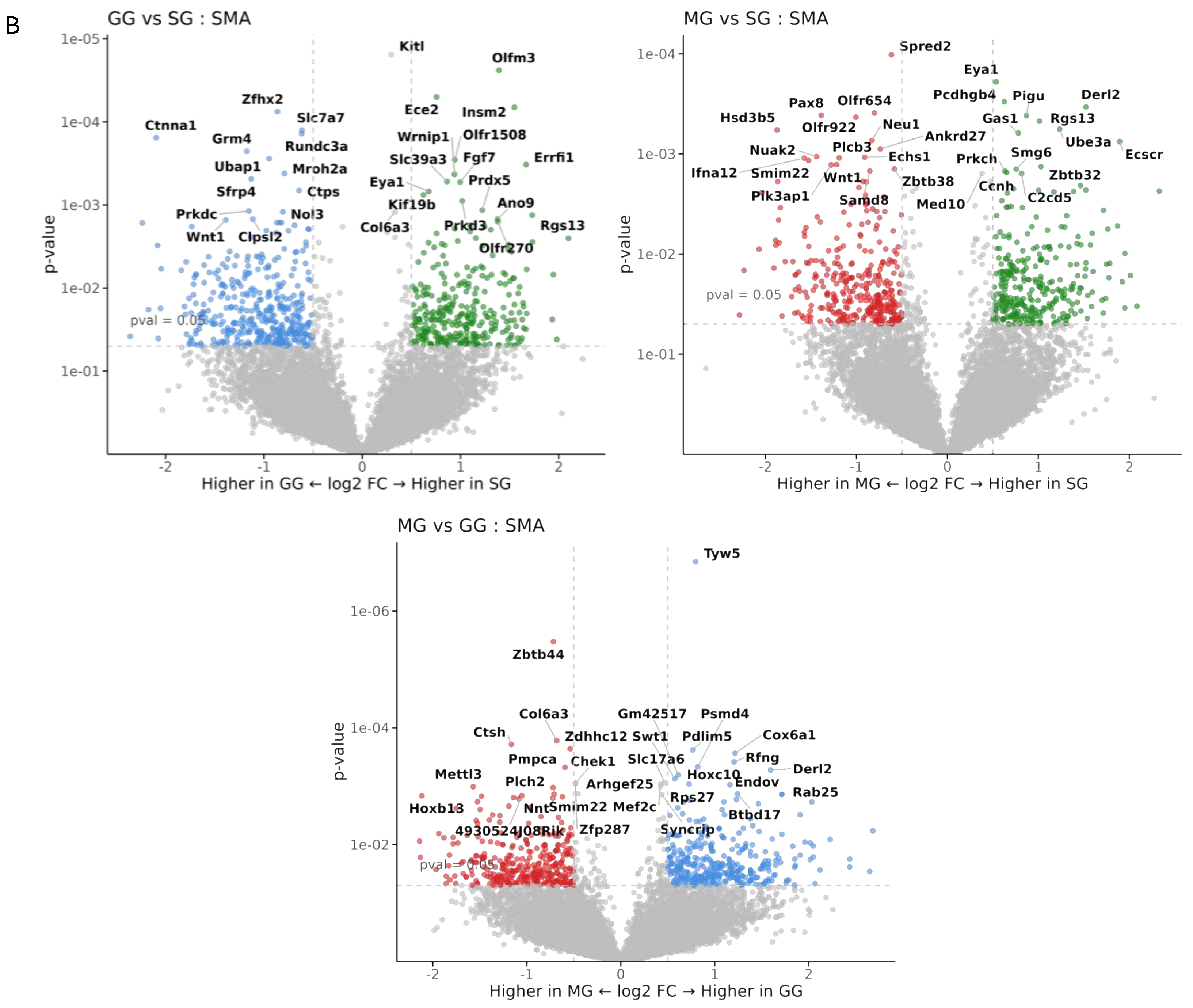
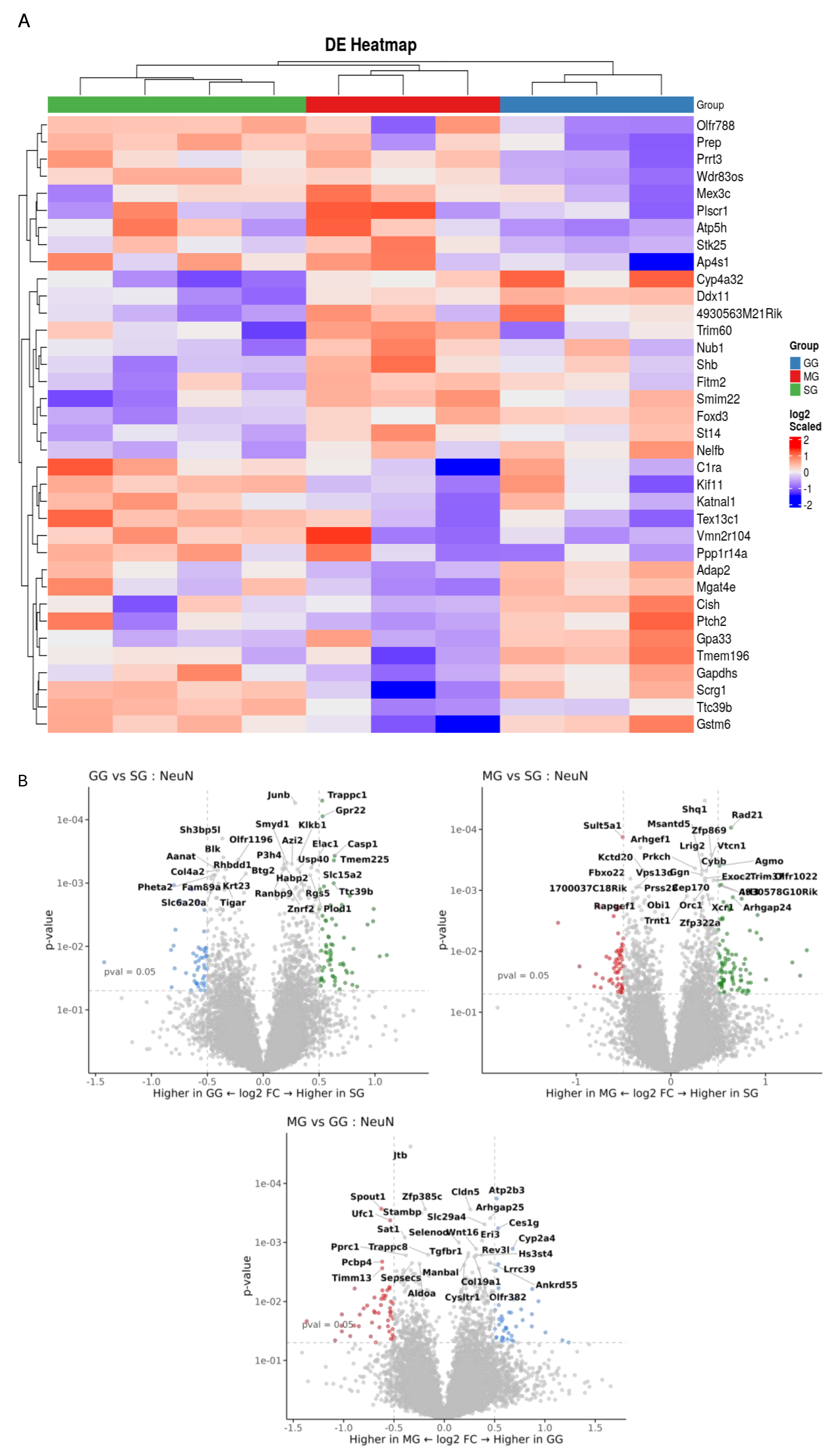
2.7. Spatial Profiling of Jejunal mRNA
2.8. Statistical Analysis
3. Results
3.1. LFR and Cumulative Lymph Volume
3.2. Lymph TG Output and Cumulative TG Amount
3.3. Lymph ApoB48 Output and Cumulative ApoB48 Amount
3.4. Spatial Transcriptomics Analysis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsieh, J.; Longuet, C.; Maida, A.; Bahrami, J.; Xu, E.; Baker, C.L.; Brubaker, P.L.; Drucker, D.J.; Adeli, K. Glucagon-Like Peptide-2 Increases Intestinal Lipid Absorption and Chylomicron Production via CD36. Gastroenterology 2009, 137, 997–1005.e4. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A.; Pott, A.; Heinze, K.; Goetze, O.; Bulut, K.; Schmidt, W.E.; Gallwitz, B.; Holst, J.J. Glucagon-Like Peptide 2 Stimulates Glucagon Secretion, Enhances Lipid Absorption, and Inhibits Gastric Acid Secretion in Humans. Gastroenterology 2006, 130, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.; Xiao, C.; Davis, X.; Tso, P.; Lewis, G.F. Glucose and GLP-2 (Glucagon-Like Peptide-2) Mobilize Intestinal Triglyceride by Distinct Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Connelly, P.W.; Patterson, B.W.; Lewis, G.F. Glucagon-Like Peptide-2 Regulates Release of Chylomicrons from the Intestine. Gastroenterology 2014, 147, 1275–1284.e4. [Google Scholar] [CrossRef]
- Hsieh, J.; Trajcevski, K.E.; Farr, S.L.; Baker, C.L.; Lake, E.J.; Taher, J.; Iqbal, J.; Hussain, M.M.; Adeli, K. Glucagon-Like Peptide 2 (GLP-2) Stimulates Postprandial Chylomicron Production and Postabsorptive Release of Intestinal Triglyceride Storage Pools via Induction of Nitric Oxide Signaling in Male Hamsters and Mice. Endocrinology 2015, 156, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.; Pedersen, N.B.; Brix, S.W.; Grunddal, K.V.; Rosenkilde, M.M.; Hartmann, B.; Ørskov, C.; Poulsen, S.S.; Holst, J.J. The Glucagon-like Peptide 2 Receptor Is Expressed in Enteric Neurons and Not in the Epithelium of the Intestine. Peptides 2015, 67, 20–28. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sharp, J.W.; Brownfield, M.S.; Raybould, H.E.; Ney, D.M. Localization and Activation of Glucagon-Like Peptide-2 Receptors on Vagal Afferents in the Rat. Endocrinology 2007, 148, 1954–1962. [Google Scholar] [CrossRef]
