Variations in Human Milk Metabolites After Gestational Diabetes: Associations with Infant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Maternal and Infant Data
2.3. Human Milk Collection and Processing
2.4. Metabolites in Human Milk
2.5. Statistical Analyses
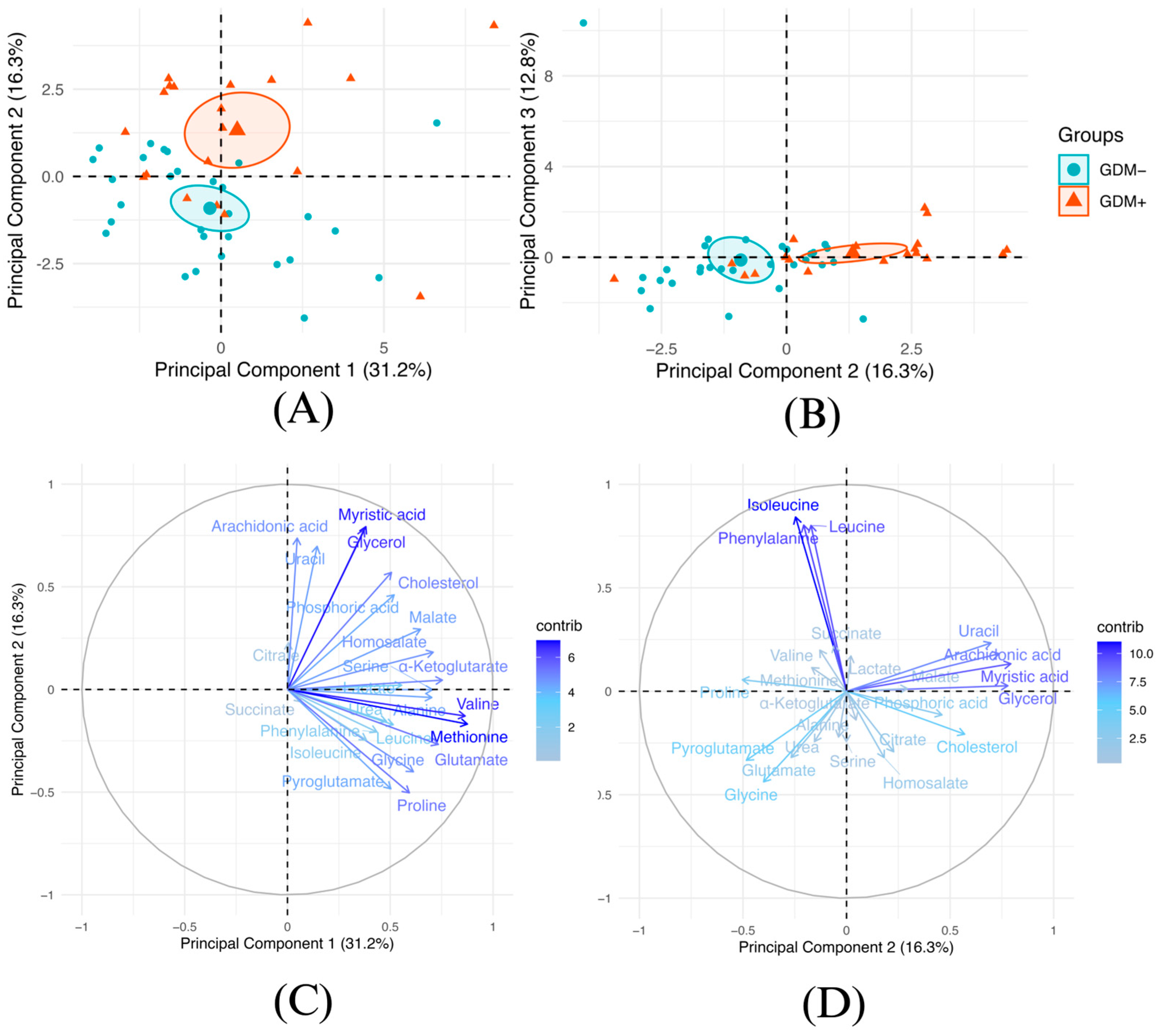
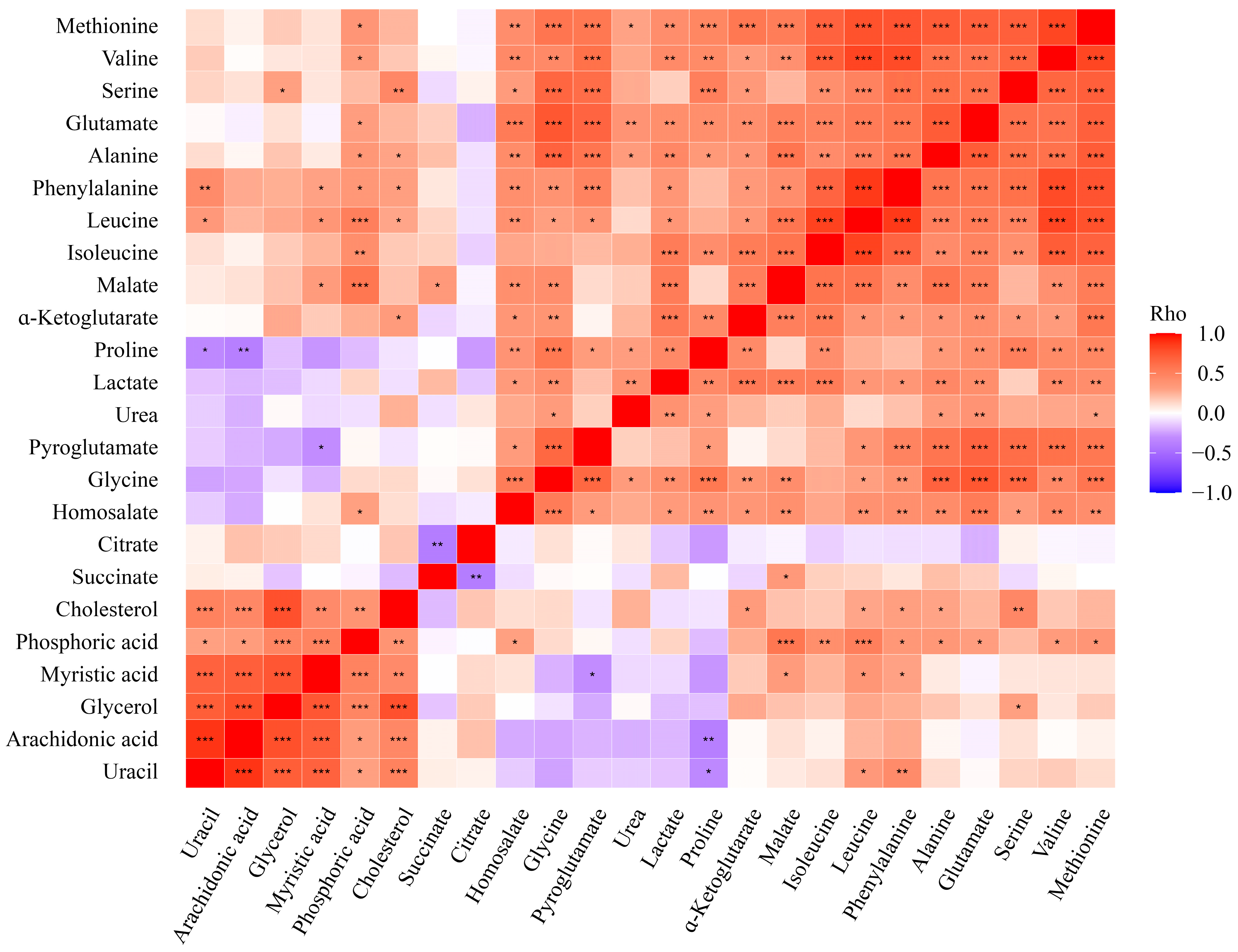
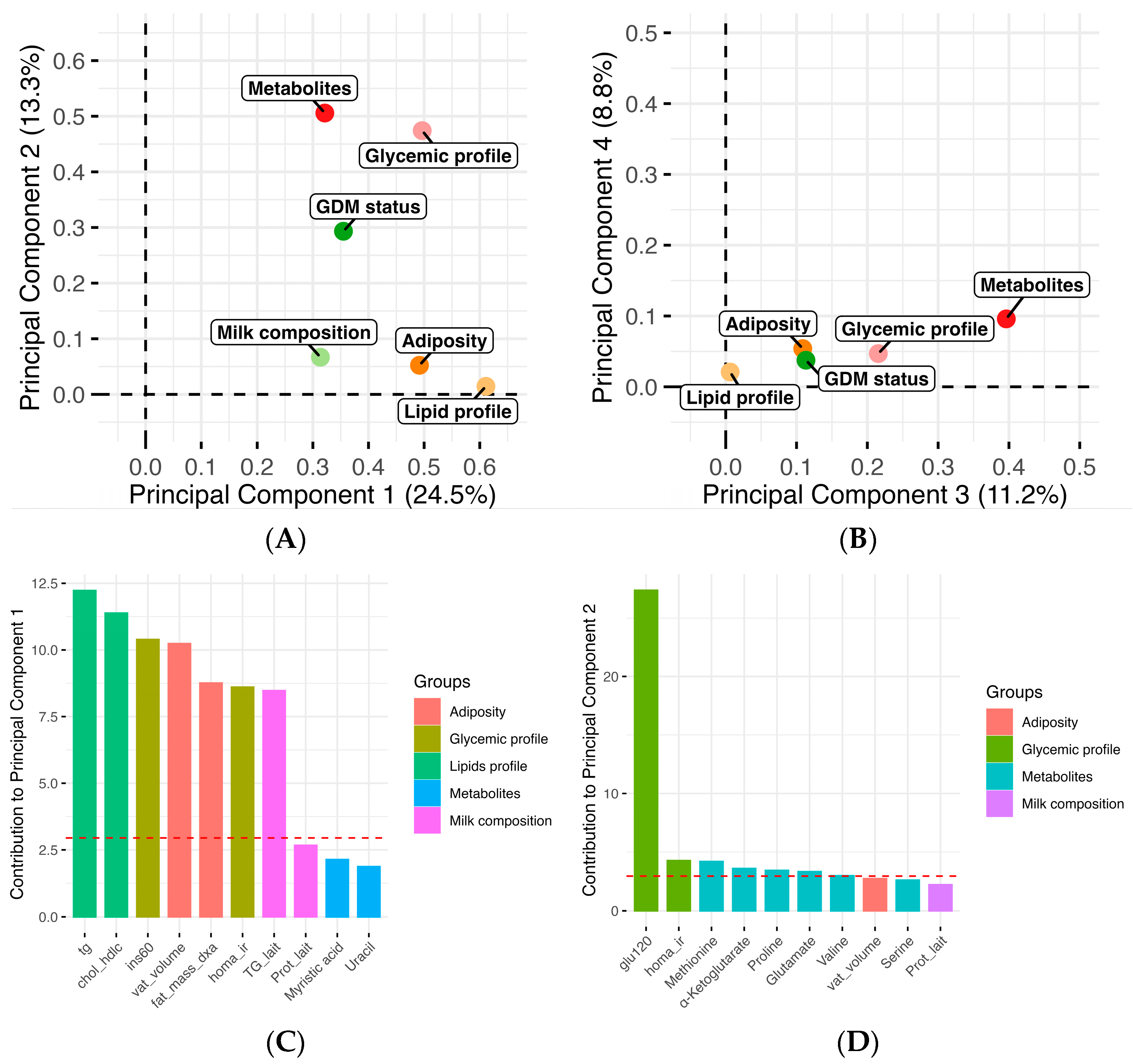
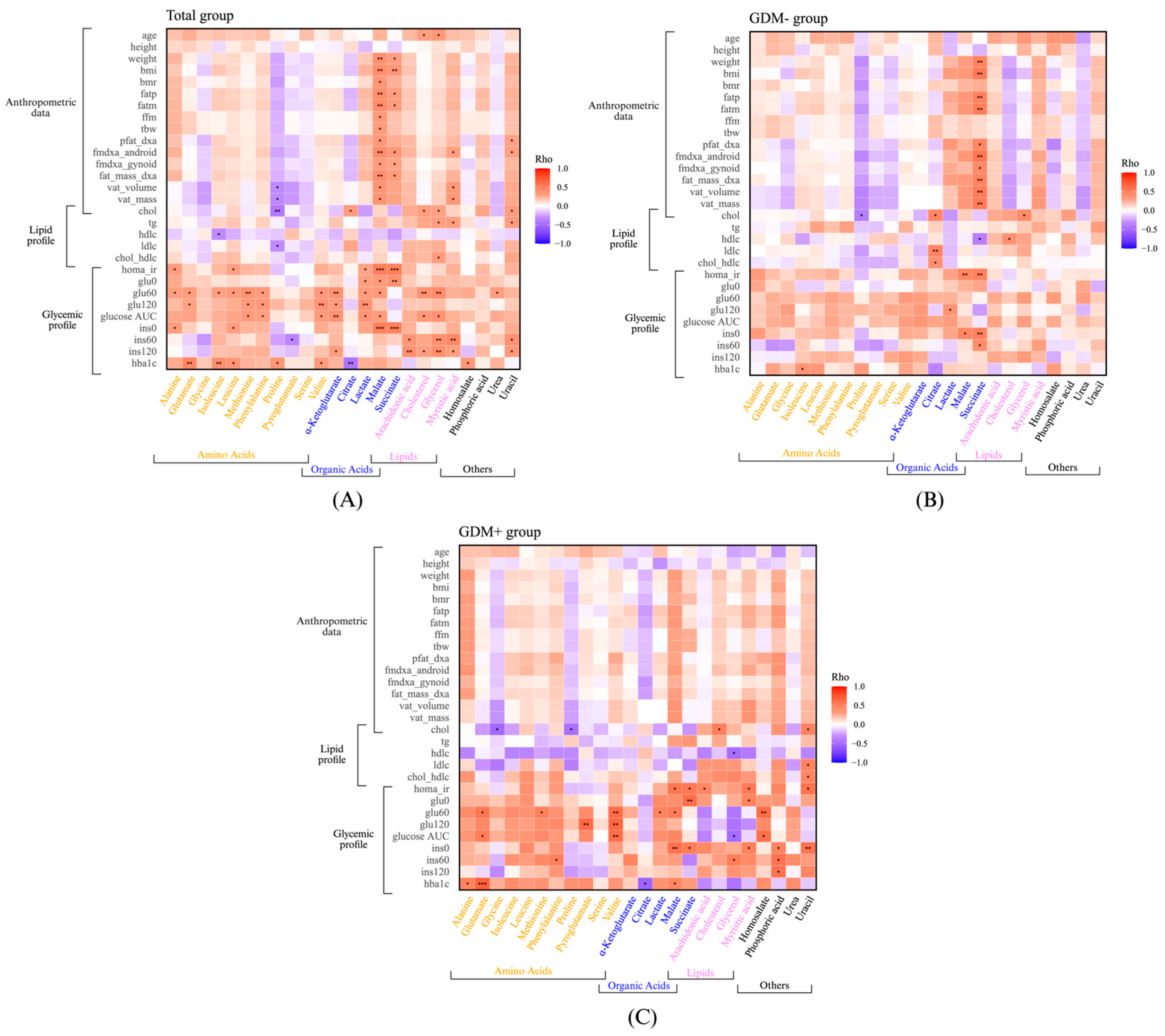
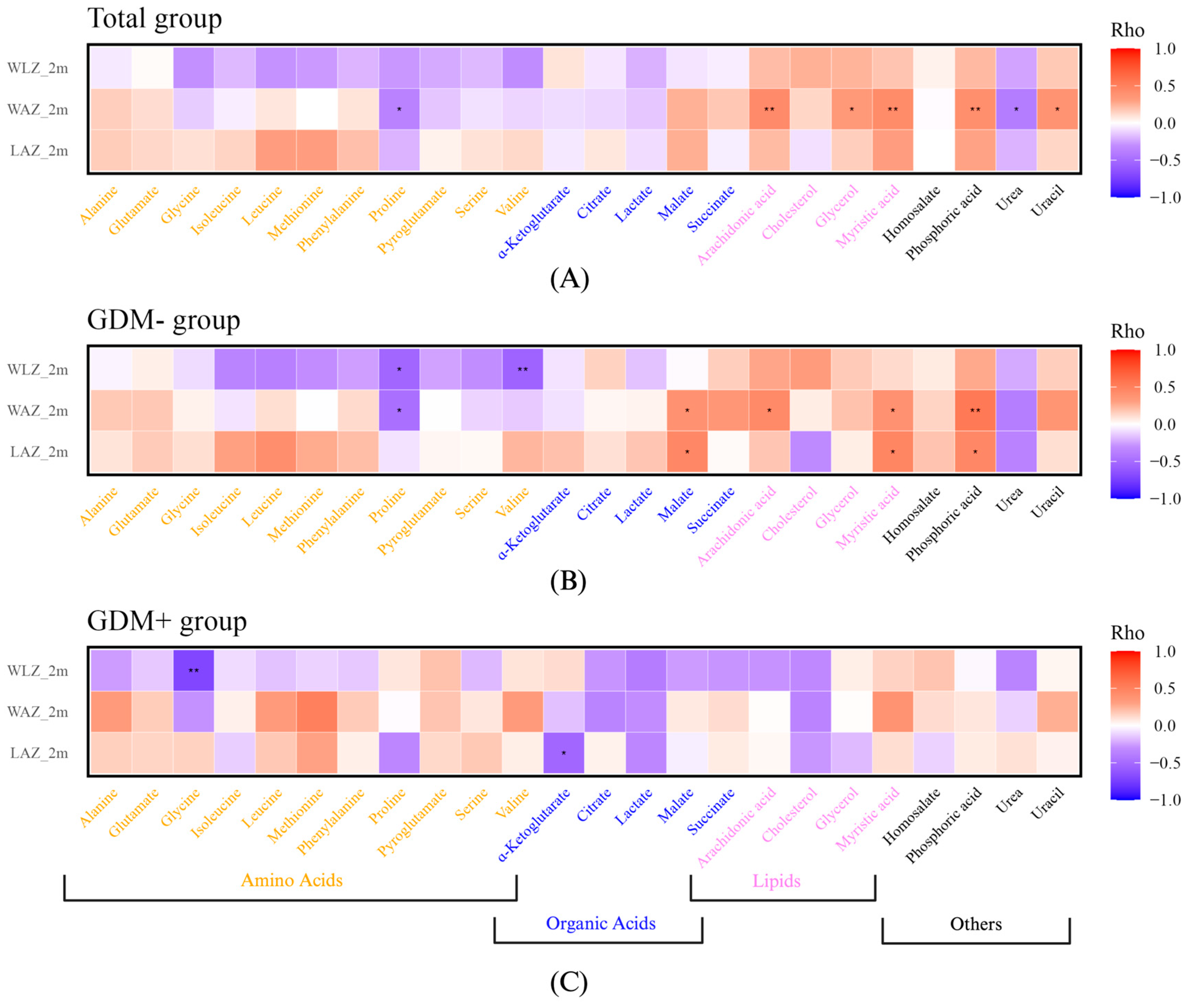
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational Diabetes Mellitus. Nat. Rev. Dis. Primers. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metabolism 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational Diabetes and the Risk of Cardiovascular Disease in Women: A Systematic Review and Meta-Analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Sheiner, E. Gestational Diabetes Mellitus: Long-Term Consequences for the Mother and Child Grand Challenge: How to Move on Towards Secondary Prevention? Front. Clin. Diabetes Healthc. 2020, 1, 546256. [Google Scholar] [CrossRef]
- Rassie, K.; Mousa, A.; Joham, A.; Teede, H.J. Metabolic Conditions Including Obesity, Diabetes, and Polycystic Ovary Syndrome: Implications for Breastfeeding and Breastmilk Composition. Semin. Reprod. Med. 2021, 39, 111–132. [Google Scholar] [CrossRef]
