Fetal Distress as a Determinant for Refeeding Syndrome in Preterm Neonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Nutritional Protocol
2.3. Data Collection
2.4. Ethics
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPD | Bronchopulmonary Dysplasia |
| BW | Birth Weight |
| CRIBB | Clinical Risk Index for Babies |
| CVO | Central Vascular Access |
| EUGR | Extrauterine Growth Restriction |
| EN | Enteral Nutrition |
| FEF | Full Enteral Feeding |
| GA | Gestational Age |
| HC | Head Circumference |
| IGF-1 | Insulin-Like Growth Factor 1 |
| IMV | Invasive Mechanical Ventilation |
| IVH | Intraventricular Hemorrhage |
| MEF | Minimal Enteral Feeding |
| MRI | Magnetic Resonance Imaging |
| NEC | Necrotizing Enterocolitis |
| NICU | Neonatal Intensive Care Unit |
| NIV | Non-Invasive Ventilation |
| PN | Parenteral Nutrition |
| PICC | Peripherally Inserted Central Catheter |
| PLV | Periventricular Leukomalacia |
| ROP | Retinopathy of Prematurity |
| RS | Refeeding Syndrome |
| VLBW | Very Low Birth Weight |
References
- Van Goudoever, J.B.; Vlaardingerbroek, H. The Present Challenges of Parenteral Nutrition in Preterm Infants and Children. J. Nutr. 2013, 143, 2059S–2060S. [Google Scholar] [PubMed]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Rigo, J.; Senterre, T. Intrauterine-like Growth Rates Can Be Achieved with Premixed Parenteral Nutrition Solution in Preterm Infants. J. Nutr. 2013, 143 (Suppl. S12), 2066S–2070S. [Google Scholar] [CrossRef]
- Hay, W.W. Aggressive Nutrition of the Preterm Infant. Curr. Pediatr. Rep. 2013, 1, 229–239. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A. Early nutritional support and outcomes in ELBW infants. Early Hum. Dev. 2010, 86 (Suppl. S1), 21–25. [Google Scholar] [CrossRef]
- Di Chiara, M.; Laccetta, G.; Regoli, D.; Dito, L.; Spiriti, C.; De Santis, B.; Travaglia, E.; Prota, R.; Parisi, P.; Brunelli, R.; et al. Delayed Macronutrients’ Target Achievement in Parenteral Nutrition Reduces the Risk of Hyperglycemia in Preterm Newborn: A Randomized Controlled Trial. Nutrients 2023, 15, 1279. [Google Scholar] [CrossRef] [PubMed]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef]
