Macronutrients, Micronutrients, and Malnutrition: Effects of Nutrition on Immune Function in Infants and Young Children
Abstract
1. Introduction
2. Infections, Allergies, Gastrointestinal Inflammation, and Malnutrition in Children
2.1. Infections
2.2. Food and Respiratory Allergies
2.3. Celiac Disease and IBD in Children
2.4. Malnutrition
3. Immunomodulation by Food
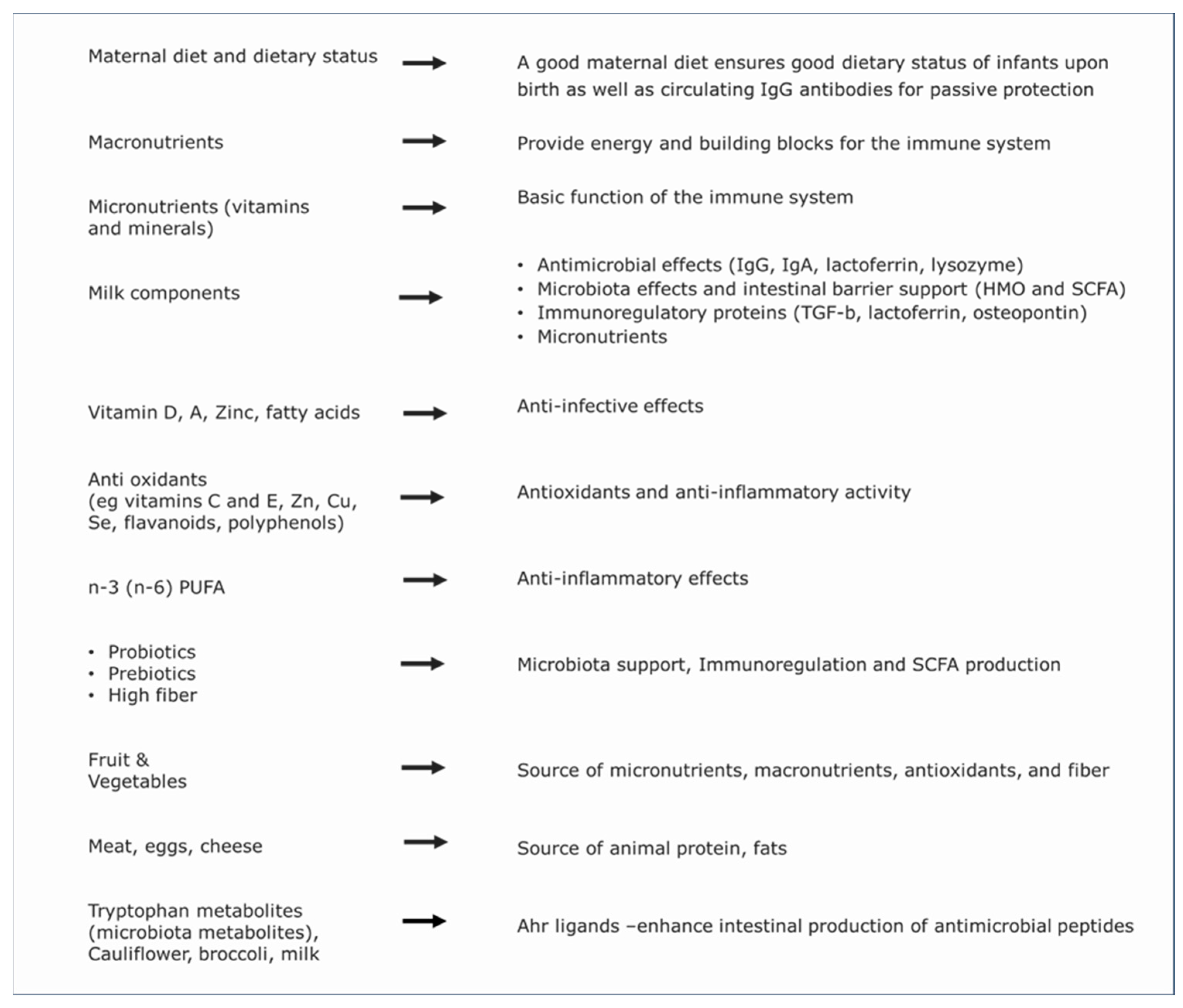
3.1. Nutritional Immune Support in Infants and Children
3.1.1. Macronutrients
3.1.2. Micronutrients
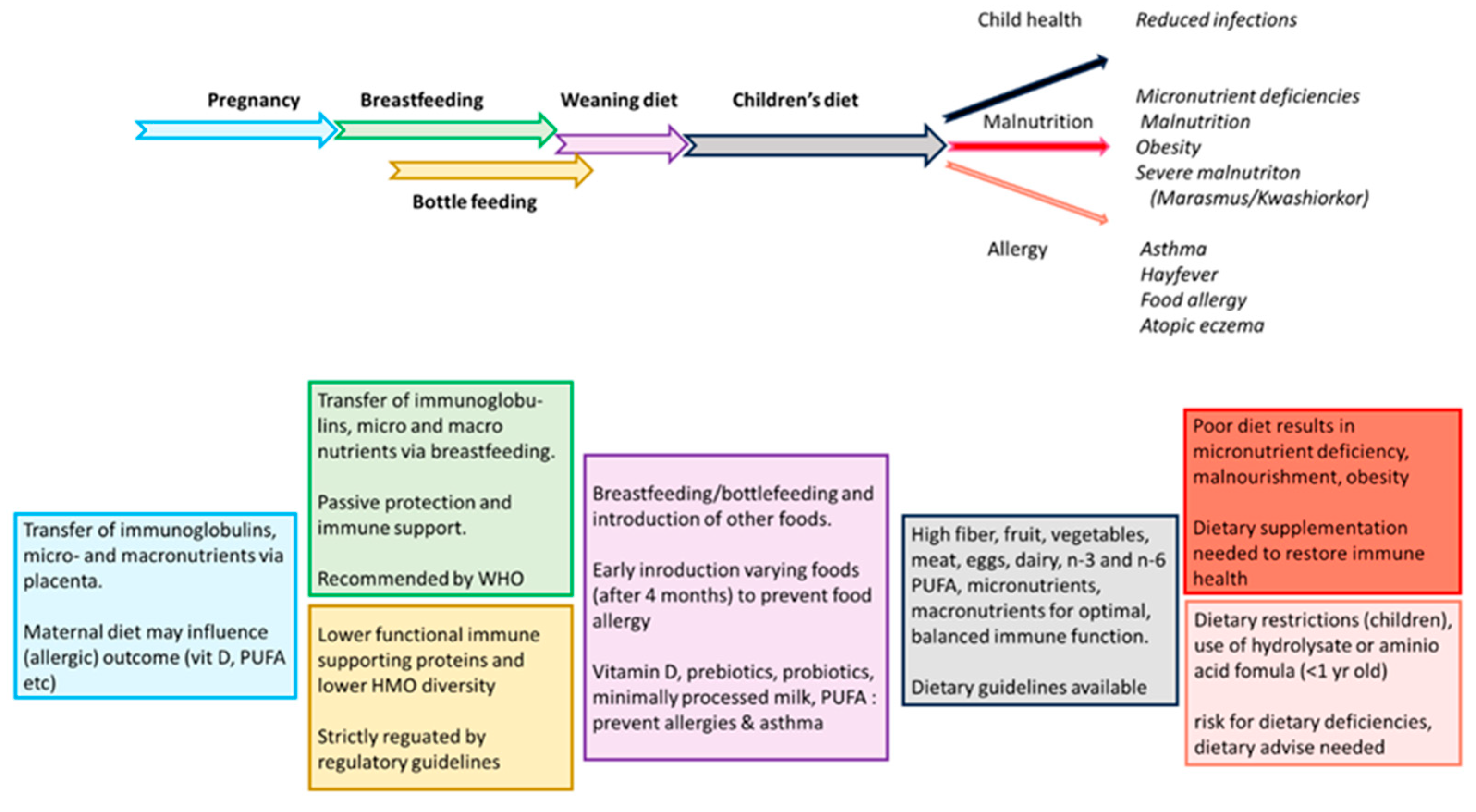
3.2. Maternal Nutrition, Breastfeeding, Cow’s Milk, and Infant Nutrition
3.2.1. Maternal Nutrition
3.2.2. Breastfeeding
3.2.3. Cow’s Milk and Infant Formula
| Component | Function | Ratio Breastmilk/Cow’s Milk * | Present in IFT |
|---|---|---|---|
| Immunoglobulins (IgA, IgG, IgM) # |
| IgA: 10 IgG: 0.1 IgM: 1.1 |
|
| Lactoferrin |
| 20 |
|
| Lactoperoxidase |
| 0.1 |
|
| TGF-β1 +, TGF-β2 + |
| TGF-β1: 0.2 TGF-β2: 0.2 |
|
| IL-10 + |
| 6 |
|
| Pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) + |
| IL-1β: 0.4 IL-6: 1 TNF-α: 0.2 |
|
| MFGM: MFGE8/Lactadherin |
| 3 |
|
| MFGM: Xantine oxidase |
| 1 |
|
| Osteopontin |
| 7 |
|
| Vitamin A, C, D and other micro-nutrients |
| Vitamin A: 2.2 Vitamin C: 1 Vitamin D: 3 |
|
| HMO |
| 3′SL: 3.8 6′SL : 21 All HMO: 70 |
|
| Prebiotics |
| n/a |
|
| n-3 PUFA (DHA, ARA, EPA) |
| DHA: 25 ARA: 2.3 EPA: 2.4 |
|
| Probiotics |
| n/a |
|
| Extracellular vesicles |
| Similar X |
|
| Alkaline phosphatase + |
| Similar X |
|
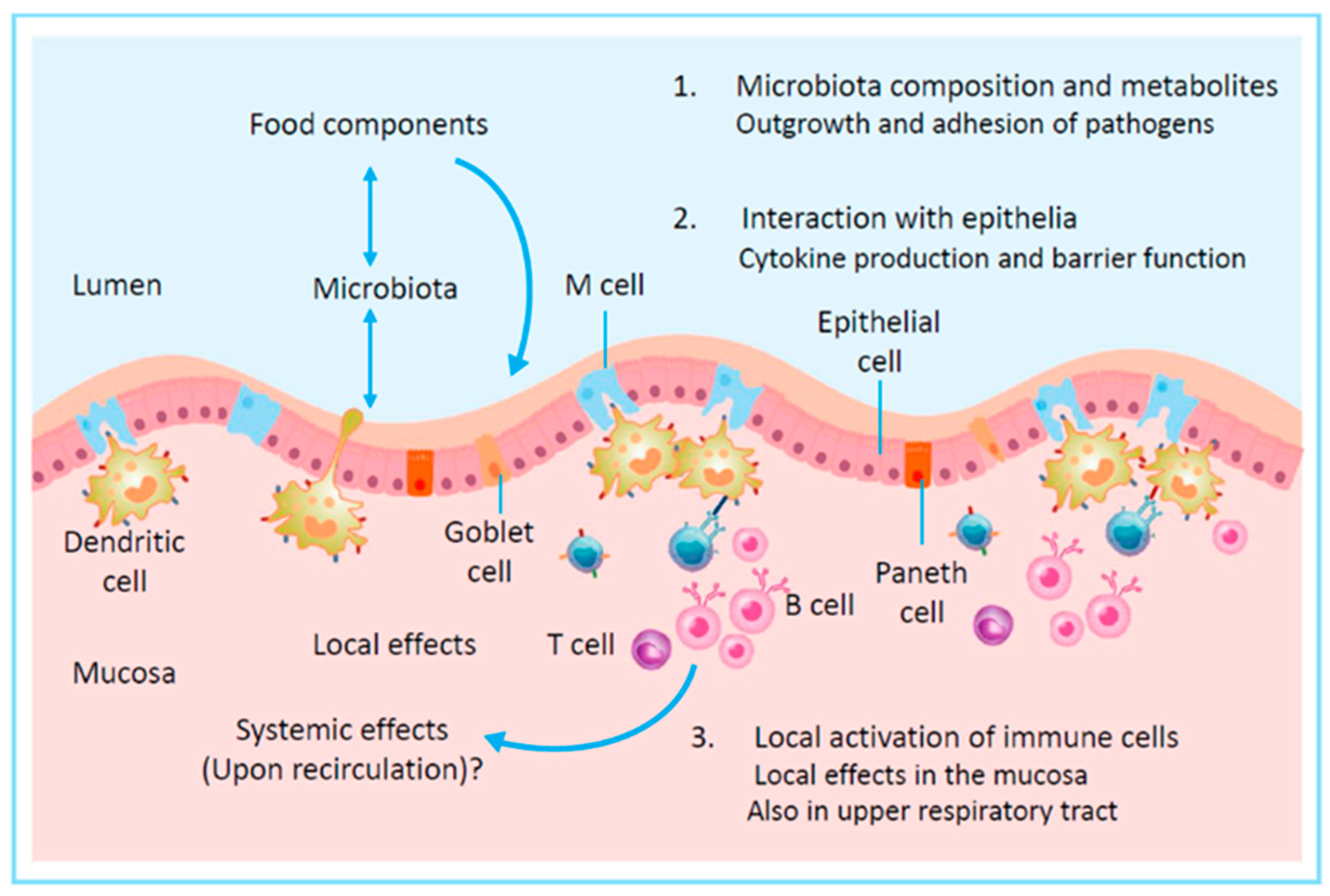
3.3. Effects of Microbiota and Their Metabolites on Immune Function
3.4. Other Immune Supportive Food Components
3.5. Nutrition and Allergy
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M. On Natural and Artificial Vaccinations. Annu. Rev. Immunol. 2003, 21, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Busse, P.J. Innate and adaptive immunosenescence. Ann. Allergy, Asthma Immunol. 2010, 104, 182–183, 210. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 2017, 102, 977–988. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef]
- De Martinis, M.; Modesti, M.; Ginaldi, L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol. Cell Biol. 2004, 82, 415–420. [Google Scholar] [CrossRef]
- Pawelec, G.; Larbi, A.; Derhovanessian, E. Senescence of the Human Immune System. J. Comp. Pathol. 2010, 142, S39–S44. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Pedersen, A.N.; Schroll, M.; Skinhøj, P.; Pedersen, B.K. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin. Exp. Immunol. 1999, 118, 235–241. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The Mother of All Pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Flasche, S.; Slack, M.; Miller, E. Long term trends introduce a potential bias when evaluating the impact of the pneumococcal conjugate vaccination programme in England and Wales. Eurosurveillance 2011, 16, 19868. [Google Scholar] [CrossRef]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef] [PubMed]
- van Daal, M.T.; Folkerts, G.; Garssen, J.; Braber, S. Pharmacological Modulation of Immune Responses by Nutritional Components. Pharmacol. Rev. 2021, 73, 198–232. [Google Scholar] [CrossRef] [PubMed]