- Hannan, F.M.; Elajnaf, T.; Vandenberg, L.N.; Kennedy, S.H.; Thakker, R.V. Hormonal Regulation of Mammary Gland Development and Lactation. Nat. Rev. Endocrinol. 2023, 19, 46–61. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary Gland Development. WIREs Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Neville, M.C.; McFadden, T.B.; Forsyth, I. Hormonal Regulation of Mammary Differentiation and Milk Secretion. J. Mammary Gland Biol. Neoplasia 2002, 7, 49–66. [Google Scholar] [CrossRef]
- Abdul Sattar, S.A.; Abdulla, A.H.; Nsaif, S.A. Gestational Diabetes Mellitus and Hormonal Alteration. IJPS 2017, 25, 37–41. [Google Scholar] [CrossRef]
- Watt, A.P.; Lefevre, C.; Wong, C.S.; Nicholas, K.R.; Sharp, J.A. Insulin Regulates Human Mammosphere Development and Function. Cell Tissue Res. 2021, 384, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Liu, Y.; Zakeri, I.F.; Mohney, R.P.; Mehta, N.; Voruganti, V.S.; Göring, H.; Cole, S.A.; Comuzzie, A.G. Global Metabolomic Profiling Targeting Childhood Obesity in the Hispanic Population. Am. J. Clin. Nutr. 2015, 102, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P. Impact of Breastfeeding on