- Bonsante, F.; Iacobelli, S.; Latorre, G.; Rigo, J.; De Felice, C.; Robillard, P.Y.; Gouyon, J.B. Initial Amino Acid Intake Influences Phosphorus and Calcium Homeostasis in Preterm Infants—It Is Time to Change the Composition of the Early Parenteral Nutrition. PLoS ONE 2013, 8, e72880. [Google Scholar] [CrossRef]
- Brener Dik, P.H.; Galletti, M.F.; Fernández Jonusas, S.A.; Alonso, G.; Mariani, G.L.; Fustiñana, C.A. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J. Perinatol. 2015, 35, 712–715. [Google Scholar] [CrossRef]
- Asfour, S.S.; Alshaikh, B.; Mathew, M.; Fouda, D.I.; Al-Mouqdad, M.M. Incidence and Risk Factors of Refeeding Syndrome in Preterm Infants. Nutrients 2024, 16, 2557. [Google Scholar] [CrossRef]
- Mizumoto, H.; Mikami, M.; Oda, H.; Hata, D. Refeeding syndrome in a small-for-dates micro-preemie receiving early parenteral nutrition. Pediatr. Int. 2012, 54, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced Feeding in Very-Low-Birth-Weight Infants May Cause Electrolyte Disturbances and Septicemia—A Randomized, Controlled Trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef]
- Ichikawa, G.; Watabe, Y.; Suzumura, H.; Sairenchi, T.; Muto, T.; Arisaka, O. Hypophosphatemia in Small for Gestational Age Extremely Low Birth Weight Infants Receiving Parenteral Nutrition in the First Week after Birth. J. Pediatr. Endocrinol. Metab. 2012, 25, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.E.; Jiang, Y.; Harding, J.E.; Crowther, C.A.; Bloomfield, F.H.; ProVIDe Trial Group. Neonatal Refeeding Syndrome and Clinical Outcome in Extremely Low-Birth-Weight Babies: Secondary Cohort Analysis from the ProVIDe Trial. JPEN J. Parenter. Enter. Nutr. 2021, 45, 65–78. [Google Scholar] [CrossRef]
- Peila, C.; Spada, E.; Giuliani, F.; Maiocco, G.; Raia, M.; Cresi, F.; Bertino, E.; Coscia, A. Extrauterine Growth Restriction: Definitions and Predictability of Outcomes in a Cohort of Very Low Birth Weight Infants or Preterm Neonates. Nutrients 2020, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Mshvildadze, M.; Mai, V. A Roadmap for Understanding and Preventing Necrotizing Enterocolitis. Curr. Gastroenterol. Rep. 2008, 10, 450–457. [Google Scholar] [CrossRef]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Metrangolo, V.; Faccioli, F.; Onestà, E.; Giancotti, A.; Di Donato, V.; Cardilli, V.; De Curtis, M. Morbidity Associated with Patent Ductus Arteriosus in Preterm Newborns: A Retrospective Case-Control Study. Ital. J. Pediatr. 