- EKaufmann, S.H. Immunology’s foundation: The 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat. Immunol. 2008, 9, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Lomax, A.R.; Calder, P.C. Prebiotics, immune function, infection and inflammation: A review of the evidence. Br. J. Nutr. 2009, 101, 633–658. [Google Scholar] [CrossRef]
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Prescott, S.L. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J. Allergy Clin. Immunol. 2013, 131, 23–30. [Google Scholar] [CrossRef]
- Adolph, T.E.; Tilg, H. Western diets and chronic diseases. Nat. Med. 2024, 30, 2133–2147. [Google Scholar] [CrossRef]
- Kolling, G.; Wu, M.; Guerrant, R.L. Enteric pathogens through life stages. Front. Cell. Infect. Microbiol. 2012, 2, 114. [Google Scholar] [CrossRef]
- Black, R.E.; Cousens, S.; Johnson, H.L.; Lawn, J.E.; Rudan, I.; Bassani, D.G.; Jha, P.; Campbell, H.; Walker, C.F.; Cibulskis, R.; et al. Global, regional, and national causes of child mortality in 2008: A systematic analysis. Lancet 2010, 375, 1969–1987. [Google Scholar] [CrossRef] [PubMed]
- Barnetson, R.S.C.; Rogers, M. Childhood atopic eczema. BMJ 2002, 324, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Alduraywish, S.A.; Lodge, C.J.; Campbell, B.; Allen, K.J.; Erbas, B.; Lowe, A.J.; Dharmage, S.C. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 77–89. [Google Scholar] [CrossRef]
- Eder, W.; Ege, M.J.; von Mutius, E. The Asthma Epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; ECampbell, D.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; ASampson, H.; Beyer, K.; Lee, B.-W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef]
- du Toit, G.; Tsakok, T.; Lack, S.; Lack, G. Prevention of food allergy. J. Allergy Clin. Immunol. 2016, 137, 998–1010. [Google Scholar] [CrossRef]
- Smits, H.H.; Hiemstra, P.S.; da Costa, C.P.; Ege, M.; Edwards, M.; Garn, H.; Howarth, P.H.; Jartti, T.; de Jong, E.C.; Maizels, R.M.; et al. Microbes and asthma: Opportunities for intervention. J. Allergy Clin. Immunol. 2016, 137, 690–697. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef]
- Briani, C.; Samaroo, D.; Alaedini, A. Celiac disease: From gluten to autoimmunity. Autoimmun. Rev. 2008, 7, 644–650. [Google Scholar] [CrossRef]
- Levescot, A.; Malamut, G.; Cerf-Bensussan, N. Immunopathogenesis and environmental triggers in coeliac disease. Gut 2022, 71, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.J.; Beattie, R.M. Inflammatory bowel disease: Recent developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; et al. Main nutritional deficiencies. J. Prev. Med. Hyg. 2022, 63, E93–E101. [Google Scholar] [CrossRef]
- Katoch, O.R. Determinants of malnutrition among children: A systematic review. Nutrition 2022, 96, 111565. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The Immune System in Children with Malnutrition—A Systematic Review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef]
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M.; Addo, O.Y.; Adu-Afarwuah, S.; Alayón, S.; Bhutta, Z.; Brown, K.H.; et al. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef]
