Maternal Nutritional Programming: Sex-Specific Cardiovascular and Immune Outcomes Following Perinatal High-Fat Diet Exposure
Abstract
1. Introduction
2. Methods
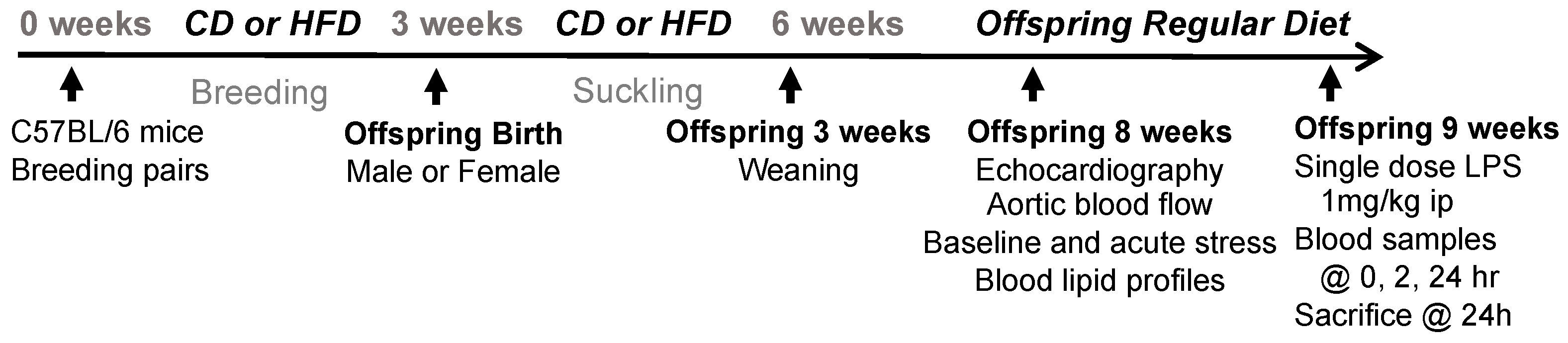
2.1. Experimental Animals
2.2. Cardiovascular Assessment: Echocardiography, Aortic Blood Flow Velocity, and Isoproterenol Stress Test
2.3. Blood Assays
2.4. Inflammatory Marker Measurements
2.5. Histological Assessment of the Heart
2.6. Statistical Analysis
3. Results
3.1. Body Weight, Organ Weight, and Blood Lipid Levels
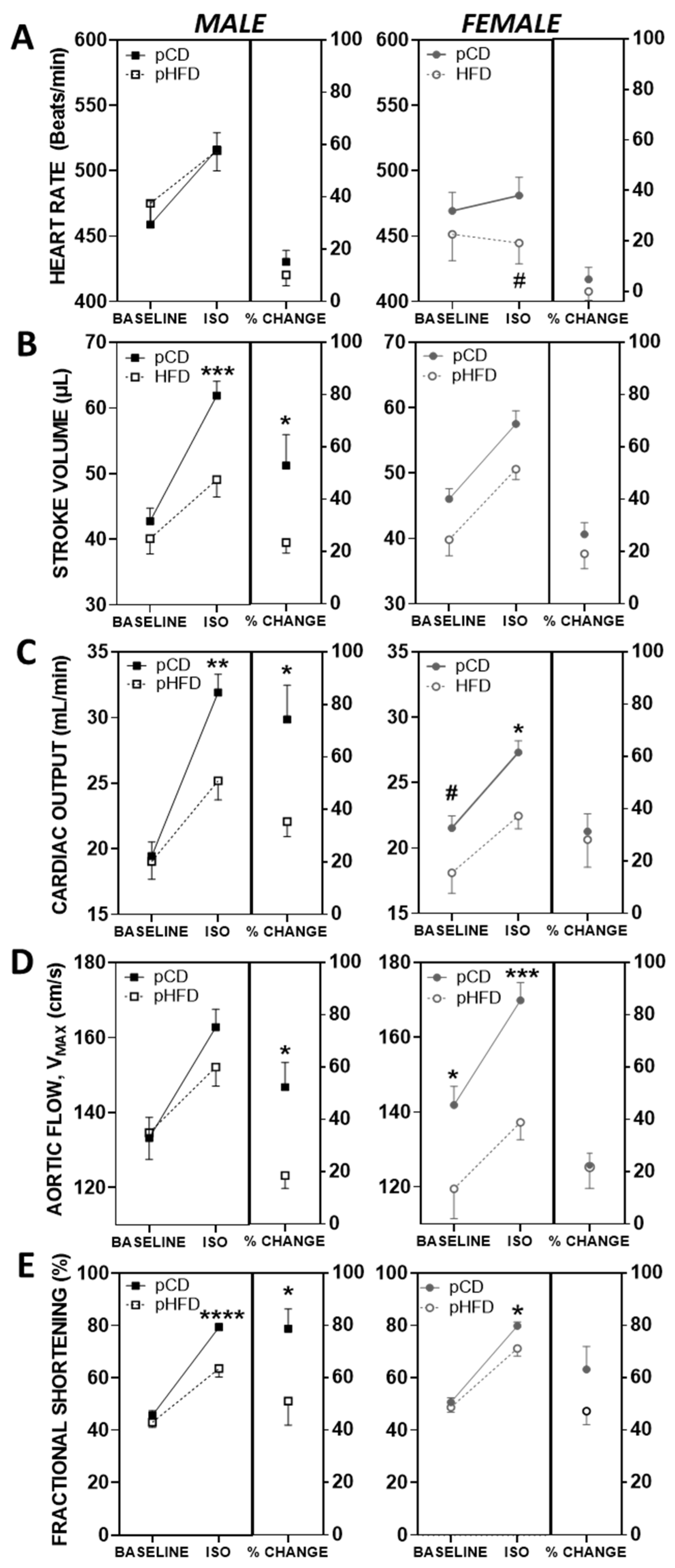
3.2. Baseline and ISO-Mediated Parameters of Cardiovascular Function
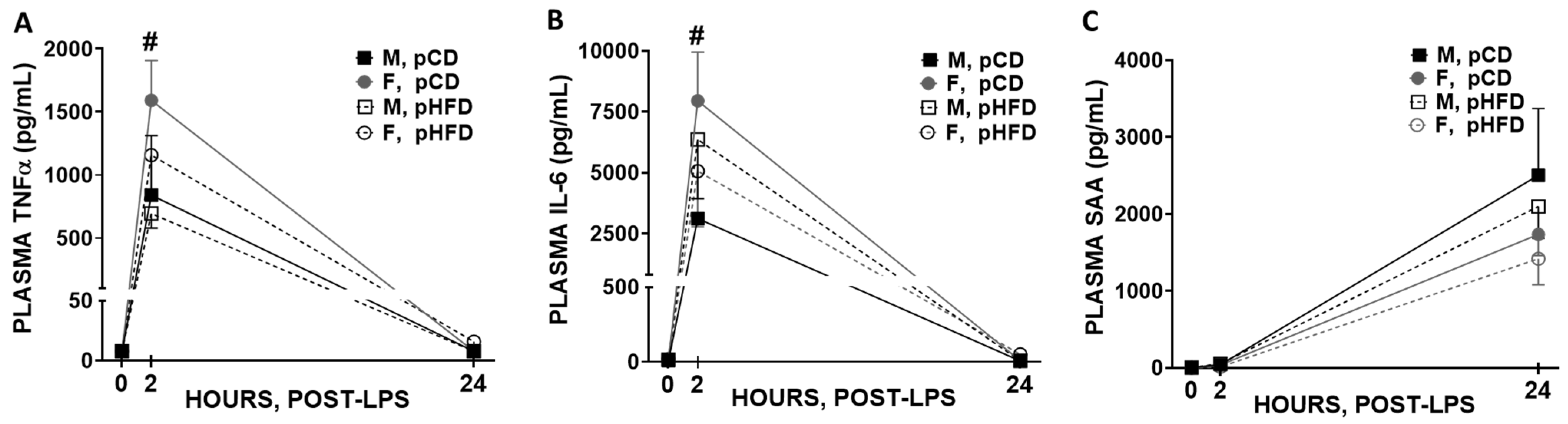
3.3. Blood Immune Response to Acute LPS
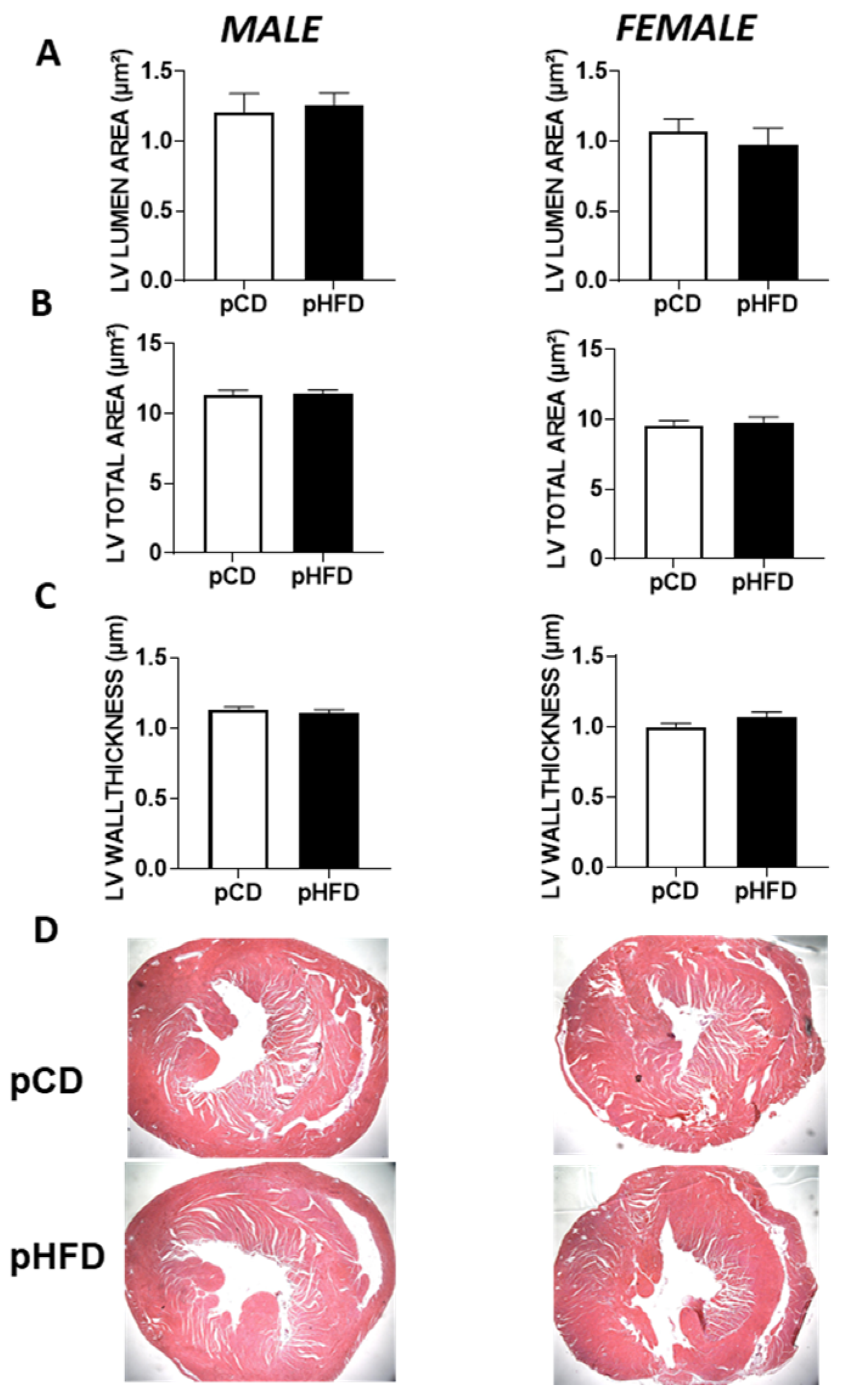
3.4. Histological Assessment of Offspring Hearts
3.5. Summary of Key Findings by Sex
4. Discussion
4.1. Overview and Major Findings
4.2. Cardiac Function and Sex-Specific Programming
4.3. Histological Findings and Structural Insights
4.4. Immune Outcomes and Inflammatory Response
4.5. Translational and Public Health Implications
4.6. Mechanistic Drivers of Sex-Specific Programming
5. Conclusions and Future Directions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| HFD | High-Fat Diet |
| CD | Control Diet |
| CV | Cardiovascular |
| pHFD | Perinatal High-Fat Diet |
| pCD | Perinatal Control Diet |
| ISO | Isoproterenol |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| SAA | Serum Amyloid A |
| CO | Cardiac Output |
| SV | Stroke Volume |
| FS | Fractional Shortening |
| LV | Left Ventricle |
| M | Male |
| F | Female |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| SEM | Standard Error of the Mean |
| ANOVA | Analysis of Variance |
References
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, E347–E913. [Google Scholar] [PubMed]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Edwards, K.C.A.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Executive Summary: Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060641. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Jaddoe, V.W.V. Maternal cardiovascular disorders before and during pregnancy and offspring cardiovascular risk across the life course. Nat. Rev. Cardiol. 2023, 20, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Desoye, G.; Leon, D.A. Reconsidering the developmental origins of adult disease paradigm: The ‘metabolic coordination of childbirth’ hypothesis. Evol. Med. Public Health 2024, 12, 50–66. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Zhang, C.X.W.; Candia, A.A.; Sferruzzi-Perri, A.N. Placental inflammation, oxidative stress, and fetal outcomes in maternal obesity. Trends. Endocrinol. Metab. 2024, 35, 638–647. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Louey, S.; Giraud, G.D. The role of growth in heart development. Nestle Nutr. Workshop Ser. Pediatr. Program 2008, 61, 39–51. [Google Scholar]
- Blackmore, H.L.; Niu, Y.; Fernandez-Twinn, D.S.; Tarry-Adkins, J.L.; Giussani, D.A.; Ozanne, S.E. Maternal Diet-induced Obesity Programs Cardiovascular Dysfunction in Adult Male Mouse Offspring Independent of Current Body Weight. Endocrinology 2014, 155, 3970–3980. [Google Scholar] [CrossRef]
- Chinas Merlin, A.; Gonzalez, K.; Mockler, S.; Perez, Y.; Jia, U.-T.A.; Chicco, A.J.; Ullevig, S.L.; Chung, E. Switching to a Standard Chow Diet at Weaning Improves the Effects of Maternal and Postnatal High-Fat and High-Sucrose Diet on Cardiometabolic Health in Adult Male Mouse Offspring. Metabolites 2022, 12, 563. [Google Scholar] [CrossRef]
- den Harink, T.; Roelofs, M.J.M.; Limpens, J.; Painter, R.C.; Roseboom, T.J.; van Deutekom, A.W. Maternal obesity in pregnancy and children’s cardiac function and structure: A systematic review and meta-analysis of evidence from human studies. PLOS ONE 2022, 17, e0275236. [Google Scholar] [CrossRef]
- Taylor, P.D.; Gu, H.; Saunders, H.; Fiori, F.; Dalrymple, K.V.; Sethupathi, P.; Yamanouchi, L.; Miller, F.; Jones, B.; Vieira, M.C.; et al. Lifestyle intervention in obese pregnancy and cardiac remodelling in 3-year olds: Children of the UPBEAT RCT. Int. J. Obes. 2022, 46, 2145–2155. [Google Scholar] [CrossRef]
