Glutamate Supplementation Regulates Nitrogen Metabolism in the Colon and Liver of Weaned Rats Fed a Low-Protein Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Fecal Sample Collection and Pretreatment
2.3. Determination of Fecal Nitrogen and Fecal Microbial Nitrogen
2.4. Quantitation of mRNA Expression by Quantitative Real-Time PCR
2.5. Western Blot Analysis
2.6. Mucin Staining
2.7. H&E Staining
2.8. DNA Extraction and 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. Effects of Glutamate Supplementation on Colon Length, Colon Morphology and Mucin Abundance in Weaned Rats with Protein Restriction
3.2. The Impacts of Glutamate Supplementation in Protein-Restricted Diets on the Abundance of Genes Associated with Na/K-ATPase, Amino Acid Transporter, and Glutamate Receptor in the Colon
3.3. The Effects of Glutamate Supplementation in Protein-Restricted Diets on the Levels of TJ Proteins in the Colon
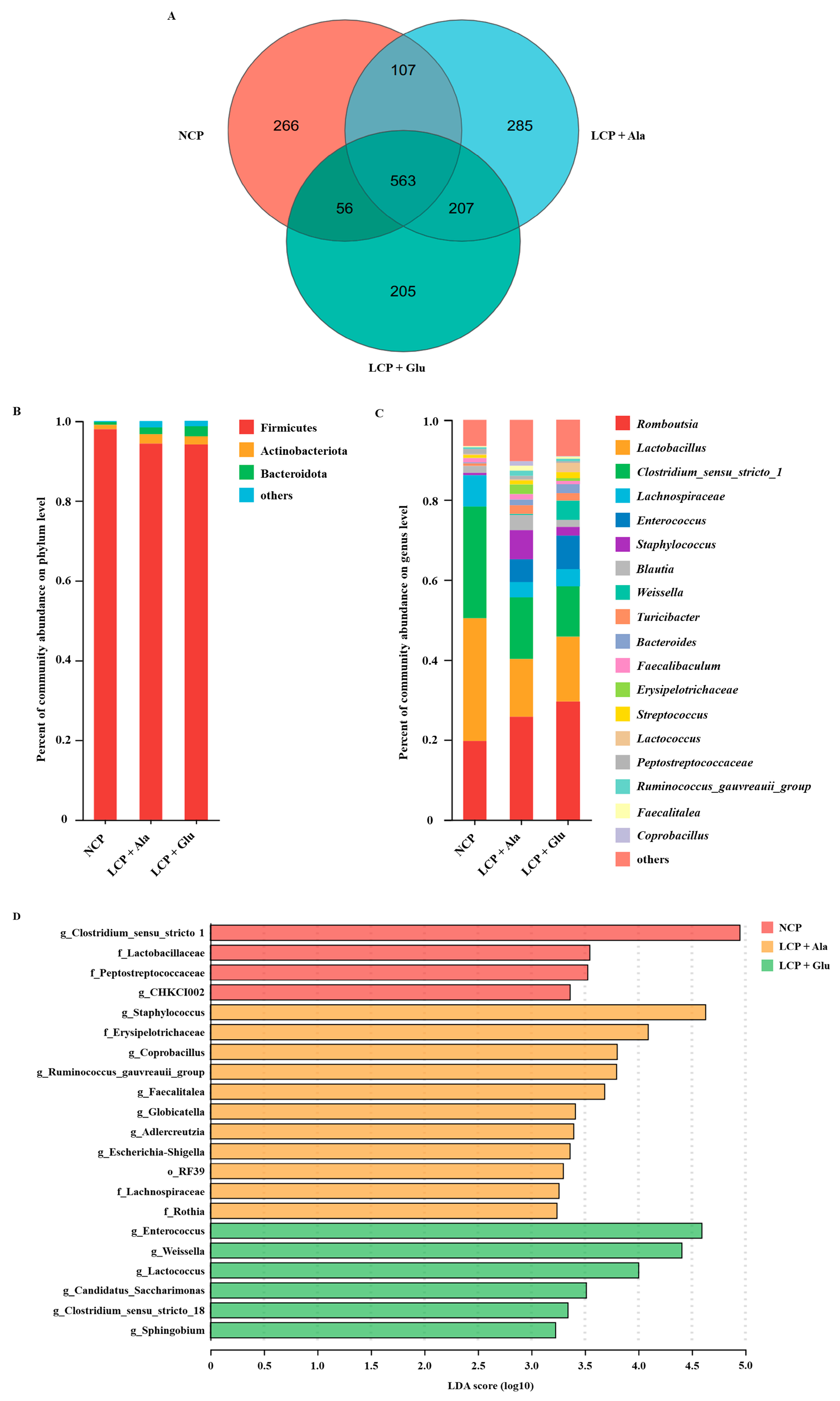
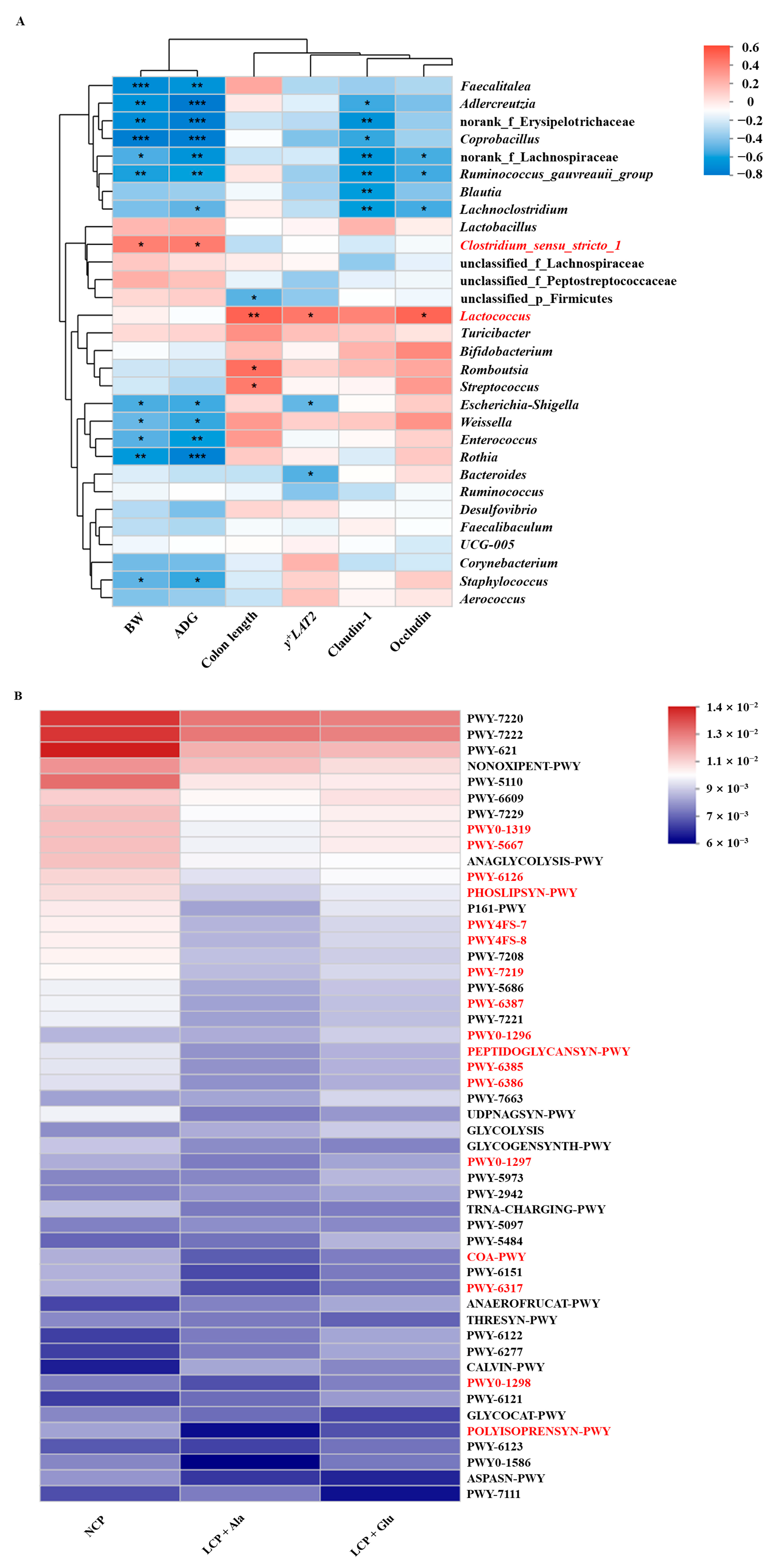
3.4. The Impacts of Glutamate on the Composition of the Microbial Community in Colon Contents
3.5. Effects of Glutamate Supplementation on Fecal Nitrogen Metabolism of Weaned Rats Fed a Low-Protein Diet
3.6. Effects of Glutamate Supplementation on the Abundance of Genes Involved in Na/K-ATPase and Amino Acid Transporters in the Liver
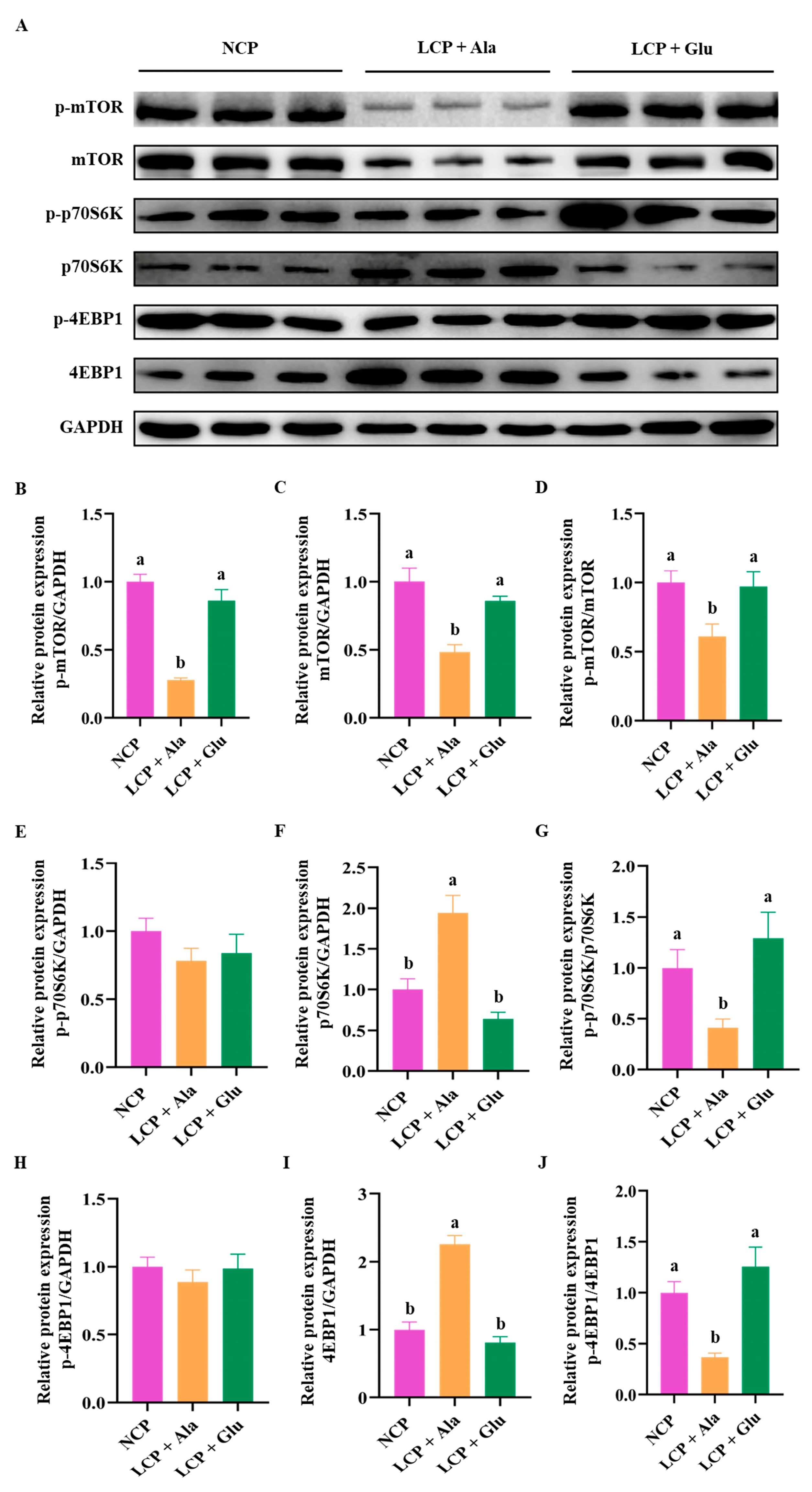
3.7. Effects of Glutamate Supplementation in Protein-Restricted Diets on the Abundance of mTOR/p70S6K/4EBP1 Signaling Pathways in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIN | American Institute of Nutrition |
| ASCT2 | Alanine-serine-cysteine transporter 2 |
| ATB0,+ | Amino acid transporter B0,+ |
| ATP | Adenosine triphosphate |
| CP | Crude protein |
| EAA | Essential amino acid |
| EAAT3 | Excitatory amino acid transporter 3 |
| H&E | Haematoxylin and eosin |
| LCP + Ala | Low-crude-protein diet supplemented with alanine |
| LCP + Glu | Low-crude-protein diet supplemented with 2.07% glutamate |
| NCP | Normal crude protein diet |
| PCR | Polymerase chain reaction |
| PEM | Protein–energy malnutrition |
| SEM | Standard error of mean |
| T1R1 | Taste receptor type 1 subunit 1 |
| TJ | Tight junction |
| ZO | Zonula occludens |
References
- Zhang, X.; Zhang, L.; Pu, Y.; Sun, M.; Zhao, Y.; Zhang, D.; Wang, X.; Li, Y.; Guo, D.; He, S. Global, Regional, and National Burden of Protein–Energy Malnutrition: A Systematic Analysis for the Global Burden of Disease Study. Nutrients 2022, 14, 2592. [Google Scholar] [CrossRef] [PubMed]
- WHO. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition. Available online: https://www.who.int/publications-detail-redirect/9789240025257 (accessed on 4 March 2025).