- Sandjaja, S.; Budiman, B.; Harahap, H.; Ernawati, F.; Soekatri, M.; Widodo, Y.; Sumedi, E.; Rustan, E.; Sofia, G.; Syarief, S.N.; et al. Food consumption and nutritional and biochemical status of 0·5–12-year-old Indonesian children: The SEANUTS study. Br. J. Nutr. 2013, 110 (Suppl. 3), S11–S20. [Google Scholar] [CrossRef]
- Rojroongwasinkul, N.; Kijboonchoo, K.; Wimonpeerapattana, W.; Purttiponthanee, S.; Yamborisut, U.; Boonpraderm, A.; Kunapan, P.; Thasanasuwan, W.; Khouw, I. SEANUTS: The nutritional status and dietary intakes of 0.5–12-year-old Thai children. Br. J. Nutr. 2013, 110 (Suppl. 3), S36–S44. [Google Scholar] [CrossRef]
- Le Nguyen, B.K.; Le Thi, H.; Do, V.A.N.; Thuy, N.T.; Huu, C.N.; Do, T.T.; Deurenberg, P.; Khouw, I. Double burden of undernutrition and overnutrition in Vietnam in 2011: Results of the SEANUTS study in 0·5–11-year-old children. Br. J. Nutr. 2013, 110, S45–S56. [Google Scholar] [CrossRef]
- Poh, B.K.; Ng, B.K.; Haslinda, M.D.S.; Shanita, S.N.; Wong, J.E.; Budin, S.B.; Ruzita, A.T.; Ng, L.O.; Khouw, I.; Norimah, A.K. Nutritional status and dietary intakes of children aged 6 months to 12 years: Findings of the Nutrition Survey of Malaysian Children (SEANUTS Malaysia). Br. J. Nutr. 2013, 110 (Suppl. 3), S21–S35. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Pongcharoen, T.; Rojroongwasinkul, N.; Tuntipopipat, S.; Winichagoon, P.; Vongvimetee, N.; Phanyotha, T.; Sukboon, P.; Muangnoi, C.; Praengam, K.; Khouw, I. South East Asian Nutrition Surveys II (SEANUTS II) Thailand: Triple burden of malnutrition among Thai children aged 6 months to 12 years. Public Health Nutr 2024, 27, e152. [Google Scholar] [CrossRef] [PubMed]
- Poh, B.K.; Wong, J.E.; Lee, S.T.; Chia, J.S.M.; Yeo, G.S.; Sharif, R.; Safii, N.S.; Jamil, N.A.; Chan, C.M.H.; Farah, N.M.; et al. Triple burden of malnutrition among Malaysian children aged 6 months to 12 years: Current findings from SEANUTS II Malaysia. Public Health Nutr. 2023, 27, e151. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Biesalski, H.K.; Bos, R.; Buskens, E.; Calder, P.C.; Saris, W.H.M.; Spieldenner, J.; Verkade, H.J.; Weber, P.; Eggersdorfer, M. Increased Intake of Foods with High Nutrient Density Can Help to Break the Intergenerational Cycle of Malnutrition and Obesity. Nutrients 2015, 7, 6016–6037. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Kelishadi, R.; Roufarshbaf, M.; Soheili, S.; Payghambarzadeh, F.; Masjedi, M. Association of Childhood Obesity and the Immune System: A Systematic Review of Reviews. Child. Obes. 2017, 13, 332–346. [Google Scholar] [CrossRef]
- Alarcon, P.C.; Damen, M.S.; Madan, R.; Deepe, G.S.; Spearman, P.; Way, S.S.; Divanovic, S. Adipocyte inflammation and pathogenesis of viral pneumonias: An overlooked contribution. Mucosal Immunol. 2021, 14, 1224–1234. [Google Scholar] [CrossRef]
- Fang, X.; Henao-Mejia, J.; Henrickson, S.E. Obesity and immune status in children. Curr. Opin. Pediatr. 2020, 32, 805–815. [Google Scholar] [CrossRef]