2021, 47, 9. [Google Scholar] [CrossRef]
- Eisenhut, M.; Choudhury, S. In Premature Newborns Intraventricular Hemorrhage Causes Cerebral Vasospasm and Associated Neurodisability via Heme-Induced Inflammasome-Mediated Interleukin-1 Production and Nitric Oxide Depletion. Front. Neurol. 2017, 8, 423. [Google Scholar] [CrossRef]
- Dammann, O.; Hartnett, M.E.; Stahl, A. Retinopathy of Prematurity. Dev. Med. Child Neurol. 2023, 65, 625–631. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Igarashi, A.; Okuno, T.; Ohta, G.; Tokuriki, S.; Ohshima, Y. Risk Factors for the Development of Refeeding Syndrome-Like Hypophosphatemia in Very Low Birth Weight Infants. Dis. Markers 2017, 2017, 9748031. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P. Umbilical Cord pH, Blood Gases, and Lactate at Birth: Normal Values, Interpretation, and Clinical Utility. Am. J. Obstet. Gynecol. 2023, 228, S1222–S1240. [Google Scholar] [CrossRef]
- Gagnon, R. Placental Insufficiency and Its Consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Boubred, F.; Herlenius, E.; Bartocci, M.; Jonsson, B.; Vanpée, M. Extremely Preterm Infants Who Are Small for Gestational Age Have a High Risk of Early Hypophosphatemia and Hypokalemia. Acta Paediatr. 2015, 104, 1077–1083. [Google Scholar] [CrossRef]
- Pająk, A.; Królak-Olejnik, B.; Szafrańska, A. Early hypophosphatemia in very low birth weight preterm infants. Adv. Clin. Exp. Med. 2018, 27, 841–847. [Google Scholar] [CrossRef]
- Sung, S.I.; Chang, Y.S.; Choi, J.H.; Ho, Y.; Kim, J.; Ahn, S.Y.; Park, W.S. Increased Risk of Refeeding Syndrome-like Hypophosphatemia with High Initial Amino Acid Intake in Small-for-Gestational-Age, Extremely-Low-Birthweight Infants. PLoS ONE 2019, 14, e0221042. [Google Scholar] [CrossRef]