- WHO. Levels and Trends in Child Malnutrition: UNICEF/WHO/The World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2021 Edition. Available online: https://www.who.int/publications/i/item/9789240025257 (accessed on 26 March 2025).
- de Onís, M.; Monteiro, C.; Akré, J.; Glugston, G. The worldwide magnitude of protein-energy malnutrition: An overview from the WHO Global Database on Child Growth. Bull World Health Organ. Bull. World Health Organ. 1993, 71, 703–712. [Google Scholar] [PubMed]
- Pelletier, D.L.; Frongillo, E.A., Jr.; Habicht, J.P. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am. J. Public Health 1993, 83, 1130–1133. [Google Scholar] [CrossRef]
- Schofield, C.; Ashworth, A. Why have mortality rates for severe malnutrition remained so high? Bull. World Health Organ. 1996, 74, 223–229. [Google Scholar]
- Young, M.; Wolfheim, C.; Marsh, D.R.; Hammamy, D. World Health Organization/United Nations Children’s Fund Joint Statement on Integrated Community Case Management: An Equity-Focused Strategy to Improve Access to Essential Treatment Services for Children. Am. J. Trop. Med. Hyg. 2012, 87, 6–10. [Google Scholar] [CrossRef]
- Bongaarts, J. The State of Food Security and Nutrition in the World 2020. Transforming food systems for affordable healthy diets. Popul. Dev. Rev. 2021, 47, 558. [Google Scholar] [CrossRef]
- Quamme, S.H.; Iversen, P.O. Prevalence of child stunting in Sub-Saharan Africa and its risk factors. Clin. Nutr. Open Sci. 2022, 42, 49–61. [Google Scholar] [CrossRef]
- Dessie, A.M.; Seretew, W.S.; Tesema, G.A.; Yirsaw, B.G.; Argaw, G.S. Prevalence of stunting and associated factors among under-five children in sub-Saharan Africa: Multilevel ordinal logistic regression analysis modeling. PLoS ONE 2024, 19, e0299310. [Google Scholar] [CrossRef]
- Reeds, P.J.; Burrin, D.G.; Stoll, B.; Jahoor, F. Intestinal glutamate metabolism. J. Nutr. 2000, 130, 978S–982S. [Google Scholar] [CrossRef]
- Pieper, R.; Kröger, S.; Richter, J.F.; Wang, J.; Martin, L.; Bindelle, J.; Htoo, J.K.; von Smolinski, D.; Vahjen, W.; Zentek, J.; et al. Fermentable fiber ameliorates fermentable protein-induced changes in microbial. J. Nutr. 2012, 142, 661–667. [Google Scholar] [CrossRef]
- Li, J.; Bai, J.; Yang, Y.; Wu, Z. Low-protein diet supplemented with 1% L-glutamine improves growth performance, serum biochemistry, redox status, plasma amino acids, and alters fecal microbiota in weaned piglets. Anim. Nutr. 2024, 17, 144–154. [Google Scholar] [CrossRef]
- Richardson, A.J.; McKain, N.; Wallace, R.J. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, M.; Ren, W.; Chen, L.; Li, T.; Yin, Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, B.; Yu, C.; Sun, H.; Gao, F.; Zhou, G. L-Glutamate supplementation improves small intestinal architecture and enhances the expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. PLoS ONE 2014, 9, e111950. [Google Scholar] [CrossRef]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Whitehead, T.R.; Price, N.P.; Drake, H.L.; Cotta, M.A. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl. Environ. Microbiol. 2008, 74, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.P.; Fang, L.D.; Sun, Y.; Su, Y.; Zhu, W.Y. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 2016, 38, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolution of Colitis in Rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Fan, P.X.; Li, L.S.; Rezaei, A.; Eslamfam, S.; Che, D.S.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch. Biochem. Biophys. 1976, 175, 670–676. [Google Scholar] [CrossRef]
- Li, L.; Zhang, P.; Zheng, P.; Bao, Z.; Wang, Y.; Huang, F.R. Hepatic cumulative net appearance of amino acids and related gene expression response to different protein diets in pigs. Livest. Sci. 2015, 182, 11–21. [Google Scholar] [CrossRef]