Maternal Metabolism: Implications for Women with Gestational Diabetes. Curr. Diab. Rep. 2014, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025; U.S. Government Publishing Office: Washington, DC, USA, 2020; pp. 107–120.
- Yi, D.; Kim, S. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef]
- Zhang, Z.; Adelman, A.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino Acid Profiles in Term and Preterm Human Milk through Lactation: A Systematic Review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W.S. Regulation and Function of the Mammalian Tricarboxylic Acid Cycle. J. Biol. Chem. 2023, 299, 102838. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A. Does Insulin Explain the Relation between Maternal Obesity and Poor Lactation Outcomes? An Overview of the Literature. Adv. Nutr. 2016, 7, 407–414. [Google Scholar] [CrossRef]
- Dugas, C.; Laberee, L.; Perron, J.; St-Arnaud, G.; Richard, V.; Perreault, V.; Leblanc, N.; Marc, I.; Di Marzo, V.; Doyen, A.; et al. Gestational Diabetes Mellitus, Human Milk Composition, and Infant Growth. Breastfeed. Med. 2023, 18, 14–22. [Google Scholar] [CrossRef]
- Fradet, A.; Castonguay-Paradis, S.; Dugas, C.; Perron, J.; St-Arnaud, G.; Marc, I.; Doyen, A.; Flamand, N.; Dahhani, F.; Di Marzo, V.; et al. The Human Milk Endocannabinoidome and Neonatal Growth in Gestational Diabetes. Front. Endocrinol. 2024, 15, 1415630. [Google Scholar] [CrossRef]
- Aydin, S.; Celik, O.; Gurates, B.; Sahin, İ.; Ulas, M.; Yilmaz, M.; Kalayci, M.; Kuloglu, T.; Catak, Z.; Aksoy, A.; et al. Concentrations of Preptin, Salusins and Hepcidins in Plasma and Milk of Lactating Women with or without Gestational Diabetes Mellitus. Peptides 2013, 49, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhu, Q.; Dong, Y.; Li, Y.; Liu, J.; Yan, Q.; Huang, M.; Niu, Y. Analysis of Serum Fatty Acid, Amino Acid, and Organic Acid Profiles in Gestational Hypertension and Gestational Diabetes Mellitus via Targeted Metabolomics. Front. Nutr. 2022, 9, 974902. [Google Scholar] [CrossRef] [PubMed]
- Simon Sarkadi, L.; Zhang, M.; Muránszky, G.; Vass, R.A.; Matsyura, O.; Benes, E.; Vari, S.G. Fatty Acid Composition of Milk from Mothers with Normal Weight, Obesity, or Gestational Diabetes. Life 2022, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Perron, J.; Marc, I.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J. Association of Prenatal Exposure to Gestational Diabetes with Offspring Body Composition and Regional Body Fat Distribution: Gestational Diabetes and Children Adiposity. Clin. Obes. 2018, 8, 81–87. [Google Scholar] [CrossRef]