- Desai, C.S.; Colangelo, L.A.; Liu, K.; Jacobs Jr, D.R.; Cook, N.L.; Lloyd-Jones, D.M.; Ogunyankin, K.O. Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: The coronary artery risk de-velopment in young adults study. Am. J. Epidemiol. 2013, 177, 20–32. [Google Scholar] [CrossRef]
- Razaz, N.; Villamor, E.; Muraca, G.M.; Bonamy, A.-K.E.; Cnattingius, S. Maternal obesity and risk of cardiovascular diseases in offspring: A population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol. 2020, 8, 572–581. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef]
- Gilardini, L.; Croci, M.; Cavaggioni, L.; Pasqualinotto, L.; Bertoli, S. Sex differences in cardiometabolic risk factors and in response to lifestyle intervention in prepubertal and pubertal subjects with obesity. Front. Pediatr. 2024, 12, 1304451. [Google Scholar] [CrossRef]
- Mahfouz, A.A.; Shatoor, A.S.; Hassanein, M.A.; Mohamed, A.; Farheen, A. Gender differences in cardiovascular risk factors among adolescents in Aseer Region, southwestern Saudi Arabia. J. Saudi Hear. Assoc. 2012, 24, 61–67. [Google Scholar] [CrossRef]
- Dasgupta, K.; O’Loughlin, J.; Chen, S.; Karp, I.; Paradis, G.; Tremblay, J.; Hamet, P.; Pilote, L. Emergence of sex differences in prevalence of high systolic blood pressure: Analysis of a longitudinal adolescent cohort. Circulation 2006, 114, 2663–2670. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Rosario, F.J.; Chan, J.; Cox, L.A.; Ferchaud-Roucher, V.; Zemski-Berry, K.A.; Reusch, J.E.B.; Keller, A.C.; Powell, T.L.; Jansson, T. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J. Physiol. 2022, 600, 3169–3191. [Google Scholar] [CrossRef]
- Huang, H.; Liu, T.; Rose, J.L.; Stevens, R.L.; Hoyt, D.G. Sensitivity of mice to lipopolysaccharide is increased by a high saturated fat and cholesterol diet. J. Inflamm. 2007, 4, 22. [Google Scholar] [CrossRef]