- Alamri, Z.Z. The role of liver in metabolism: An updated review with physiological emphasis. Int. J. Basic Clin. Pharmacol. 2018, 7, 2271–2276. [Google Scholar] [CrossRef]
- Rostom, H.; Shine, B. Basic metabolism: Proteins. Surgery 2023, 41, 761–766. [Google Scholar]
- Wang, D.; Xu, C.C.; Yang, W.Y.; Chen, J.; Ou, Y.H.; Guan, Y.Y.; Guan, J.L.; Liu, Y. E3 ligase RNF167 and deubiquitinase STAMBPL1 modulate mTOR and cancer progression. Mol. Cell 2022, 82, 770–784.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Wang, Y.; Wei, X.Y.; Cai, W.F.; Wu, J.F.; Zhu, M.X.; Wang, Y.L.; Liu, Y.H.; Xiong, J.Y.; Qu, Q.; et al. AMPK targets PDZD8 to trigger carbon source shift from glucose to glutamine. Cell Res. 2024, 34, 683–706. [Google Scholar] [CrossRef]
- Hu, S.H.; Feng, Y.Y.; Yang, Y.X.; Ma, H.D.; Zhou, S.X.; Qiao, Y.N.; Zhang, K.H.; Zhang, L.; Huang, L.; Yuan, Y.Y.; et al. Amino acids downregulate SIRT4 to detoxify ammonia through the urea cycle. Nat. Metab. 2023, 5, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Shon, W.J.; Park, M.N.; Lee, J.; Shin, J.H.; Shin, D.M. Renal transcriptome profiles in mice reveal the need for sufficient water intake irrespective of the drinking water type. Sci. Rep. 2022, 12, 10911. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli, J. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Liu, T.; Peng, J.; Xiong, Y.H.; Zhou, S.Q.; Cheng, X.H. Effects of dietary glutamine and glutamate supplementation on small intestinal structure, active absorption and DNA, RNA concentrations in skeletal muscle tissue of weaned piglets during d 28 to 42 of age. Asian-Australas. J. Anim. Sci. 2002, 15, 238–242. [Google Scholar] [CrossRef]
- Li, Y.Y.; Han, H.; Yin, J.; Zheng, J.; Zhu, X.T.; Li, T.J.; Yin, Y.L. Effects of glutamate and aspartate on growth performance, serum amino acids, and amino acid transporters in piglets. Food Agric. Immunol. 2018, 29, 675–687. [Google Scholar] [CrossRef]
- Yue, L.Y.; Qiao, S.Y. Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning. Livest. Sci. 2008, 115, 144–152. [Google Scholar] [CrossRef]
- Zhang, S.H.; Qiao, S.Y.; Ren, M.; Zeng, X.F.; Ma, X.; Wu, Z.L.; Thacker, P.; Wu, G.Y. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 2013, 45, 1191–1205. [Google Scholar] [CrossRef]
- Nechvatal, J.M.; Ram, J.L.; Basson, M.D.; Namprachan, P.; Niec, S.R.; Badsha, K.Z.; Matherly, L.H.; Majumdar, A.P.N.; Kato, I. Fecal collection, ambient preservation, and DNA extraction for PCR amplification of bacterial and human markers from human feces. J. Microbiol. Methods 2008, 72, 124–132. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6432-94; Method for the Determination of Crude Protein in Feedstuffs. National Standards of the People’s Republic of China: Beijing, China, 1994.
- Miner-Williams, W.; Moughan, P.J.; Fuller, M.F. Endogenous components of digesta protein from the terminal ileum of pigs fed a casein-based diet. J. Agric. Food Chem. 2009, 57, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, J.Q.; Ji, Y.; Yang, Q.; Chen, Y.F.; Wang, F.C.; Wu, Z.L. Amuc attenuates high-fat diet-induced metabolic disorders linked to the regulation of fatty acid metabolism, bile acid metabolism, and the gut microbiota in mice. Int. J. Biol. Macromol. 2023, 242, 124650. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.Z.; Deng, S.W.; Yang, Y.; Chen, S.; Wu, Z.L. Microplastics caused embryonic growth retardation and placental dysfunction in pregnant mice by activating GRP78/IRE1α/JNK axis induced apoptosis and endoplasmic reticulum stress. Part. Fibre Toxicol. 2024, 21, 36. [Google Scholar] [CrossRef]
- Tang, Z.N.; Yang, Y.; Yang, M.R.; Jiang, D.; Ge, Y.; Zhang, X.Y.; Liu, H.Z.; Fu, Q.Y.; Liu, X.Y.; Yang, Y.; et al. Elucidating the modulatory role of dietary hydroxyproline on the integrity and functional performance of the intestinal barrier in early-weaned piglets: A comprehensive analysis of its interplay with the gut microbiota and metabolites. Int. Immunopharmacol. 2024, 134, 112268. [Google Scholar] [CrossRef]