- Wells, J.M.; Gao, Y.; de Groot, N.; Vonk, M.M.; Ulfman, L.; van Neerven, R.J. Babies, Bugs, and Barriers: Dietary Modulation of Intestinal Barrier Function in Early Life. Annu. Rev. Nutr. 2022, 42, 165–200. [Google Scholar] [CrossRef]
- Govers, C.; Calder, P.C.; Savelkoul, H.F.J.; Albers, R.; van Neerven, R.J.J. Ingestion, Immunity, and Infection: Nutrition and Viral Respiratory Tract Infections. Front. Immunol. 2022, 13, 841532. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Willemsen, L.E. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186. [Google Scholar] [CrossRef]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Day, L.; Cakebread, J.A.; Loveday, S.M. Food proteins from animals and plants: Differences in the nutritional and functional properties. Trends Food Sci. Technol. 2022, 119, 428–442. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Prentice, S. They Are What You Eat: Can Nutritional Factors during Gestation and Early Infancy Modulate the Neonatal Immune Response? Front. Immunol. 2017, 8, 1641. [Google Scholar] [CrossRef]
- Mestecky, J.; Strober, W.; Russell, M.W.; Cheroutre, H.; Lambrecht, B.N.; Kelsall, B.L. (Eds.) Mucosal Immunology, 4th ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Boerstra, B.V.; de Jong, N.; Meyer, R.; Agostoni, C.; De Cosmi, V.; Grimshaw, K.; Milani, G.P.; Muraro, A.; Elberink, H.O.; Schöll, I.P.; et al. Nutrient supplementation for prevention of viral respiratory tract infections in healthy subjects: A systematic review and meta-analysis. Allergy 2021, 77, 1373–1388. [Google Scholar] [CrossRef]
- Scherbaum, V.; Srour, M.L. The role of breastfeeding in the prevention of childhood malnutrition. World Rev. Nutr. Diet. 2016, 115, 82–97. [Google Scholar] [CrossRef]
- Richard, C.; Lewis, E.D.; Field, C.J. Evidence for the essentiality of arachidonic and docosahexaenoic acid in the postnatal maternal and infant diet for the development of the infant’s immune system early in life. Appl. Physiol. Nutr. Metab. 2016, 41, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P.C.; Karaviti, D.; Zemlin, M.; Skevaki, C. Impact of Early Life Nutrition on Children’s Immune System and Noncommunicable Diseases Through Its Effects on the Bacterial Microbiome, Virome and Mycobiome. Front. Immunol. 2021, 12, 644269. [Google Scholar] [CrossRef] [PubMed]
- Jeurink, P.V.; Knipping, K.; Wiens, F.; Barańska, K.; Stahl, B.; Garssen, J.; Krolak-Olejnik, B. Importance of maternal diet in the training of the infant’s immune system during gestation and lactation. Crit. Rev. Food Sci. Nutr. 2018, 59, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Munblit, D.; Boyle, R.J.; Warner, J.O. Factors affecting breast milk composition and potential consequences for development of the allergic phenotype. Clin. Exp. Allergy 2014, 45, 583–601. [Google Scholar] [CrossRef]
- Munblit, D.; Treneva, M.; Peroni, D.G.; Colicino, S.; Chow, L.Y.; Dissanayeke, S.; Pampura, A.; Boner, A.L.; Geddes, D.T.; Boyle, R.J.; et al. Immune Components in Human Milk Are Associated with Early Infant Immunological Health Outcomes: A Prospective Three-Country Analysis. Nutrients 2017, 9, 532. [Google Scholar] [CrossRef]