- Lovshin, J.; Estall, J.; Yusta, B.; Brown, T.J.; Drucker, D.J. Glucagon-like Peptide (GLP)-2 Action in the Murine Central Nervous System Is Enhanced by Elimination of GLP-1 Receptor Signaling. J. Biol. Chem. 2001, 276, 21489–21499. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Belsham, D.D. Glucagon-Like Peptide-2 Directly Regulates Hypothalamic Neurons Expressing Neuropeptides Linked to Appetite Control in Vivo and in Vitro. Endocrinology 2012, 153, 2385–2397. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, F.; Li, X.; Chang, B.; Li, D.; Wang, Y.; Tong, Q.; Xu, Y.; Fukuda, M.; Zhao, J.J.; et al. Central GLP-2 Enhances Hepatic Insulin Sensitivity via Activating PI3K Signaling in POMC Neurons. Cell Metab. 2013, 18, 86–98. [Google Scholar] [CrossRef]
- El-Jamal, N.; Erdual, E.; Neunlist, M.; Koriche, D.; Dubuquoy, C.; Maggiotto, F.; Chevalier, J.; Berrebi, D.; Dubuquoy, L.; Boulanger, E.; et al. Glugacon-like Peptide-2: Broad Receptor Expression, Limited Therapeutic Effect on Intestinal Inflammation and Novel Role in Liver Regeneration. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G274–G285. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.M.; Raka, F.; Hoffman, S.; Adeli, K. GLP-2 Regulation of Dietary Fat Absorption and Intestinal Chylomicron Production via Neuronal Nitric Oxide Synthase (NNOS) Signaling. Diabetes 2022, 71, 1388–1399. [Google Scholar] [CrossRef]
- Mukherjee, K.; Xiao, C. GLP-2 Regulation of Intestinal Lipid Handling. Front. Physiol. 2024, 15, 1358625. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Wang, R.; Xiao, C. Release of Lipids Stored in the Intestine by Glucagon-Like Peptide-2 Involves a Gut-Brain Neural Pathway. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Shi, X.; Li, X.; Chang, B.; Wang, Y.; Li, D.; Chan, L. GLP-2 Receptor in POMC Neurons Suppresses Feeding Behavior and Gastric Motility. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E853–E864. [Google Scholar] [CrossRef]
- Nogueiras, R.; Wiedmer, P.; Perez-Tilve, D.; Veyrat-Durebex, C.; Keogh, J.M.; Sutton, G.M.; Pfluger, P.T.; Castaneda, T.R.; Neschen, S.; Hofmann, S.M.; et al. The Central Melanocortin System Directly Controls Peripheral Lipid Metabolism. J. Clin. Investig. 2007, 117, 3475–3488. [Google Scholar] [CrossRef]
- Richardson, J.; Cruz, M.T.; Majumdar, U.; Lewin, A.; Kingsbury, K.A.; Dezfuli, G.; Vicini, S.; Verbalis, J.G.; Dretchen, K.L.; Gillis, R.A.; et al. Melanocortin Signaling in the Brainstem Influences Vagal Outflow to the Stomach. J. Neurosci. 2013, 33, 13286–13299. [Google Scholar] [CrossRef]