- Li, J.; Bai, J.; Song, Z.; Ji, Y.; Chen, Z.H.; Yang, Y.; Wu, Z.L. Dietary pectin attenuates Salmonella typhimurium-induced colitis by modulating the TLR2-NF-κB pathway and intestinal microbiota in mice. Food Chem. Toxicol. 2023, 182, 114100. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, X.X.; Zhang, Y.C.; Li, J.; Dai, Z.L.; Wu, Z.L. Glycine regulates mucosal immunity and the intestinal microbial composition in weaned piglets. Amino Acids 2022, 54, 385–398. [Google Scholar] [CrossRef]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol 2022, 201, 115074. [Google Scholar] [CrossRef]
- Davila, A.-M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.-H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, J.-Y. The role of colonic microbiota amino acid metabolism in gut health regulation. Cell Insight 2025, 4, 100227. [Google Scholar] [CrossRef]
- de Oliveira, K.M.; Soares, G.M.; da Silva, J.A., Jr.; Alves, B.L.; Freitas, I.N.; Bem, K.C.P.; Mousovich-Neto, F.; Ribeiro, R.A.; Carneiro, E.M. Prolonged post-weaning protein restriction induces gut dysbiosis and colonic dysfunction in male mice. Am. J. Physiology. Endocrinol. Metab. 2025, 328, E599–E610. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Song, P.X.; Fan, P.X.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.B.; Ma, N.; Chen, Y.Q.; et al. Moderate Dietary Protein Restriction Optimized Gut Microbiota and Mucosal Barrier in Growing Pig Model. Front. Cell. Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef]
- Kurashima, Y.; Kiyono, H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu. Rev. Immunol. 2017, 35, 119–147. [Google Scholar] [CrossRef]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Torii, K.; Uneyama, H.; Nakamura, E. Physiological roles of dietary glutamate signaling via gut-brain axis due to efficient digestion and absorption. J. Gastroenterol. 2013, 48, 442–451. [Google Scholar] [CrossRef]
- Kitamura, A.; Tsurugizawa, T.; Uematsu, A.; Torii, K.; Uneyama, H. New therapeutic strategy for amino acid medicine: Effects of dietary glutamate on gut and brain function. J. Pharmacol. Sci. 2012, 118, 138–144. [Google Scholar] [CrossRef]
- Xia, R.; Peng, H.F.; Zhang, X.; Zhang, H.S. Comprehensive review of amino acid transporters as therapeutic targets. Int. J. Biol. Macromol. 2024, 260, 129646. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.A.; de Jong, J.; Vaessen, M.J.; van Leeuwen, P.A.; Houdijk, A.P. Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J. Gastroenterol. 2011, 17, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Dinges, M.M.; Green, A.; Cramer, S.E.; Larive, C.K.; Lytle, C. Absorptive transport of amino acids by the rat colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G189–G202. [Google Scholar] [CrossRef]
- Matsumoto, M.; Ooga, T.; Kibe, R.; Aiba, Y.; Koga, Y.; Benno, Y. Colonic Absorption of Low-Molecular-Weight Metabolites Influenced by the Intestinal Microbiome: A Pilot Study. PLoS ONE 2017, 12, e0169207. [Google Scholar] [CrossRef] [PubMed]
- Scharrer, E. Concentrative amino-acid uptake at serosal side of colon mucosa. Pflug. Arch.-Eur. J. Physiol. 1978, 376, 245–249. [Google Scholar] [CrossRef]
- Walter, J.; Ley, R. The Human Gut Microbiome: Ecology and Recent Evolutionary Changes. In Annual Review of Microbiology; Gottesman, S., Harwood, C.S., Eds.; Annual Review of Microbiology: Palo Alto, CA, USA, 2011; Volume 65, pp. 411–429. [Google Scholar]
- Ma, J.Y.; Piao, X.S.; Mahfuz, S.; Long, S.F.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Reese, A.T.; Pereira, F.C.; Schintlmeister, A.; Berry, D.; Wagner, M.; Hale, L.P.; Wu, A.C.; Jiang, S.R.; Durand, H.K.; Zhou, X.Y.; et al. Microbial nitrogen limitation in the mammalian large intestine. Nat. Microbiol. 2018, 3, 1441–1450. [Google Scholar] [CrossRef]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef]