- Frisk, G.; Diderholm, H. Animal serum factor(s) causing adverse effects on RIAs of human enterovirus IgM. J. Virol. Methods 1991, 31, 353–358. [Google Scholar] [CrossRef]
- Morrissey, R.L.; Siu, S.C.; Guerrero, J.L.; Newell, J.B.; Weyman, A.E.; Picard, M.H. Automated Assessment of Ventricular Volume and Function by Echocardiography: Validation of Automated Border Detection. J. Am. Soc. Echocardiogr. 1994, 7, 107–115. [Google Scholar] [CrossRef]
- Mihm, M.J.; Seifert, J.L.; Coyle, C.M.; Bauer, J.A. Diabetes related cardiomyopathy Time dependent echocardiographic evaluation in an experimental rat model. Life Sci. 2001, 69, 527–542. [Google Scholar] [CrossRef]
- Han, B.; Baliga, R.; Huang, H.; Giannone, P.J.; Bauer, J.A. Decreased cardiac expression of vascular endothelial growth factor and redox imbalance in murine diabetic cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2009, 297, H829–H835. [Google Scholar] [CrossRef]
- Combs, D.T.; Martin, C.M. Evaluation of isoproterenol as a method of stress testing. Am. Hear. J. 1974, 87, 711–715. [Google Scholar] [CrossRef]
- Chaves, A.A.; Weinstein, D.M.; Bauer, J.A. Non-invasive echocardiographic studies in mice: Influence of anesthetic regimen. Life Sci. 2001, 69, 213–222. [Google Scholar] [CrossRef]
- Alves, F.C.R.; Moreira, A.; Moutinho, O. Maternal and long-term offspring outcomes of obesity during pregnancy. Arch. Gynecol. Obstet. 2024, 309, 2315–2321. [Google Scholar] [CrossRef]
- Junior, M.D.F.; Cavalcante, K.V.N.; Ferreira, L.A.; Lopes, P.R.; Pontes, C.N.R.; Bessa, A.d.S.M.d.; Neves, Â.R.; Francisco, F.A.; Pedrino, G.R.; Xavier, C.H.; et al. Postnatal early overfeeding induces cardiovascular dysfunction by oxidative stress in adult male Wistar rats. Life Sci. 2019, 226, 173–184. [Google Scholar] [CrossRef]
- Chavaglia Cavalet, L.; Dos Santos Ribeiro, L.C.; Rosa, G.B.; Sousa, K.K.; de Melo, A.B.S.; Campos, D.B.T.; Ferreira, L.A.; Amaral, N.O.; Calisto, Y.T.V.; de Castro, A.G.; et al. Long-term effects of early overfeeding and food restriction during puberty on cardiac remodeling in adult rats. J. Dev. Orig. Health Dis. 2020, 11, 492–498. [Google Scholar] [CrossRef]