- Rahdar, P.; Khazali, H. Rfamide-Related Peptide-3 Suppresses the Substance P-Induced Promotion of the Reproductive Performance in Female Rats Modulating Hypothalamic Kisspeptin Expression. Exp. Brain Res. 2020, 238, 2457–2467. [Google Scholar] [CrossRef]
- Do Carmo, J.M.; Da Silva, A.A.; Moak, S.P.; Da Silva, F.S.; Spradley, F.T.; Hall, J.E. Role of Melanocortin 4 Receptor in Hypertension Induced by Chronic Intermittent Hypoxia. Acta Physiol. 2019, 225, e13222. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain, in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997; ISBN 9780123919496. [Google Scholar]
- Baldassano, S.; Gasbjerg, L.S.; Kizilkaya, H.S.; Rosenkilde, M.M.; Holst, J.J.; Hartmann, B. Increased Body Weight and Fat Mass After Subchronic GIP Receptor Antagonist, but Not GLP-2 Receptor Antagonist, Administration in Rats. Front. Endocrinol. 2019, 10, 492. [Google Scholar] [CrossRef]
- Hwa, J.J.; Ghibaudi, L.; Gao, J.; Parker, E.M. Central Melanocortin System Modulates Energy Intake and Expenditure of Obese and Lean Zucker Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 281, R444–R451. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.M.; Yu, F.; Printz, R.; Hasty, A.H.; Swift, L.L.; Niswender, K.D. Central Nervous System Neuropeptide Y Signaling Modulates VLDL Triglyceride Secretion. Diabetes 2008, 57, 1482–1490. [Google Scholar] [CrossRef]
- Blevins, J.E.; Morton, G.J.; Williams, D.L.; Caldwell, D.W.; Bastian, L.S.; Wisse, B.E.; Schwartz, M.W.; Baskin, D.G. Forebrain Melanocortin Signaling Enhances the Hindbrain Satiety Response to CCK-8. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R476–R484. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Stahel, P.; Morgantini, C.; Nahmias, A.; Dash, S.; Lewis, G.F. Glucagon-like Peptide-2 Mobilizes Lipids from the Intestine by a Systemic Nitric Oxide-independent Mechanism. Diabetes Obes. Metab. 2019, 21, 2535–2541. [Google Scholar] [CrossRef]
- Kooijman, S.; Boon, M.R.; Parlevliet, E.T.; Geerling, J.J.; van de Pol, V.; Romijn, J.A.; Havekes, L.M.; Meurs, I.; Rensen, P.C.N. Inhibition of the Central Melanocortin System Decreases Brown Adipose Tissue Activity. J. Lipid Res. 2014, 55, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Xue, W.; Liu, R.; Ma, Q.; Kasaragod, V.B.; Sun, S.; Li, Q.; Chen, Y.; Yuan, M.; Yang, Y.; et al. A Brain-to-Gut Signal Controls Intestinal Fat Absorption. Nature 2024, 634, 936–943. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; von Scheidt, M.; Li, S.; Steiner, A.; Güldener, U.; Koplev, S.; Ma, A.; Hao, K.; Pan, C.; et al. Transcriptome-Wide Association Study of Coronary Artery Disease Identifies Novel Susceptibility Genes. Basic Res. Cardiol. 2022, 117, 6. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Qin, C.; Wang, Z.; Chen, J.; Wang, R.; Hu, J.; Zou, Q.; Niu, X. Resveratrol Attenuate Myocardial Injury by Inhibiting Ferroptosis Via Inducing KAT5/GPX4 in Myocardial Infarction. Front. Pharmacol. 2022, 13, 906073. [Google Scholar] [CrossRef]
- Chen, W.; Yu, F.; Di, M.; Li, M.; Chen, Y.; Zhang, Y.; Liu, X.; Huang, X.; Zhang, M. MicroRNA-124-3p Inhibits Collagen Synthesis in Atherosclerotic Plaques by Targeting Prolyl 4-Hydroxylase Subunit Alpha-1 (P4HA1) in Vascular Smooth Muscle Cells. Atherosclerosis 2018, 277, 98–107. [Google Scholar] [CrossRef]
- Cao, X.; Liu, X.; Li, M.; Zhang, Y.; Chen, L.; Wang, L.; Di, M.; Zhang, M. Overexpression of Prolyl-4-Hydroxylase-α1 Stabilizes but Increases Shear Stress-Induced Atherosclerotic Plaque in Apolipoprotein E-Deficient Mice. Dis. Markers 2016, 2016, 1701637. [Google Scholar] [CrossRef]
- Jung, I.-H.; Elenbaas, J.S.; Alisio, A.; Santana, K.; Young, E.P.; Kang, C.J.; Kachroo, P.; Lavine, K.J.; Razani, B.; Mecham, R.P.; et al. SVEP1 Is a Human Coronary Artery Disease Locus That Promotes Atherosclerosis. Sci. Transl. Med. 2021, 13, eabe0357. [Google Scholar] [CrossRef] [PubMed]
- Cresci, S.; Wu, J.; Province, M.A.; Spertus, J.A.; Steffes, M.; McGill, J.B.; Alderman, E.L.; Brooks, M.M.; Kelsey, S.F.; Frye, R.L.; et al. Peroxisome Proliferator-Activated Receptor Pathway Gene Polymorphism Associated with Extent of Coronary Artery Disease in Patients with Type 2 Diabetes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial. Circulation 2011, 124, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Kolodgie, F. Morphological Characteristics of Coronary Atherosclerosis in Diabetes Mellitus. Can. J. Cardiol. 2006, 22, 81B–84B. [Google Scholar] [CrossRef]