- Boscarino, G.; Di Chiara, M.; Cellitti, R.; De Nardo, M.C.; Conti, M.G.; Parisi, P.; Spalice, A.; Di Mario, C.; Ronchi, B.; Russo, A.; et al. Effects of Early Energy Intake on Neonatal Cerebral Growth of Preterm Newborn: An Observational Study. Sci. Rep. 2021, 11, 18457. [Google Scholar] [CrossRef]
- Bonsante, F.; Iacobelli, S.; Chantegret, C.; Martin, D.; Gouyon, J.B. The effect of parenteral nitrogen and energy intake on electrolyte balance in the preterm infant. Eur. J. Clin. Nutr. 2011, 65, 1088–1093. [Google Scholar] [CrossRef]
- Wright, T.B.; Bloomfield, F.H.; Alexander, T.; Cormack, B.E. Association between Early Phosphate Intake and Refeeding Syndrome in Extremely Low-birth-weight Infants: A Retrospective Cohort Study. J. Parenter. Enter. Nutr. 2025, 2025, jpen.2739. [Google Scholar] [CrossRef]
- Goyale, A.; Ashley, S.L.; Taylor, D.R.; Elnenaei, M.O.; Alaghband-Zadeh, J.; Sherwood, R.A.; Le Roux, C.W.; Vincent, R.P. Predicting Refeeding Hypophosphataemia: Insulin Growth Factor 1 (IGF-1) as a Diagnostic Biochemical Marker for Clinical Practice. Ann. Clin. Biochem. 2015, 52, 82–87. [Google Scholar] [CrossRef]
- Cakir, B.; Hellström, W.; Tomita, Y.; Fu, Z.; Liegl, R.; Winberg, A.; Hansen-Pupp, I.; Ley, D.; Hellström, A.; Löfqvist, C.; et al. IGF1, Serum Glucose, and Retinopathy of Prematurity in Extremely Preterm Infants. JCI Insight 2020, 5, e140363. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Kermorvant-Duchemin, E.; Johnson, M.; Sáenz De Pipaón, M.; Smith, L.E.; Hård, A.-L.; Zachariassen, G.; Fusch, C.; Iacobelli, S.; Johnson, M.J.; et al. Nutritional Interventions to Prevent Retinopathy of Prematurity. Pediatr. Res. 2024, 96, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lei, C.; Qibo, R.; Huang, X.; Chen, Y.; Wang, M.; Zhang, M. Insulin-like Growth Factor-1 and Retinopathy of Prematurity: A Systemic Review and Meta-Analysis. Surv. Ophthalmol. 2023, 68, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Vorum, H.; Ditzel, J. Disturbance of Inorganic Phosphate Metabolism in Diabetes Mellitus: Its Relevance to the Pathogenesis of Diabetic Retinopathy. J. Ophthalmol. 2014, 2014, 135287. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of Insulin-like Growth Factors in Embryonic and Postnatal Growth. Cell 1993, 75, 73–82. [Google Scholar]