- Richterich, R.; Dauwalder, H. Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method. Schweiz. Med. Wochenschr. 1971, 101, 615–618. [Google Scholar]
- Desbuquois, B.; Aurbach, G.D. Use of Polyethylene Glycol to Separate Free and Antibody-Bound Peptide Hormones in Radioimmunoassays. J. Clin. Endocrinol. Metab. 1971, 33, 732–738. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and ?-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Gingras, V.; Vigneault, J.; Weisnagel, S.J.; Tchernof, A.; Robitaille, J. Accelerometry-Measured Physical Activity and Inflammation after Gestational Diabetes. Med. Sci. Sports Exerc. 2013, 45, 1307–1312. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- WHO MULTICENTRE GROWTH REFERENCE STUDY GROUP; De Onis, M. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar] [CrossRef]
- Frégeau-Proulx, L.; Lacouture, A.; Berthiaume, L.; Weidmann, C.; Harvey, M.; Gonthier, K.; Pelletier, J.-F.; Neveu, B.; Jobin, C.; Bastien, D.; et al. Multiple Metabolic Pathways Fuel the Truncated Tricarboxylic Acid Cycle of the Prostate to Sustain Constant Citrate Production and Secretion. Mol. Metab. 2022, 62, 101516. [Google Scholar] [CrossRef] [PubMed]