- Bauer, R.C.; Tohyama, J.; Cui, J.; Cheng, L.; Yang, J.; Zhang, X.; Ou, K.; Paschos, G.K.; Zheng, X.L.; Parmacek, M.S.; et al. Knockout of Adamts7, a Novel Coronary Artery Disease Locus in Humans, Reduces Atherosclerosis in Mice. Circulation 2015, 131, 1202–1213. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Z.S.; Xiang, B.; Wei, C.; Wang, Y.; Sun, K.; Chen, G.; Lan, M.S.; Carmona, G.N.; Notkins, A.L.; et al. Targeted Deletion of Insm2 in Mice Result in Reduced Insulin Secretion and Glucose Intolerance. J. Transl. Med. 2018, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhuo, R.; Zhang, Z.; Wang, J.; Tao, Y.; Yang, R.; Guo, X.; Chen, Y.; Jia, S.; Yao, Y.; et al. Super-Enhancer-Associated INSM2 Regulates Lipid Metabolism by Modulating MTOR Signaling Pathway in Neuroblastoma. Cell Biosci. 2022, 12, 158. [Google Scholar] [CrossRef]
- Mei, L.; Chen, Y.; Chen, P.; Chen, H.; He, S.; Jin, C.; Wang, Y.; Hu, Z.; Li, W.; Jin, L.; et al. Fibroblast Growth Factor 7 Alleviates Myocardial Infarction by Improving Oxidative Stress via PI3Kα/AKT-Mediated Regulation of Nrf2 and HXK2. Redox Biol. 2022, 56, 102468. [Google Scholar] [CrossRef]
- Jiang, H.; Song, D.; Zhou, X.; Chen, F.; Yu, Q.; Ren, L.; Dai, Q.; Zeng, M. Maresin1 Ameliorates MSU Crystal-Induced Inflammation by Upregulating Prdx5 Expression. Mol. Med. 2023, 29, 158. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Liang, Z.; Wang, M.; Hu, C.; Chang, C.; Shi, L.; Ji, Q.; Liu, L. Secreted Frizzled-Related Protein 4 Exerts Anti-Atherosclerotic Effects by Reducing Inflammation and Oxidative Stress. Eur. J. Pharmacol. 2022, 923, 174901. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, F.; Tong, Y.; Huang, Y.; Zhang, J. ATF3-SLC7A7 Axis Regulates MTORC1 Signaling to Suppress Lipogenesis and Tumorigenesis in Hepatocellular Carcinoma. Cells 2025, 14, 253. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-H.; Hsu, S.-Y.; Leu, S. Cardiomyocyte-Specific Overexpression of GPR22 Ameliorates Cardiac Injury in Mice with Acute Myocardial Infarction. BMC Cardiovasc. Disord. 2024, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, L.; Meng, L.; Lv, C.; Tian, X. High Expression of CASP1 Induces Atherosclerosis. Medicine 2024, 103, e37616. [Google Scholar] [CrossRef]
- Beli, E.; Yan, Y.; Moldovan, L.; Lydic, T.A.; Krishman, P.; Tersey, S.A.; Duan, Y.; Salazar, T.E.; Dominguez, J.M.; Nguyen, D.V.; et al. Reshaping Lipid Metabolism with Long-Term Alternate Day Feeding in Type 2 Diabetes Mice. Npj Metab. Health Dis. 2025, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, X.; Lv, Y.; Wang, X.; Cui, Y. The Integrated Analysis Identifies Three Critical Genes as Novel Diagnostic Biomarkers Involved in Immune Infiltration in Atherosclerosis. Front. Immunol. 2022, 13, 905921. [Google Scholar] [CrossRef]