- Ohkawa, N.; Shoji, H.; Ikeda, N.; Suganuma, H.; Shimizu, T. Relationship between Insulin-like Growth Factor 1, Leptin and Ghrelin Levels and Catch-up Growth in Small for Gestational Age Infants of 27–31 Weeks during Neonatal Intensive Care Unit Admission. J. Paediatr. Child Health 2017, 53, 62–67. [Google Scholar] [CrossRef]
| Cases | Controls | OR | p | |
|---|---|---|---|---|
| n = 53 | n = 359 | |||
| Cesarean section, No. (%) | 44 (83) | 303 (86.8) | 0.74 (0.34–1.62) | 0.450 |
| Twin pregnancy, No. (%) | 14 (26.4) | 111 (31.8) | 0.77 (0.40–1.47) | 0.431 |
| Maternal age ≥ 35 years, No. (%) | 33.8 (32.21 to 35.8) | 34.07 (33.4 to 34.73) | - | 0.762 |
| Gestational diabetes, No. (%) | 3 (5.7) | 50 (13.9) | 0.45 (1.13–1.51) | 0.184 |
| Pregnancy-induced hypertension, No. (%) | 15 (28.3) | 75 (20.9) | 1.49 (0.78–2.60) | 0.221 |
| Thyroid disorders, No. (%) | 5 (9.4) | 47 (13.1) | 0.69 (0.26–1.82) | 0.593 |
| Risk of infection 1, No. (%) | 12 (22.6) | 126 (35.1) | 0.54 (0.27–1.06) | 0.073 |
| Placental Abruption, No. (%) | 3 (5.7) | 30 (8.4) | 0.65 (0.19–2.30) | 0.517 |
| Antenatal corticosteroids 2, No. (%) | 30 (56.6) | 231 (64.7) | 0.86 (0.32–1.78) | 0.224 |
| IUGR 3, No. (%) | 11 (20.8) | 53 (14.8) | 1.51 (0.73–3.12) | 0.260 |
| Gestational age, weeks | 28.79 (28.06 to 29.52) | 29.68 (29.42 to 29.94) | - | 0.016 * |
| ELBW 4, No. (%) | 21 (39.6) | 68 (19.2) | 2.80 (1.38–4.72) | <0.001 * |
| Birth Weight, Z-score | −0.68 (−0.99 to −0.38) | −0.31 (−0.43 to −0.19) | - | 0.029 * |
| Birth Length, Z-score | −0.58 (−0.98 to −0.18) | −0.25 (−0.46 to −0.11) | - | 0.116 |
| HC 5 at birth, Z-score | −0.36 (−0.66 to −0.07) | −0.08 (−0.05 to 0.22) | - | 0.018 |
| SGA 6, No. (%) | 14 (26.4) | 64 (17.8) | 1.65 (0.84–3.22) | 0.139 |
| Male Gender, No. (%) | 28 (52.8) | 192 (53.8) | 0.96 (0.54–1.71) | 0.894 |
| Apgar Score below 5, No. (%) | 12 (22.6) | 30 (8.8) | 3.02 (1.43–6.36) | 0.002 * |
| Fetal distress 7, No. (%) | 6 (60.0) | 4 (40.0) | 8.70 (2.3–31.6) | 0.002 * |
| Surfactant, No. (%) | 26 (49.1) | 143 (39.8) | 1.45 (0.81–2.59) | 0.202 |
| Caffeine, No. (%) | 49 (92.5) | 295 (82.2) | 2.65 (0.92–7.62) | 0.061 |
| Cases | Controls | OR | p | |
