Effects of Time-Restricted Fasting–Nicotinamide Mononucleotide Combination on Exercise Capacity via Mitochondrial Activation and Gut Microbiota Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Cell Treatment
2.3. Exercise Capacity Testing
2.3.1. Treadmill Exhaustion Test
2.3.2. Forced Swimming Test
2.3.3. Wire Hang Test
2.3.4. Climbing-Pole Test
2.3.5. Rotarod Test
2.3.6. Strength Test
2.4. Histology Staining, Immunohistochemistry, and Immunofluorescence
2.5. Measurement of Oxidative Damage and Antioxidant Markers
2.6. Detection of Energy Metabolism-Related Indicators
2.7. Determination of Mitochondrial Respiratory Function
2.8. Detection of Mitochondrial DNA Copy Number
2.9. Detection of Gene Transcription
2.10. Western Blot Analysis
2.11. Analysis of Gut Microbiota and Short-Chain Fatty Acids (SCFAs)
2.12. Statistical Analysis
3. Results
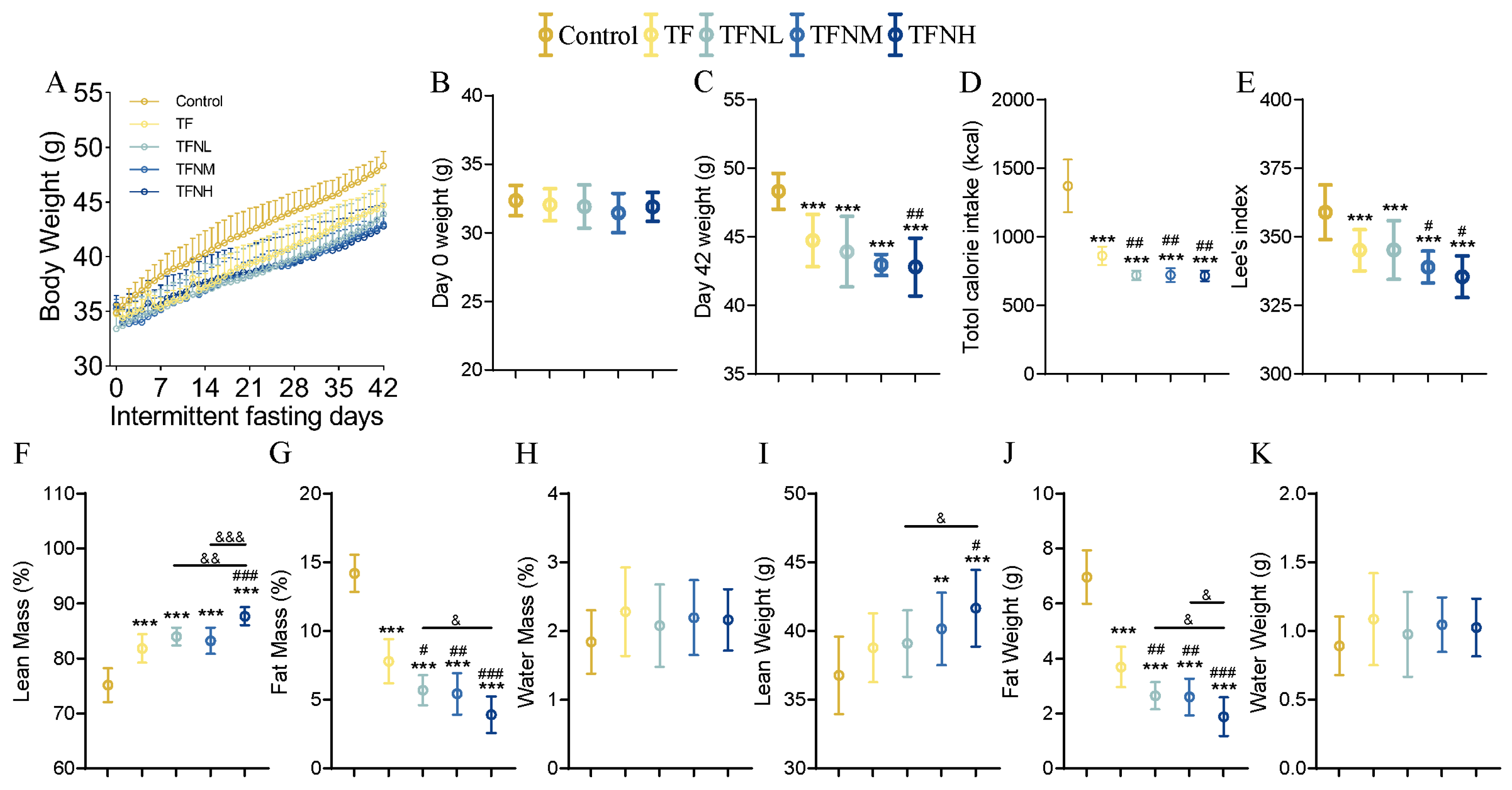
3.1. Effect of TF and NMN Supplementation on Physiological Index of Mice
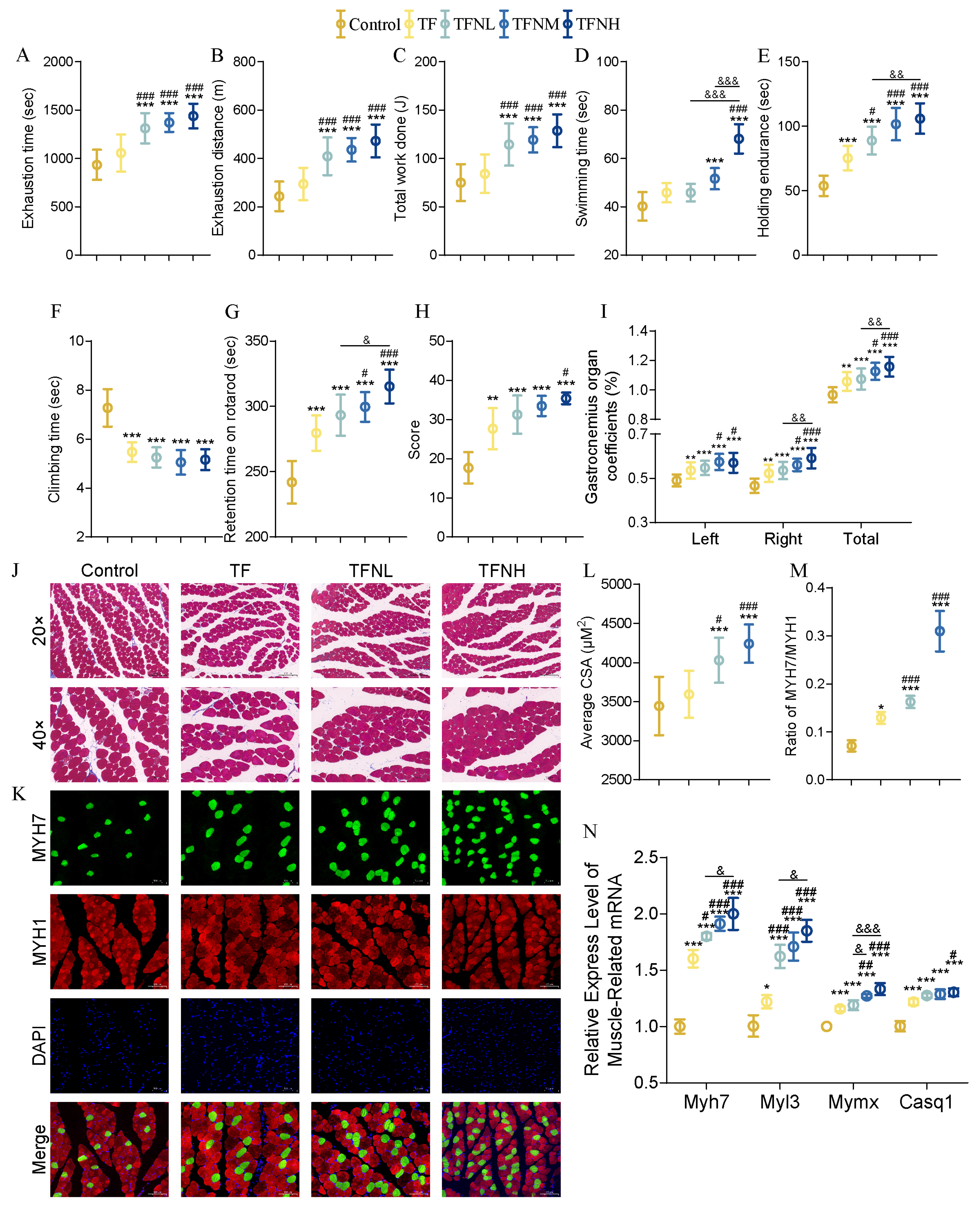