- Thomas, G.H.; Mullins, J.G.; Merrick, M. Membrane topology of the Mep/Amt family of ammonium transporters. Mol. Microbiol. 2000, 37, 331–344. [Google Scholar] [CrossRef]
- Pi, Y.; Gao, K.; Peng, Y.; Mu, C.L.; Zhu, W.Y. Antibiotic-induced alterations of the gut microbiota and microbial fermentation in protein parallel the changes in host nitrogen metabolism of growing pigs. Animal 2019, 13, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecologyand host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. Biomed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, C.; Yu, S.; Zhu, L.; Wang, Y.; Hu, H.; Deng, J. Progress in Mycotoxins Affecting Intestinal Mucosal Barrier Function. Int. J. Mol. Sci. 2019, 20, 2777. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.; Eberhard, M.; Brunner, R.M.; Hennig, U.; Kuhla, S.; Kleessen, B.; Metges, C.C. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J. Nutr. 2006, 136, 1198–1202. [Google Scholar] [CrossRef]
- Marras, L.; Caputo, M.; Bisicchia, S.; Soato, M.; Bertolino, G.; Vaccaro, S.; Inturri, R. The Role of Bifidobacteria in Predictive and Preventive Medicine: A Focus on Eczema and Hypercholesterolemia. Microorganisms 2021, 9, 836. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wu, H.; Wu, L.; Yu, Q. The effect of dietary supplementation of low crude protein on intestinal morphology in pigs. Res. Vet. Sci. 2019, 122, 15–21. [Google Scholar] [CrossRef]
- Portejoie, S.; Martinez, J.; Landmann, G. Ammonia of farm origin: Impact on human and animal health and on the natural habitat. Prod. Anim. 2002, 15, 151–160. [Google Scholar]
- Leip, A.; Weiss, F.; Lesschen, J.P.; Westhoek, H. The Nitrogen Footprint of Food Products in the European Union; Cambridge University Press: Cambridge, UK, 2014; Volume 152, pp. 20–33. [Google Scholar]
- Wrong, O.M.; Vince, A. Urea and ammonia metabolism in the human large intestine. Proc. Nutr. Soc. 1984, 43, 77–86. [Google Scholar] [CrossRef]
- Nahm, K.H. Influences of Fermentable Carbohydrates on Shifting Nitrogen Excretion and Reducing Ammonia Emission of Pigs. Crit. Rev. Environ. Sci. Technol. 2003, 33, 165–186. [Google Scholar] [CrossRef]
- Hyde, R.; Taylor, P.M.; Hundal, H.S. Amino acid transporters: Roles in amino acid sensing and signalling in animal cells. Biochem. J. 2003, 373, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Aliaga, I. Nerveless and gutsy: Intestinal nutrient sensing from invertebrates to humans. Semin. Cell Dev. Biol. 2012, 23, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef]
- Yoon, M.S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Ye, J.L.; Gao, C.Q.; Li, X.G.; Jin, C.L.; Wang, D.; Shu, G.; Wang, W.C.; Kong, X.F.; Yao, K.; Yan, H.C. EAAT3 promotes amino acid transport and proliferation of porcine intestinal epithelial cells. Oncotarget 2016, 7, 38681–38692. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 2017, 171, 642–654.e12. [Google Scholar] [CrossRef]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araujo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.-Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Han, H.; Yin, J.; He, X.G.; Tang, Z.Y.; Li, T.J.; Yao, K.; Yin, Y.L. d- and l-Aspartate regulates growth performance, inflammation and intestinal microbial community in young pigs. Food Funct. 2019, 10, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, L.; Jin, S.S.; Hou, R.X.; Chen, M.Z.; Liu, Y.H.; Tang, W.J.; Li, T.J.; Yin, Y.L.; He, L.Q. d-Aspartate in Low-Protein Diets Improves the Pork Quality by Regulating Energy and Lipid Metabolism via the Gut Microbes. J. Agric. Food Chem. 2023, 71, 12417–12430. [Google Scholar] [CrossRef]