|---|---|---|---|---|
| n = 53 | n = 359 | |||
| MEF 1 0–7 days, No. (%) | 23 (43.4) | 228 (63.5) | 0.44 (0.24–0.79) | 0.005 * |
| FEF 2 7 days, No. (%) | 7 (13.2) | 98 (27.3) | 0.40 (0.17–0.92) | 0.028 * |
| EN 3 0–7 days, g/kg/day | 127.0 (82.8 to 171.3) | 205.4 (184.0 to 226.8) | - | 0.008 * |
| EN 0–3 days, g/kg/day | 30.7 (21.2 to 43.1) | 40.1 (28.1 to 50.3) | - | 0.181 |
| EN 4–7 days, g/kg/day | 97.3 (84.8 to 134.4) | 143.4 (131.6 to 159.1) | - | 0.041 * |
| Prolonged PN 4, No. (%) | 42 (79.2) | 208 (57.9) | 2.77 (1.38–5.56) | 0.003 * |
| PN 0–7 days, g/kg/day | 673.9 (609.2 to 738.7) | 481.2 (452.0 to 51.5) | - | <0.001 * |
| PN 0–3 days, g/kg/day | 267.4 (94.4 to 300.5) | 200.5 (117.7 to 267.9) | - | <0.001 * |
| PN 4–7 days, g/kg/day | 406.5 (388.7 to 456.2) | 280.6 (192.2 to 334.7) | - | <0.001 * |
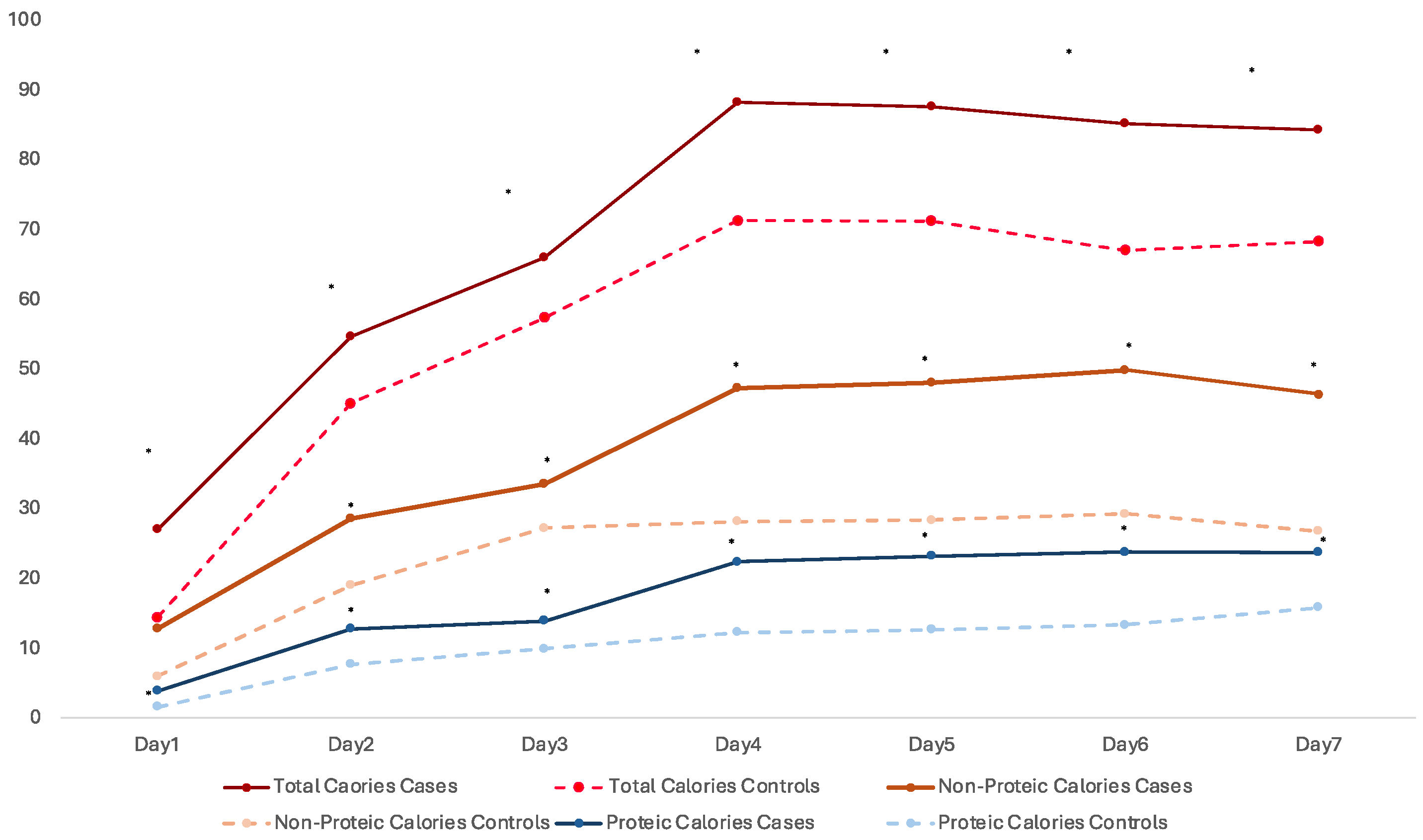
| Total Calories PN 5, g·kg·wk−1 | 312.2 (257.1–367.3) | 186.3 (166.4–206.2) | - | <0.001 * |
| Non-proteic Cal PN 6, g·kg·wk−1 | 231.9 (186.6–277.2) | 133.8 (118.2–149.5) | - | <0.001 * |
| Proteic Cal PN 7, g·kg·wk−1 | 94.1 (78.8–109.4) | 55.6 (50.5–60.7) | - | <0.001 * |
| Calcium PN 8, g·kg·wk−1 | 312.2 (257.1–367.3) | 186.3 (166.4–206.2) | - | <0001 * |
| Phosphorus PN 9, g·kg·wk−1 | 334.0 (273.3–394.7) | 239.0 (162.1–315.9) | - | 0.355 |
| Calcium/Phosphorus PN 10 | 28.0 (52.8) | 147.0 (40.9) | 1.61 (1.43–1.98) | 1.020 |
| Variables | β | Wald | p-Value | Odds Ratio (OR) | 95 C.I for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model I | ||||||
| GA 1 | 0.037 | 0.008 | 0.927 | 1.038 | 0.469 | 2.299 |
| Male sex | 0.013 | 0.002 | 0.968 | 1.013 | 0.550 | 1.864 |
| Fetal distress 2 | 0.798 | 4.818 | 0.028 * | 2.220 | 1.089 | 4.527 |
| PN Calories 3 | 0.512 | 1.181 | 0.277 | 1.668 | 0.663 | 4.200 |
| PN Calcium 4 | 0.420 | 0.642 | 0.423 | 1.521 | 0.545 | 4.245 |
| PN Phosphorus 5 | 0.005 | 0.000 | 0.993 | 1.005 | 0.337 | 2.996 |
| MEF 6 | −0.150 | 0.169 | 0.681 | 0.861 | 0.422 | 1.758 |
| Model II | ||||||
| ELBW 7 | 0.087 | 0.043 | 0.835 | 1.091 | 0.481 | 2.476 |
| Male sex | 0.016 | 0.003 | 0.959 | 1.016 | 0.551 | 1.873 |
| Fetal distress | 0.795 | 4.952 | 0.026 * | 2.214 | 1.099 | 4.458 |