- Mirpuri, J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: Implications for public health initiatives. Pediatr. Res. 2021, 89, 301–306. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Maternal diet and its influence on the development of allergic disease. Clin. Exp. Allergy 2014, 45, 63–74. [Google Scholar] [CrossRef]
- Gunaratne, A.W.W.; Makrides, M.; Collins, C.T.T. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst. Rev. 2015, 7, CD010085. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.W.; Nwaru, B.I.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.A.; Bischoff, S.C.; et al. EAACI position paper: Influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 1429–1444. [Google Scholar] [CrossRef]
- Abrams, E.M.; Shaker, M.S.; Chan, E.S.; Brough, H.A.; Greenhawt, M. Prevention of food allergy in infancy: The role of maternal interventions and exposures during pregnancy and lactation. Lancet Child Adolesc. Health 2023, 7, 358–366. [Google Scholar] [CrossRef] [PubMed]
- van Neerven, R.; Savelkoul, H. Nutrition and Allergic Diseases. Nutrients 2017, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Keelan, J.; Garssen, J.; Simmer, K.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Prescott, S.L. Study Protocol for a Randomised Controlled Trial Investigating the Effects of Maternal Prebiotic Fibre Dietary Supplementation from Mid-Pregnancy to Six Months’ Post-Partum on Child Allergic Disease Outcomes. Nutrients 2022, 14, 2753. [Google Scholar] [CrossRef]
- Venter, C.; Pickett-Nairne, K.; Leung, D.; Fleischer, D.; O’Mahony, L.; Glueck, D.H.; Dabelea, D. Maternal allergy-preventive diet index, offspring infant diet diversity, and childhood allergic diseases. Allergy 2024, 79, 3475–3488. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.M. Maternal antibodies, childhood infections, and autoimmune diseases. N. Engl. J. Med. 2001, 345, 1331–1335. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding#:~:text=WHO%20and%20UNICEF%20recommend%3A,years%20of%20age%20or%20beyond (accessed on 30 September 2021).
- Gura, T. Nature’s first functional food. Science 2014, 345, 747–749. [Google Scholar] [CrossRef]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef]
- Duijts, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A. Prolonged and Exclusive Breastfeeding Reduces the Risk of Infectious Diseases in Infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef]
- Verhasselt, V. Neonatal tolerance under breastfeeding influence. Curr. Opin. Immunol. 2010, 22, 623–630. [Google Scholar] [CrossRef]
- Betrán, A.P.; de Onís, M.; ALauer, J.; Villar, J. Ecological study of effect of breast feeding on infant mortality in Latin America. BMJ 2001, 323, 303. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, L.M.; Walker, C.L.F.; Noiman, A.; Victora, C.; Black, R.E. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health 2011, 11 (Suppl. 3), S15. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulou, E.; Feketea, G.; Koumbi, L.; Mesiari, C.; Berghea, E.C.; Konstantinou, G.N. Breastfeeding and COVID-19: From Nutrition to Immunity. Front. Immunol. 2021, 12, 661806. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.M.; Murphy, V.E.; Whalen, O.M.; Gibson, P.G.; Jensen, M.E. Breastfeeding and wheeze-related outcomes in high-risk infants: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 1609–1618. [Google Scholar] [CrossRef]