- Wellington, M.O.; Hulshof, T.G.; Ernst, K.; Balemans, A.; Page, G.; Van Hees, H.M.J. Impact of L-Arginine and L-Glutamine supplementation on growth performance and immune status in weanling pigs challenged with Escherichia coli F4. J. Anim. Sci. 2023, 101, skad138. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.J.; Hou, Y.Q.; Zhu, H.L.; Zhao, S.J.; Ding, B.Y.; Yin, Y.L.; Yi, G.F.; Shi, J.X.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef]
- Holecek, M. Side Effects of Amino Acid Supplements. Physiol. Res. 2022, 71, 29–45. [Google Scholar] [CrossRef]
- Holecek, M. Side Effects of Long-Term Glutamine Supplementation. J. Parenter. Enter. Nutr. 2013, 37, 607–616. [Google Scholar] [CrossRef]





| Items | NCP | LCP + Ala | LCP + Glu |
|---|---|---|---|
| Ingredients (%) | |||
| Corn starch | 39.75 | 42.64 | 41.82 |
| Casein | 20.00 | 10.00 | 10.00 |
| Sucrose | 10.00 | 10.00 | 10.00 |
| Maltodextrin | 13.20 | 13.20 | 13.20 |
| Soybean oil | 7.00 | 8.25 | 8.25 |
| Cellulose | 5.00 | 5.00 | 5.00 |
| t-Butylhydroquinone | 0.0014 | 0.0014 | 0.0014 |
| AIN-93G mineral mix 1 | 3.50 | 3.50 | 3.50 |
| AIN-93G vitamin mix 2 | 1.00 | 1.00 | 1.00 |
| Choline | 0.25 | 0.25 | 0.25 |
| Lysine | 0.00 | 0.94 | 0.94 |
| L-Arginine hydrochloride | 0.00 | 0.41 | 0.41 |
| L-Threonine | 0.00 | 0.41 | 0.41 |
| L-Tryptophan | 0.00 | 0.12 | 0.12 |
| DL-Methionine | 0.00 | 0.27 | 0.27 |
| L-Leucine | 0.00 | 0.89 | 0.89 |
| L-Isoleucine | 0.00 | 0.49 | 0.49 |
| L-Valine | 0.00 | 0.61 | 0.61 |
| L-Histidine | 0.00 | 0.28 | 0.28 |
| Cystine | 0.30 | 0.00 | 0.00 |
| Phenylalanine | 0.00 | 0.49 | 0.49 |
| L-Alanine | 0.00 | 1.25 | 0.00 |
| L-Glutamate | 0.00 | 0.00 | 2.07 |
| Calculated nutrient level (%) | |||
| Crude fat | 6.98 | 8.19 | 8.19 |
| Carbohydrate | 61.69 | 64.52 | 63.72 |
| Crude protein | 18.14 | 14.52 | 14.51 |
| Gross energy, kcal/g | 4.35 | 4.35 | 4.34 |
| Analyzed nutrient level (%) | |||
| Crude fat | 7.11 | 9.61 | 9.21 |
| Crude protein | 18.22 | 15.41 | 14.83 |
| Crude ash | 2.79 | 2.67 | 2.73 |
| Dry matter | 86.91 | 86.95 | 86.80 |
| Items | 1: NCP | 2: LCP + Ala | 3: LCP + Glu | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 2 vs. 3 | 1 vs. 3 | |||||
| Feed intake, g | 64.689 a1 | 52.867 b | 53.956 b | 3.591 | 0.071 | 0.847 | 0.094 |
| Nitrogen intake, g | 1.886 a | 1.303 b | 1.280 b | 0.091 | 0.005 | 0.868 | 0.004 |
| Fecal nitrogen, g | 0.232 a | 0.164 b | 0.110 c | 0.006 | <0.001 | 0.001 | <0.001 |
| Fecal microbial nitrogen, g | 0.035 ab | 0.024 b | 0.048 a | 0.003 | 0.070 | 0.004 | 0.054 |
| Fecal nitrogen/nitrogen intake, % | 12.306 a | 12.629 a | 8.748 b | 0.381 | 0.634 | 0.001 | 0.002 |
| Fecal microbial nitrogen/fecal nitrogen, % | 15.296 b | 14.710 b | 44.079 a | 2.862 | 0.918 | 0.002 | 0.002 |
| Digestible nitrogen, g | 1.654 a | 1.139 b | 1.170 b | 0.086 | 0.007 | 0.817 | 0.009 |
| Apparent nitrogen digestibility, % | 87.7 b | 87.4 b | 91.3 a | 0.004 | 0.598 | 0.001 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Zhang, J.; Ji, Y.; Dai, Z.; Yang, Y.; Wu, Z. Glutamate Supplementation Regulates Nitrogen Metabolism in the Colon and Liver of Weaned Rats Fed a Low-Protein Diet. Nutrients 2025, 17, 1465. https://doi.org/10.3390/nu17091465
Jiang D, Zhang J, Ji Y, Dai Z, Yang Y, Wu Z. Glutamate Supplementation Regulates Nitrogen Metabolism in the Colon and Liver of Weaned Rats Fed a Low-Protein Diet. Nutrients. 2025; 17(9):1465. https://doi.org/10.3390/nu17091465
Chicago/Turabian StyleJiang, Da, Jing Zhang, Yun Ji, Zhaolai Dai, Ying Yang, and Zhenlong Wu. 2025. "Glutamate Supplementation Regulates Nitrogen Metabolism in the Colon and Liver of Weaned Rats Fed a Low-Protein Diet" Nutrients 17, no. 9: 1465. https://doi.org/10.3390/nu17091465
APA StyleJiang, D., Zhang, J., Ji, Y., Dai, Z., Yang, Y., & Wu, Z. (2025). Glutamate Supplementation Regulates Nitrogen Metabolism in the Colon and Liver of Weaned Rats Fed a Low-Protein Diet. Nutrients, 17(9), 1465. https://doi.org/10.3390/nu17091465