| PN Calories | 0.404 | 0.580 | 0.446 | 1.498 | 0.529 | 4.240 |
| PN Calcium | 0.001 | 0.000 | 0.999 | 1.001 | 0.338 | 2.963 |
| PN Phosphorus | 0.134 | 0.130 | 0.719 | 0.874 | 0.421 | 1.817 |
| MEF | 0.087 | 0.043 | 0.835 | 1.091 | 0.481 | 2.476 |
| Model III | ||||||
| GA | 0.276 | 0.435 | 0.509 | 1.318 | 0.581 | 0.276 |
| Male sex | 0.100 | 0.095 | 0.758 | 1.105 | 0.584 | 0.100 |
| Fetal distress | 0.831 | 4.824 | 0.028 * | 2.295 | 1.093 | 0.831 |
| Non-proteic calories 8 | 0.955 | 3.346 | 0.067 | 2.598 | 0.934 | 7.224 |
| Proteic calories 9 | 0.522 | 0.898 | 0.343 | 1.686 | 0.572 | 4.963 |
| PN Calcium | 0.001 | 0.000 | 0.999 | 1.001 | 0.309 | 0.001 |
| PN Phosphorus | −0.184 | 0.204 | 0.651 | 0.832 | 0.376 | −0.184 |
| MEF | 0.299 | 0.220 | 0.639 | 1.349 | 0.387 | 0.299 |
| Model IV | ||||||
| ELBW | −0.250 | 0.234 | 0.628 | 0.779 | 0.283 | 2.142 |
| Male sex | 0.091 | 0.079 | 0.779 | 1.096 | 0.579 | 2.073 |
| Fetal distress | 0.785 | 4.489 | 0.034 * | 2.192 | 1.061 | 4.531 |
| Non-proteic calories | 0.944 | 3.199 | 0.074 | 2.569 | 0.914 | 7.225 |
| Proteic calories | 0.531 | 0.910 | 0.340 | 1.700 | 0.571 | 5.057 |
| PN Calcium | 0.346 | 0.293 | 0.588 | 1.414 | 0.404 | 4.953 |
| PN Phosphorus | 0.114 | 0.037 | 0.847 | 1.021 | 0.352 | 3.563 |
| MEF | −0.256 | 0.385 | 0.535 | 0.774 | 0.344 | 1.739 |
| Variables | β | Wald | p-Value | Odds Ratio (OR) | 95 C.I for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model I | ||||||
| GA 1 | −1.039 | 15.839 | 0.000 * | 0.354 | 0.212 | 3.135 |
| Male sex | 0.060 | 0.073 | 0.786 | 1.062 | 0.688 | 1.714 |
| Fetal distress 2 | −0.095 | 0.099 | 0.753 | 0.909 | 0.502 | 1.287 |
| IUGR 3 | 1.264 | 12.735 | 0.000 * | 3.539 | 1.768 | 7.967 |
| Prolonged PN 4 | 1.138 | 9.677 | 0.002 * | 3.120 | 1.523 | 4.403 |
| FEF 5 | 0.135 | 0.118 | 0.731 | 1.145 | 0.529 | 3.338 |
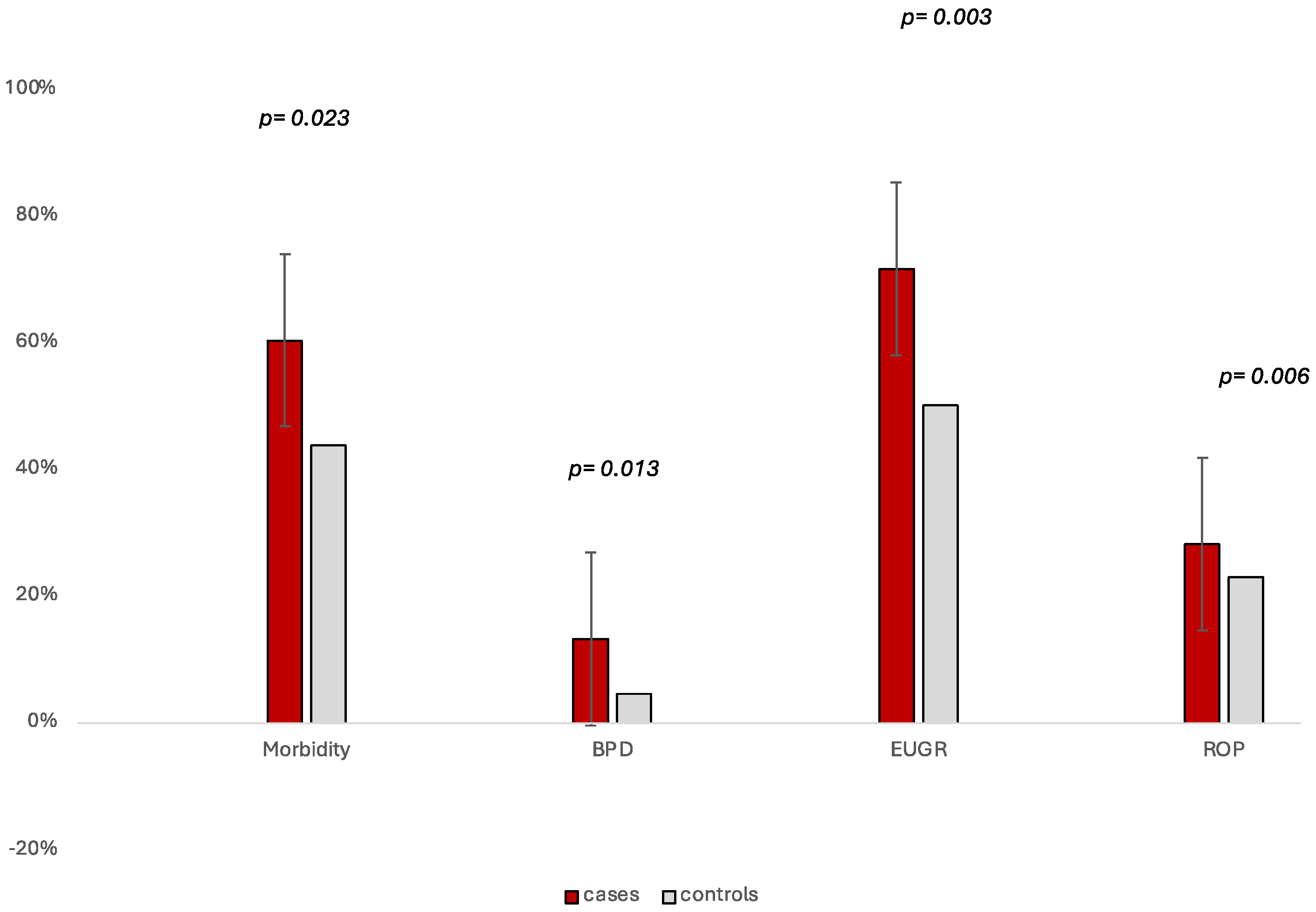
| RS 6 | 0.913 | 6.585 | 0.010 * | 2.493 | 1.241 | 4.155 |
| Model II | ||||||
| ELBW 7 | 0.589 | 4.335 | 0.037 | 1.801 | 1.035 | 3.135 |
| Male sex | 0.112 | 0.267 | 0.605 | 1.119 | 0.730 | 1.714 |
| Fetal distress | −0.337 | 1.256 | 0.262 | 0.714 | 0.396 | 1.287 |