- Munblit, D.; Peroni, D.G.; Boix-Amoros, A.; Hsu, P.S.; Land, B.V.; Gay, M.C.L.; Kolotilina, A.; Skevaki, C.; Boyle, R.J.; Collado, M.C.; et al. Human Milk and Allergic Diseases: An Unsolved Puzzle. Nutrients 2017, 9, 894. [Google Scholar] [CrossRef]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef]
- Turfkruyer, M.; Verhasselt, V. Breast milk and its impact on maturation of the neonatal immune system. Curr. Opin. Infect. Dis. 2015, 28, 199–206. [Google Scholar] [CrossRef]
- van Neerven, R.J.; Knol, E.F.; Heck, J.M.; Savelkoul, H.F. Which factors in raw cow’s milk contribute to protection against allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- von Mutius, E.; Vercelli, D. Farm living: Effects on childhood asthma and allergy. Nat. Rev. Immunol. 2010, 10, 861–868. [Google Scholar] [CrossRef]
- Deckers, J.; Marsland, B.J.; von Mutius, E. Protection against allergies: Microbes, immunity, and the farming effect. Eur. J. Immunol. 2021, 51, 2387–2398. [Google Scholar] [CrossRef]
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469. [Google Scholar] [CrossRef]
- Riedler, J.; Braun-Fahrländer, C.; Eder, W.; Schreuer, M.; Waser, M.; Maisch, S.; Carr, D.; Schierl, R.; Nowak, D.; von Mutius, E. ALEX Study Team Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet 2001, 358, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Waser, M.; Michels, K.B.; Bieli, C.; Flöistrup, H.; Pershagen, G.; Von Mutius, E.; Ege, M.; Riedler, J.; Schram-Bijkerk, D.; Brunekreef, B.; et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin. Exp. Allergy 2007, 37, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.W.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 878–889.e3. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773.e4. [Google Scholar] [CrossRef]
- Sozańska, B.; Pearce, N.; Dudek, K.; Cullinan, P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural Poland. Allergy 2013, 68, 644–650. [Google Scholar] [CrossRef]
- Perkin, M.; Strachan, D. Which aspects of the farming lifestyle explain the inverse association with childhood allergy? J. Allergy Clin. Immunol. 2006, 117, 1374–1381. [Google Scholar] [CrossRef]
- Loss, G.; Depner, M.; Ulfman, L.H.; van Neerven, R.J.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Kaulek, V.; Roduit, C.; et al. Consumption of unprocessed cow’s milk protects infants from common respiratory infections. J. Allergy Clin. Immunol. 2015, 135, 56–62.e2. [Google Scholar] [CrossRef]
- Hartog, G.D.; Savelkoul, H.F.J.; Schoemaker, R.; Tijhaar, E.; Westphal, A.H.; de Ruiter, T.; van de Weg-Schrijver, E.; van Neerven, R.J.J. Modulation of Human Immune Responses by Bovine interleukin-10. PLoS ONE 2011, 6, e18188. [Google Scholar] [CrossRef]
- Hartog, G.D.; van Altena, C.; Savelkoul, H.F.; van Neerven, R.J. The Mucosal Factors Retinoic Acid and TGF-β1 Induce Phenotypically and Functionally Distinct Dendritic Cell Types. Int. Arch. Allergy Immunol. 2013, 162, 225–236. [Google Scholar] [CrossRef]