- do Carmo, J.M.; Omoto, A.C.M.; Dai, X.; Moak, S.P.; Mega, G.S.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E.; da Silva, A.A. Sex differences in the impact of parental obesity on offspring cardiac SIRT3 expression, mitochondrial efficiency, and diastolic function early in life. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H485–H495. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, G.A.; Farias, R.D.S.; Pedro, S.S.; Rocha, N.N.; Brito, F.C.F.; Scaramello, C.B.V. Overweight during lactation and its implications for biometric, nutritional and cardiovascular parameters of young and adult male and female rats. J. Nutr. Sci. 2020, 9, e27. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Ferreira-Junior, M.D.; Amaral, K.d.J.V.; Cavalcante, K.V.N.; Pontes, C.N.R.; Cristin, L.; Ribeiro, L.C.d.S.; dos Santos, B.G.; Xavier, C.H.; Mathias, P.C.d.F.; et al. Maternal postnatal early overfeeding induces sex-related cardiac dysfunction and alters sexually hormones levels in young offspring. J. Nutr. Biochem. 2022, 103, 108969. [Google Scholar] [CrossRef]
- Wang, W.; Huo, Y.; Zhang, J.; Xu, D.; Bai, F.; Gui, Y. Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis. Nutrients 2022, 14, 4237. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Gori, M.; Lam, C.S.P.; Gupta, D.K.; Santos, A.B.S.; Cheng, S.; Shah, A.M.; Claggett, B.; Zile, M.R.; Kraigher-Krainer, E.; Pieske, B.; et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur. J. Hear. Fail. 2014, 16, 535–542. [Google Scholar] [CrossRef]
- Nirala, S.; Tan, X.-R.; Shafiq, M.; Basnet, R.; Singh, A. Maternal High Fat Diet and its Expressions in the Heart and Liver in the Mice Embryogenesis. Curr. Mol. Med. 2024, 24, 889–898. [Google Scholar] [CrossRef]
- Preston, C.C.; Larsen, T.D.; Eclov, J.A.; Louwagie, E.J.; Gandy, T.C.T.; Faustino, R.S.; Baack, M.L. Maternal High Fat Diet and Diabetes Disrupts Transcriptomic Pathways That Regulate Cardiac Metabolism and Cell Fate in Newborn Rat Hearts. Front. Endocrinol. 2020, 11, 570846. [Google Scholar] [CrossRef]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence 2014, 5, 12–19. [Google Scholar] [CrossRef]