- Lafront, C.; Germain, L.; Campolina-Silva, G.H.; Weidmann, C.; Berthiaume, L.; Hovington, H.; Brisson, H.; Jobin, C.; Frégeau-Proulx, L.; Cotau, R.; et al. The Estrogen Signaling Pathway Reprograms Prostate Cancer Cell Metabolism and Supports Proliferation and Disease Progression. J. Clin. Investig. 2024, 134, e170809. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. CP Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Krausz, K.W.; Xie, C.; Beyoğlu, D.; Gonzalez, F.J.; Idle, J.R. Metabolic Profiling by Gas Chromatography-Mass Spectrometry of Energy Metabolism in High-Fat Diet-Fed Obese Mice. PLoS ONE 2017, 12, e0177953. [Google Scholar] [CrossRef]
- Gonthier, K.; Weidmann, C.; Berthiaume, L.; Jobin, C.; Lacouture, A.; Lafront, C.; Harvey, M.; Neveu, B.; Loehr, J.; Bergeron, A.; et al. Isocitrate Dehydrogenase 1 Sustains a Hybrid Cytoplasmic–Mitochondrial Tricarboxylic Acid Cycle That Can Be Targeted for Therapeutic Purposes in Prostate Cancer. Mol. Oncol. 2023, 17, 2109–2125. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology NIST/EPA/NIH Mass Spectral Library (NIST 17). 2017. Available online: https://www.sisweb.com/software/ms/nist17.pdf (accessed on 31 March 2025).