- Perdijk, O.; Van Neerven, R.J.J.; Brink, E.V.D.; Savelkoul, H.F.J.; Brugman, S. Bovine Lactoferrin Modulates Dendritic Cell Differentiation and Function. Nutrients 2018, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Hartog, G.D.; Jacobino, S.; Bont, L.; Cox, L.; Ulfman, L.H.; Leusen, J.H.W.; van Neerven, R.J.J. Specificity and Effector Functions of Human RSV-Specific IgG from Bovine Milk. PLoS ONE 2014, 9, e112047. [Google Scholar] [CrossRef] [PubMed]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; van Neerven, R.J. Immunological Effects of Human Milk Oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef]
- Abbring, S.; Hols, G.; Garssen, J.; van Esch, B.C. Raw cow’s milk consumption and allergic diseases—The potential role of bioactive whey proteins. Eur. J. Pharmacol. 2019, 843, 55–65. [Google Scholar] [CrossRef]
- Kleinjan, M.; van Herwijnen, M.J.; Libregts, S.F.; van Neerven, R.J.; Feitsma, A.L.; Wauben, M.H. Regular Industrial Processing of Bovine Milk Impacts the Integrity and Molecular Composition of Extracellular Vesicles. J. Nutr. 2021, 151, 1416–1425. [Google Scholar] [CrossRef]
- Perdijk, O.; van Splunter, M.; Savelkoul, H.F.J.; Brugman, S.; van Neerven, R.J.J. Cow’s Milk and Immune Function in the Respiratory Tract: Potential Mechanisms. Front. Immunol. 2018, 9, 143. [Google Scholar] [CrossRef]
- Perdijk, O.; van Baarlen, P.; Fernandez-Gutierrez, M.M.; Brink, E.v.D.; Schuren, F.H.J.; Brugman, S.; Savelkoul, H.F.J.; Kleerebezem, M.; van Neerven, R.J.J. Sialyllactose and Galactooligosaccharides Promote Epithelial Barrier Functioning and Distinctly Modulate Microbiota Composition and Short Chain Fatty Acid Production In Vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef]
- Demmelmair, H.; Prell, C.; Timby, N.; Lönnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef]
- Leiferman, A.; Shu, J.; Upadhyaya, B.; Cui, J.; Zempleni, J. Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Porbahaie, M.; Hummel, A.; Saouadogo, H.; Coelho, R.; Savelkoul, H.; Teodorowicz, M.; van Neerven, R. Short-chain fatty acids inhibit the activation of T lymphocytes and myeloid cells and induce innate immune tolerance. Benef. Microbes 2023, 14, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef]
- Cuello-Garcia, C.; Brozek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergies: A systematic review and meta-analysis of randomized controlled trials. Clin. Exp. Allergy 2017, 47, 1468–1477. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef]
- Kassai, S.; de Vos, P. Gastrointestinal barrier function, immunity, and neurocognition: The role of human milk oligosaccharide (hMO) supplementation in infant formula. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13271. [Google Scholar] [CrossRef]