| IUGR | 1.390 | 15.832 | 0.000 * | 4.017 | 2.025 | 7.967 |
| Prolonged PN | 0.794 | 5.111 | 0.024 * | 2.212 | 1.111 | 4.403 |
| FEF | 0.463 | 1.489 | 0.222 | 1.588 | 0.756 | 3.338 |
| RS | 0.734 | 4.352 | 0.037 * | 2.084 | 1.045 | 4.155 |
| Variables | β | Wald | p-Value | Odds Ratio (OR) | 95 C.I for OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model I | ||||||
| GA 1 | 2.256 | 20.440 | 0.000 * | 9.545 | 3.589 | 3.589 |
| Male sex | −0.382 | 1.666 | 0.197 | 0.683 | 0.382 | 0.382 |
| Fetal distress 2 | −0.646 | 2.581 | 0.108 | 0.524 | 0.238 | 0.238 |
| IUGR 3 | 0.349 | 0.670 | 0.413 | 1.418 | 0.614 | 0.614 |
| IMV 4 | 1.001 | 11.198 | 0.001 * | 2.720 | 1.514 | 1.514 |
| RS 5 | 1.171 | 7.287 | 0.007 * | 2.764 | 1.321 | 1.321 |
| Model II | ||||||
| ELBW 6 | 1.513 | 22.922 | 0.000 * | 4.541 | 2.444 | 8.437 |
| Male sex | −0.366 | 1.531 | 0.216 | 0.693 | 0.388 | 1.238 |
| Fetal distress | −0.565 | 1.890 | 0.169 | 0.569 | 0.254 | 1.272 |
| IUGR | −0.310 | 0.560 | 0.454 | 0.733 | 0.325 | 1.652 |
| IMV | 0.953 | 9.310 | 0.002 * | 2.593 | 1.406 | 4.782 |
| RS | 0.952 | 6.482 | 0.011 * | 2.590 | 1.245 | 5.390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Chiara, M.; Spiriti, C.; Gloria, F.; Laccetta, G.; Dito, L.; Gharbiya, M.; Rizzo, G.; Terrin, G. Fetal Distress as a Determinant for Refeeding Syndrome in Preterm Neonates. Nutrients 2025, 17, 1417. https://doi.org/10.3390/nu17091417
Di Chiara M, Spiriti C, Gloria F, Laccetta G, Dito L, Gharbiya M, Rizzo G, Terrin G. Fetal Distress as a Determinant for Refeeding Syndrome in Preterm Neonates. Nutrients. 2025; 17(9):1417. https://doi.org/10.3390/nu17091417
Chicago/Turabian StyleDi Chiara, Maria, Caterina Spiriti, Flavia Gloria, Gianluigi Laccetta, Lucia Dito, Magda Gharbiya, Giuseppe Rizzo, and Gianluca Terrin. 2025. "Fetal Distress as a Determinant for Refeeding Syndrome in Preterm Neonates" Nutrients 17, no. 9: 1417. https://doi.org/10.3390/nu17091417
APA StyleDi Chiara, M., Spiriti, C., Gloria, F., Laccetta, G., Dito, L., Gharbiya, M., Rizzo, G., & Terrin, G. (2025). Fetal Distress as a Determinant for Refeeding Syndrome in Preterm Neonates. Nutrients, 17(9), 1417. https://doi.org/10.3390/nu17091417