- Adu-Amankwaah, J.; Adekunle, A.O.; Tang, Z.; Bushi, A.; Tan, R.; Fu, L.; Gong, Z.; Ma, Z.; Mprah, R.; Noah, M.L.N.; et al. Estradiol contributes to sex differences in resilience to sepsis-induced metabolic dysregulation and dysfunction in the heart via GPER-1-mediated PPARδ/NLRP3 signaling. Metabolism 2024, 156, 155934. [Google Scholar] [CrossRef]
- Abuiessa, S.A.; Wedn, A.M.; El-Gowilly, S.M.; Helmy, M.M.; El-Mas, M.M. Pre-eclamptic Fetal Programming Alters Neuroinflammatory and Cardiovascular Consequences of Endotoxemia in Sex-Specific Manners. J. Pharmacol. Exp. Ther. 2020, 373, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Xerri, A.; Gallardo, F.; Kober, F.; Mathieu, C.; Fourny, N.; Tran, T.T.; Mege, J.-L.; Singer, M.; Lalevée, N.; Bernard, M.; et al. Female hormones prevent sepsis-induced cardiac dysfunction: An experimental randomized study. Sci. Rep. 2022, 12, 4939. [Google Scholar] [CrossRef]
- Yang, Y.; Rivera, L.; Fang, S.; Cavalier, M.; Suris, A.; Zhou, Y.; Huang, Y. Maternal high-fat diet alters Tet-mediated epigenetic regulation during heart development. iScience 2024, 27, 110631. [Google Scholar] [CrossRef] [PubMed]
- Alsiraj, Y.; Thatcher, S.E.; Charnigo, R.; Chen, K.; Blalock, E.; Daugherty, A.; Cassis, L.A. Female Mice with an XY Sex Chromosome Complement Develop Severe Angiotensin II–Induced Abdominal Aortic Aneurysms. Circulation 2017, 135, 379–391. [Google Scholar] [CrossRef]
- AlSiraj, Y.; Chen, X.; Thatcher, S.E.; Temel, R.E.; Cai, L.; Blalock, E.; Katz, W.; Ali, H.M.; Petriello, M.; Deng, P.; et al. XX sex chromosome complement promotes atherosclerosis in mice. Nat. Commun. 2019, 10, 2631. [Google Scholar] [CrossRef]
- Li, K.; Qian, W.; Zhang, F.; Zhang, W.; Lv, H.; Quan, M.; Sun, W.; Liu, R.; Cao, X.; Xian, Z.; et al. Maternal high-fat diet exacerbates atherosclerosis development in offspring through epigenetic memory. Nat. Cardiovasc. Res. 2025, 4, 362–379. [Google Scholar] [CrossRef]