- Quispe, R.; Elshazly, M.B.; Zhao, D.; Toth, P.P.; Puri, R.; Virani, S.S.; Blumenthal, R.S.; Martin, S.S.; Jones, S.R.; Michos, E.D. Total Cholesterol/HDL-Cholesterol Ratio Discordance with LDL-Cholesterol and Non-HDL-Cholesterol and Incidence of Atherosclerotic Cardiovascular Disease in Primary Prevention: The ARIC Study. Eur. J. Prev. Cardiol. 2020, 27, 1597–1605. [Google Scholar] [CrossRef]
- Jain, S.; Jacobson, K.A. Adipocyte Purinergic Receptors Activated by Uracil Nucleotides as Obesity and Type 2 Diabetes Targets. Curr. Opin. Pharmacol. 2022, 63, 102190. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. IJMS 2020, 21, 9628. [Google Scholar] [CrossRef]
- Takato, T.; Iwata, K.; Murakami, C.; Wada, Y.; Sakane, F. Chronic Administration of Myristic Acid Improves Hyperglycaemia in the Nagoya–Shibata–Yasuda Mouse Model of Congenital Type 2 Diabetes. Diabetologia 2017, 60, 2076–2083. [Google Scholar] [CrossRef]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte Lipolysis and Insulin Resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.-M.; Cline, G.W.; Nakahara, K.; et al. Metformin, Phenformin, and Galegine Inhibit Complex IV Activity and Reduce Glycerol-Derived Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef] [PubMed]
- Zeljković, A.; Ardalić, D.; Vekić, J.; Antonić, T.; Vladimirov, S.; Rizzo, M.; Gojković, T.; Ivanišević, J.; Mihajlović, M.; Vujčić, S.; et al. Effects of Gestational Diabetes Mellitus on Cholesterol Metabolism in Women with High-Risk Pregnancies: Possible Implications for Neonatal Outcome. Metabolites 2022, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Fall, C. Infant Nutrition and Later Health: A Review of Current Evidence. Nutrients 2012, 4, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Bayley, T.M.; Alasmi, M.; Thorkelson, T.; Krug-Wispe, S.; Jones, P.J.H.; Bulani, J.L.; Tsang, R.C. Influence of Formula versus Breast Milk on Cholesterol Synthesis Rates in Four-Month-Old Infants. Pediatr. Res. 1998, 44, 60–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Liu, B.; Shu, H.; Zhang, L.; Bao, M.; Yi, W.; Tan, Y.; Ji, X.; Zhang, C.; et al. Human Serum Metabolomic Analysis Reveals Progression for High Blood Pressure in Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2021, 9, e002337. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A.J. Succinate: A Metabolic Signal in Inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Sabadell-Basallote, J.; Astiarraga, B.; Castaño, C.; Ejarque, M.; Repollés-de-Dalmau, M.; Quesada, I.; Blanco, J.; Núñez-Roa, C.; Rodríguez-Peña, M.-M.; Martínez, L.; et al. SUCNR1 Regulates Insulin Secretion and Glucose Elevates the Succinate Response in People with Prediabetes. J. Clin. Investig. 2024, 134, e173214. [Google Scholar] [CrossRef]
- Shen, D.; Kruger, L.; Deatherage, T.; Denton, T.T. Synthesis of α-Ketoglutaramic Acid. Anal. Biochem. 2020, 607, 113862. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Steinacker, J.M. α-Ketoglutarate Stimulates Cell Growth through the Improvement of Glucose and Glutamine Metabolism in C2C12 Cell Culture. Front. Nutr. 2023, 10, 1145236. [Google Scholar] [CrossRef]
- Kostiuchenko, O.; Lushnikova, I.; Kowalczyk, M.; Skibo, G. mTOR/α-Ketoglutarate-Mediated Signaling Pathways in the Context of Brain Neurodegeneration and Neuroprotection. BBA Adv. 2022, 2, 100066. [Google Scholar] [CrossRef]
- Hall, J.C. Review: Glycine. J. Parenter. Enter. Nutr. 1998, 22, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, C.; Azab, S.; De Souza, R.J.; Shanmuganathan, M.; Desai, D.; Teo, K.; Atkinson, S.A.; Morrison, K.; Gupta, M.; Britz-McKibbin, P.; et al. Serum Metabolomic Signatures of Gestational Diabetes in South Asian and White European Women. BMJ Open Diabetes Res. Care 2022, 10, e002733. [Google Scholar] [CrossRef]