- Bernier-Latmani, J.; Petrova, T.V. Intestinal Lymphatic Vasculature: Structure, Mechanisms and Functions. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 510–526. [Google Scholar] [CrossRef]
- Xiao, C.; Stahel, P.; Nahmias, A.; Lewis, G.F. Emerging Role of Lymphatics in the Regulation of Intestinal Lipid Mobilization. Front. Physiol. 2020, 10, 1604. [Google Scholar] [CrossRef]
- Zarkada, G.; Chen, X.; Zhou, X.; Lange, M.; Zeng, L.; Lv, W.; Zhang, X.; Li, Y.; Zhou, W.; Liu, K.; et al. Chylomicrons Regulate Lacteal Permeability and Intestinal Lipid Absorption. Circ. Res. 2023, 133, 333–349. [Google Scholar] [CrossRef]
- Nurmi, H.; Saharinen, P.; Zarkada, G.; Zheng, W.; Robciuc, M.R.; Alitalo, K. VEGF-C Is Required for Intestinal Lymphatic Vessel Maintenance and Lipid Absorption. EMBO Mol. Med. 2015, 7, 1418–1425. [Google Scholar] [CrossRef]
- Breslin, J.W.; Gaudreault, N.; Watson, K.D.; Reynoso, R.; Yuan, S.Y.; Wu, M.H. Vascular Endothelial Growth Factor-C Stimulates the Lymphatic Pump by a VEGF Receptor-3-Dependent Mechanism. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H709–H718. [Google Scholar] [CrossRef]
- Zhang, F.; Zarkada, G.; Han, J.; Li, J.; Dubrac, A.; Ola, R.; Genet, G.; Boyé, K.; Michon, P.; Künzel, S.E.; et al. Lacteal Junction Zippering Protects against Diet-Induced Obesity. Science 1979 2018, 361, 599–603. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, K.; Khan, M.S.A.; Howland, J.G.; Xiao, C. Glucagon-like Peptide-2 Acts Partially Through Central GLP-2R and MC4R in Mobilizing Stored Lipids from the Intestine. Nutrients 2025, 17, 1416. https://doi.org/10.3390/nu17091416
Mukherjee K, Khan MSA, Howland JG, Xiao C. Glucagon-like Peptide-2 Acts Partially Through Central GLP-2R and MC4R in Mobilizing Stored Lipids from the Intestine. Nutrients. 2025; 17(9):1416. https://doi.org/10.3390/nu17091416
Chicago/Turabian StyleMukherjee, Kundanika, Muhammad Saad Abdullah Khan, John G. Howland, and Changting Xiao. 2025. "Glucagon-like Peptide-2 Acts Partially Through Central GLP-2R and MC4R in Mobilizing Stored Lipids from the Intestine" Nutrients 17, no. 9: 1416. https://doi.org/10.3390/nu17091416
APA StyleMukherjee, K., Khan, M. S. A., Howland, J. G., & Xiao, C. (2025). Glucagon-like Peptide-2 Acts Partially Through Central GLP-2R and MC4R in Mobilizing Stored Lipids from the Intestine. Nutrients, 17(9), 1416. https://doi.org/10.3390/nu17091416