| Male Offspring | Female Offspring | |||
|---|---|---|---|---|
| Prenatal (Maternal) Diet | ||||
| Parameters | pCD | pHFD | pCD | pHFD |
| Body Weight (g) | 21.68 ± 0.76 | 19.5 ± 0.5 * | 16.0 ± 0.4 # | 15.2 ± 0.4 # |
| Heart (%) | 0.6 ± 0.01 | 0.62 ± 0.01 | 0.71 ± 0.019 # | 0.69 ± 0.013 # |
| Liver (%) | 5.04 ± 0.1 | 5.35 ± 0.1 | 5.1 ± 0.11 | 5.3 ± 0.12 |
| Spleen (%) | 0.44 ± 0.02 | 0.49 ± 0.02 | 0.44 ± 0.02 | 0.52 ± 0.019 |
| Pancreas (%) | 0.88 ± 0.03 | 0.82 ± 0.04 | 0.88 ± 0.033 | 0.90 ± 0.28 |
| Total Cholesterol | 166 ± 7.2 | 148 ± 4.9 | 110 ± 4.4 # | 105 ± 2.5 # |
| Triglycerides | 74 ± 7.8 | 69 ± 7.7 | 54 ± 3.1 | 59 ± 6.3 |
| HDL | 65 ± 1.8 | 65 ± 4.4 | 45 ± 1.7 # | 50 ± 3.9 # |
| LDL | 93 ± 4.8 | 86 ± 3.9 | 72 ± 3.6 # | 72 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsiraj, Y.; Huang, H.; Shoemaker, R.; Schanbacher, B.; Murphy, M.; Giannone, P.; Bauer, J.A. Maternal Nutritional Programming: Sex-Specific Cardiovascular and Immune Outcomes Following Perinatal High-Fat Diet Exposure. Nutrients 2025, 17, 1464. https://doi.org/10.3390/nu17091464
Alsiraj Y, Huang H, Shoemaker R, Schanbacher B, Murphy M, Giannone P, Bauer JA. Maternal Nutritional Programming: Sex-Specific Cardiovascular and Immune Outcomes Following Perinatal High-Fat Diet Exposure. Nutrients. 2025; 17(9):1464. https://doi.org/10.3390/nu17091464
Chicago/Turabian StyleAlsiraj, Yasir, Hong Huang, Robin Shoemaker, Brandon Schanbacher, Margaret Murphy, Peter Giannone, and John A. Bauer. 2025. "Maternal Nutritional Programming: Sex-Specific Cardiovascular and Immune Outcomes Following Perinatal High-Fat Diet Exposure" Nutrients 17, no. 9: 1464. https://doi.org/10.3390/nu17091464
APA StyleAlsiraj, Y., Huang, H., Shoemaker, R., Schanbacher, B., Murphy, M., Giannone, P., & Bauer, J. A. (2025). Maternal Nutritional Programming: Sex-Specific Cardiovascular and Immune Outcomes Following Perinatal High-Fat Diet Exposure. Nutrients, 17(9), 1464. https://doi.org/10.3390/nu17091464