- White, P.J.; Lapworth, A.L.; McGarrah, R.W.; Kwee, L.C.; Crown, S.B.; Ilkayeva, O.; An, J.; Carson, M.W.; Christopher, B.A.; Ball, J.R.; et al. Muscle-Liver Trafficking of BCAA-Derived Nitrogen Underlies Obesity-Related Glycine Depletion. Cell Rep. 2020, 33, 108375. [Google Scholar] [CrossRef]

| GDM- (n = 29) | GDM+ (n = 24) | p Value | |
|---|---|---|---|
| Maternal | |||
| Metabolic data | |||
| Age (years) | 30.0 ± 3.1 | 33.6 ± 3.6 | <0.001 *** |
| Height (m) | 1.64 ± 0.07 | 1.65 ± 0.04 | 0.60 A |
| Weight (Kg) | 74.6 ± 17.6 | 84.8 ± 19.7 | 0.067 |
| BMI (kg/m2) | 27.7 ± 5.9 | 31.2 ± 7.1 | 0.07 |
| Fat mass (%) | 38.0 ± 8.6 | 42.8 ± 6.8 | 0.04 * |
| Fasting glucose (mmol/L) | 4.80 ± 0.31 | 5.04 ± 0.42 | 0.05 |
| Glucose AUC (mmol.min/L) | 670 ± 101 | 804 ± 118 | <0.001 *** |
| Fasting insulin (pmol/L) | 51.0 ± 35.34 | 50.9 ± 20.5 | 0.64 A |
| Hba1c (%) | 5.18 ± 0.37 | 5.31 ± 0.24 | 0.14 A |
| Cholesterol (mmol/L) | 5.09 ± 1.04 | 5.24 ± 1.13 | 0.62 |
| Triglycerides (mmol/L) | 0.84 ± 0.40 | 1.15 ± 0.46 | 0.007 **A |
| Hdlc (mmol/L) | 1.74 ± 0.37 | 1.56 ± 0.29 | 0.04 * |
| Ldlc (mmol/L) | 2.96 ± 0.90 | 3.15 ± 1.05 | 0.423 |
| Chol/hdlc | 3.02 ± 0.80 | 3.45 ± 0.94 | 0.047 * |
| GDM treatment | |||
| Diet only | — | 9 (45%) | — |
| Insulin or oral hypoglycemic agent | — | 11 (55%) | — |
| Gestational age (weeks) | 39.41 ± 1.1 | 38.6 ± 1.0 | 0.01 * |
| Breastfeeding | 0.23 B | ||
| Exclusive | 29 (100%) | 22 (91.7%) | |
| Non-exclusive | 0 (0%) | 2 (8.3%) | |
| Timing of human milk collection | 0.28 | ||
| Day (6:00 a.m. to 6:00 p.m.) | 25 (86%) | 14 (70%) | |
| Night (6:00 p.m. to 6:00 a.m.) | 4 (14%) | 6 (30%) | |
| Delivery | 1 B | ||
| Vaginal Birth | 27 (93.1%) | 22 (91.7%) | |
| Cesarean | 2 (6.9%) | 2 (8.3%) | |
| Infant | |||
| Sex | 0.01 *C | ||
| Boys | 12 (41%) | 17 (74%) | |
| Girls | 17 (59%) | 6 (26%) | |
| Length (cm) | |||
| Birth | 50.70 ± 1.60 | 50 ± 4.17 | 0.42 |
| 2 months | 57.63 ± 1.73 | 57.77 ± 2.95 | 0.86 |
| Weight (kg) | |||
| Birth | 3.34 ± 0.38 | 3.37 ± 0.34 | 0.75 |
| 2 months | 5.06 ± 0.71 | 5.48 ± 0.78 | 0.08 |
| LAZ | |||
| Birth | 0.68 ± 0.88 | 0.64 ± 0.91 | 0.89 |
| 2 months | 0.04 ± 0.83 | 0.17 ± 1.07 | 0.66 |
| WAZ | |||
| Birth | 0.10 ± 0.84 | 0.11 ± 0.69 | 0.98 |
| 2 months | −0.32 ± 0.88 | 0.23 ± 0.72 | 0.03 * |
| WLZ | |||
| Birth | −0.64 ± 1.26 | −0.52 ±1.42 | 0.79 |
| 2 months | −0.33 ± 1.07 | 0.04 ± 0.73 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fradet, A.; Berthiaume, L.; Laroche, L.-A.; Dugas, C.; Perron, J.; Doyen, A.; Audet-Walsh, É.; Robitaille, J. Variations in Human Milk Metabolites After Gestational Diabetes: Associations with Infant Growth. Nutrients 2025, 17, 1466. https://doi.org/10.3390/nu17091466
Fradet A, Berthiaume L, Laroche L-A, Dugas C, Perron J, Doyen A, Audet-Walsh É, Robitaille J. Variations in Human Milk Metabolites After Gestational Diabetes: Associations with Infant Growth. Nutrients. 2025; 17(9):1466. https://doi.org/10.3390/nu17091466
Chicago/Turabian StyleFradet, Alice, Line Berthiaume, Laurie-Anne Laroche, Camille Dugas, Julie Perron, Alain Doyen, Étienne Audet-Walsh, and Julie Robitaille. 2025. "Variations in Human Milk Metabolites After Gestational Diabetes: Associations with Infant Growth" Nutrients 17, no. 9: 1466. https://doi.org/10.3390/nu17091466
APA StyleFradet, A., Berthiaume, L., Laroche, L.-A., Dugas, C., Perron, J., Doyen, A., Audet-Walsh, É., & Robitaille, J. (2025). Variations in Human Milk Metabolites After Gestational Diabetes: Associations with Infant Growth. Nutrients, 17(9), 1466. https://doi.org/10.3390/nu17091466