- Hooper, L.V. You AhR What You Eat: Linking Diet and Immunity. Cell 2011, 147, 489–491. [Google Scholar] [CrossRef]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Suaini, N.H.A.; Afghani, J.; Heye, K.N.; O’Mahony, L.; Venter, C.; Lauener, R.; Frei, R.; Roduit, C. Systematic review of the association between short-chain fatty acids and allergic diseases. Allergy Eur. J. Allergy Clin. Immunol. 2024, 79, 1789–1811. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.; Calder, P. Omega-6 and Omega-3 Polyunsaturated Fatty Acids and Allergic Diseases in Infancy and Childhood. Curr. Pharm. Des. 2014, 20, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Z.; Lai, C. Significance of Increasing n-3 PUFA Content in Pork on Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 858–870. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- West, C. Probiotics for allergy prevention. Benef. Microbes 2016, 7, 171–180. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733.e30. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.; Groetch, M.; Vassilopoulou, E.; Meyer, R.; Laitinen, K.; Swain, A.; Durban, R.; Benjamin, O.; Bottse, R.; Grimshaw, K.; et al. The immune-supportive diet in allergy management: A narrative review and proposal. Allergy Eur. J. Allergy Clin. Immunol. 2023, 78, 1441–1458. [Google Scholar] [CrossRef]
- Smith, P.K.; Masilamani, M.; Li, X.-M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.B.; Zenker, H.E.; Croes, C.-A.; Ewaz, A.; Ruinemans-Koerts, J.; Savelkoul, H.F.J.; van Neerven, R.J.J.; Teodorowicz, M. Receptor Mediated Effects of Advanced Glycation End Products (AGEs) on Innate and Adaptative Immunity: Relevance for Food Allergy. Nutrients 2022, 14, 371. [Google Scholar] [CrossRef] [PubMed]
- Teodorowicz, M.; Van Neerven, J.; Savelkoul, H. Food Processing: The Influence of the Maillard Reaction on Immunogenicity and Allergenicity of Food Proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Ulfman, L.; Tsuang, A.; Sprikkelman, A.B.; Goh, A.; van Neerven, R.J.J. Relevance of Early Introduction of Cow’s Milk Proteins for Prevention of Cow’s Milk Allergy. Nutrients 2022, 14, 2659. [Google Scholar] [CrossRef]
- Ierodiakonou, D.; Larsen, V.G.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-analysis. JAMA J. Am. Med. Assoc. 2016, 316, 1181–1192. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064.e7. [Google Scholar] [CrossRef]
- Du Toit, G.; Huffaker, M.F.; Radulovic, S.; Feeney, M.; Fisher, H.R.; Byron, M.; Dunaway, L.; Calatroni, A.; Johnson, M.; Foong, R.X.; et al. Follow-up to Adolescence after Early Peanut Introduction for Allergy Prevention. NEJM Evid. 2024, 3, EVIDoa2300311. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Neerven, R.J.J. Macronutrients, Micronutrients, and Malnutrition: Effects of Nutrition on Immune Function in Infants and Young Children. Nutrients 2025, 17, 1469. https://doi.org/10.3390/nu17091469
van Neerven RJJ. Macronutrients, Micronutrients, and Malnutrition: Effects of Nutrition on Immune Function in Infants and Young Children. Nutrients. 2025; 17(9):1469. https://doi.org/10.3390/nu17091469
Chicago/Turabian Stylevan Neerven, R. J. Joost. 2025. "Macronutrients, Micronutrients, and Malnutrition: Effects of Nutrition on Immune Function in Infants and Young Children" Nutrients 17, no. 9: 1469. https://doi.org/10.3390/nu17091469
APA Stylevan Neerven, R. J. J. (2025). Macronutrients, Micronutrients, and Malnutrition: Effects of Nutrition on Immune Function in Infants and Young Children. Nutrients, 17(9), 1469. https://doi.org/10.3390/nu17091469