3.2. Effect of TF and TFN on Exercise Performance and Relative Indicators of Mice
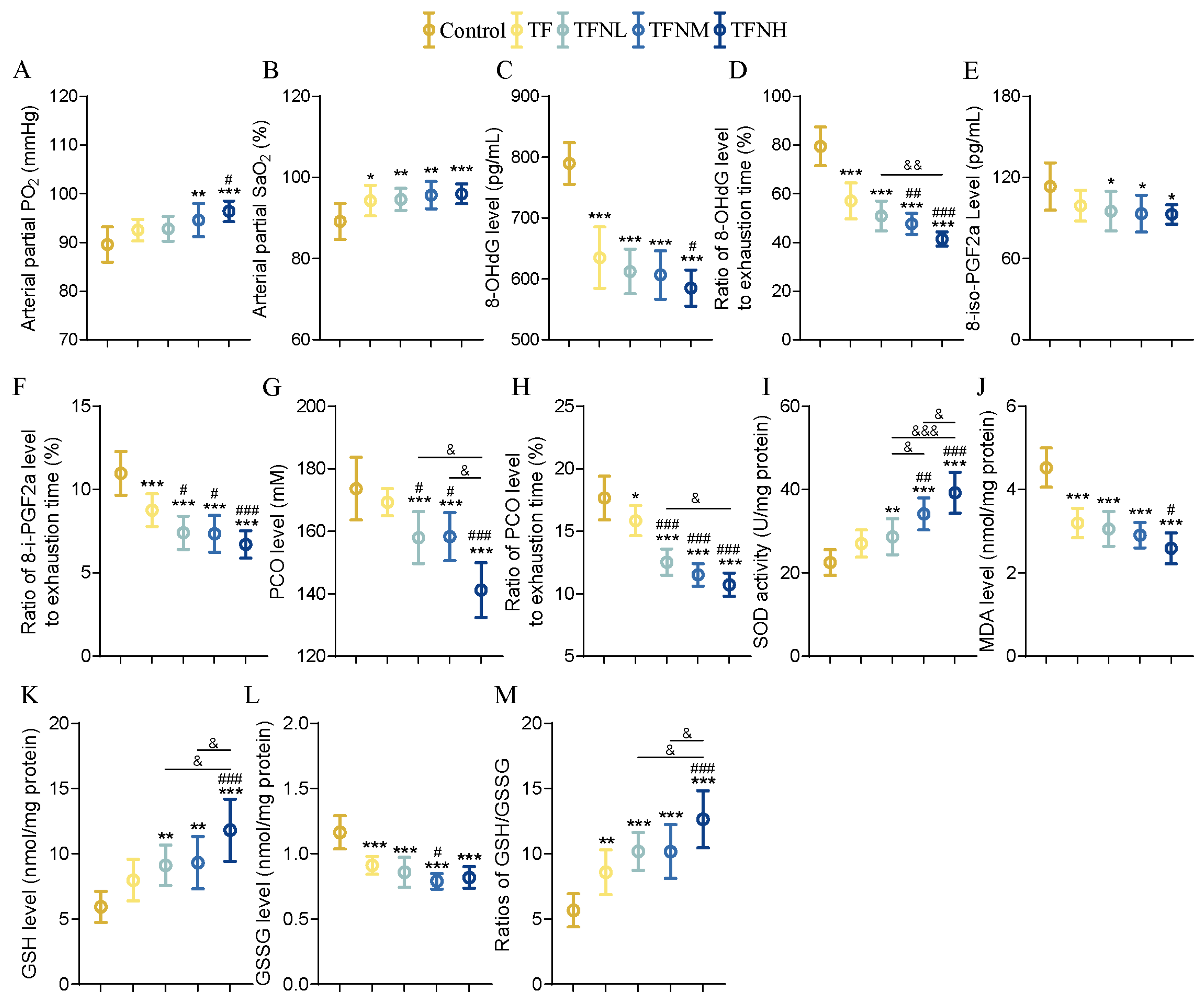
3.3. Effect of TF and TFN on Oxidation and Antioxidant Levels of Mice
3.4. Effect of TF and TFN on Energy Metabolism of Mice
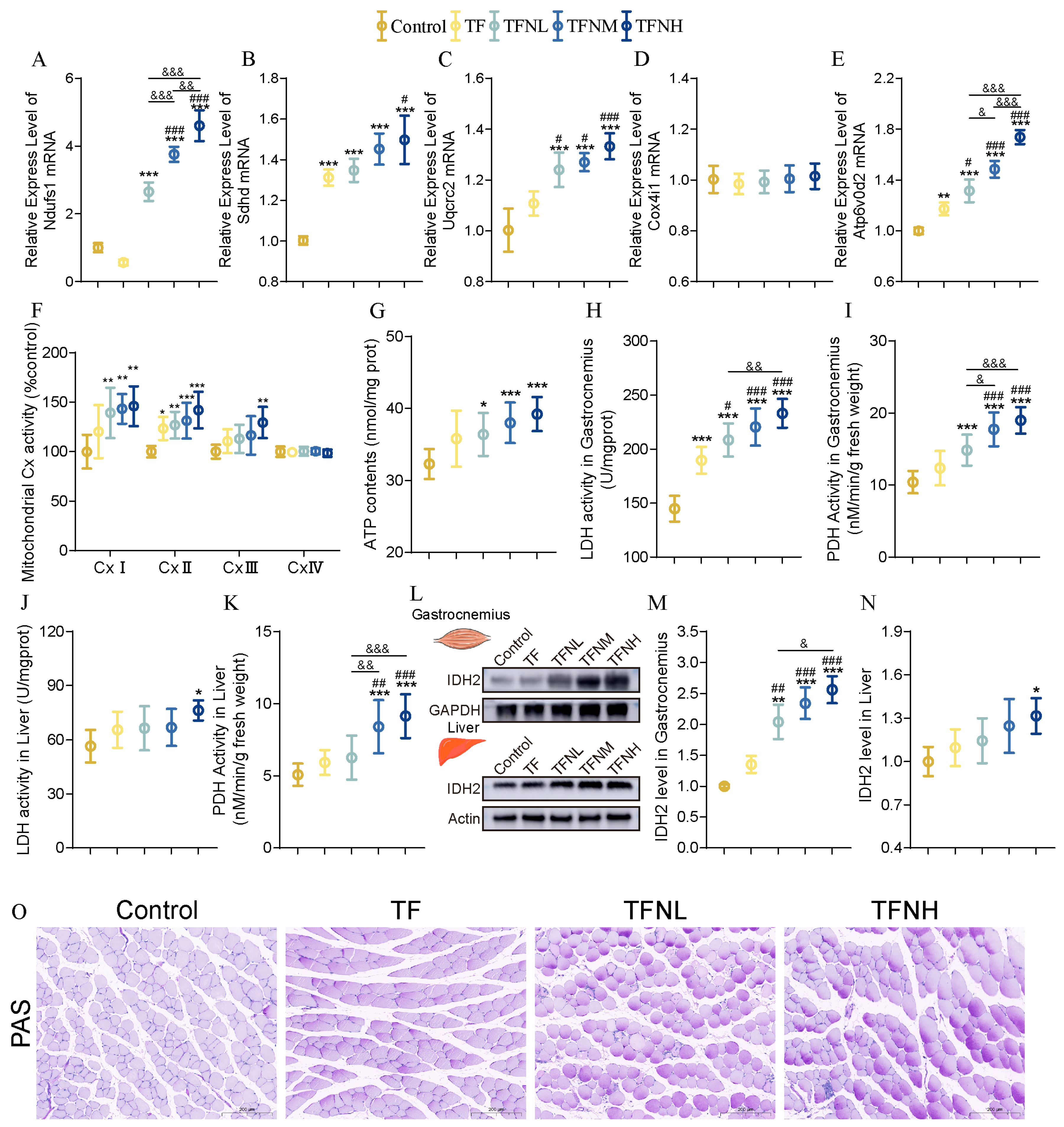
3.5. Effect of TF and TFN on Mitochondrial Function of Mice
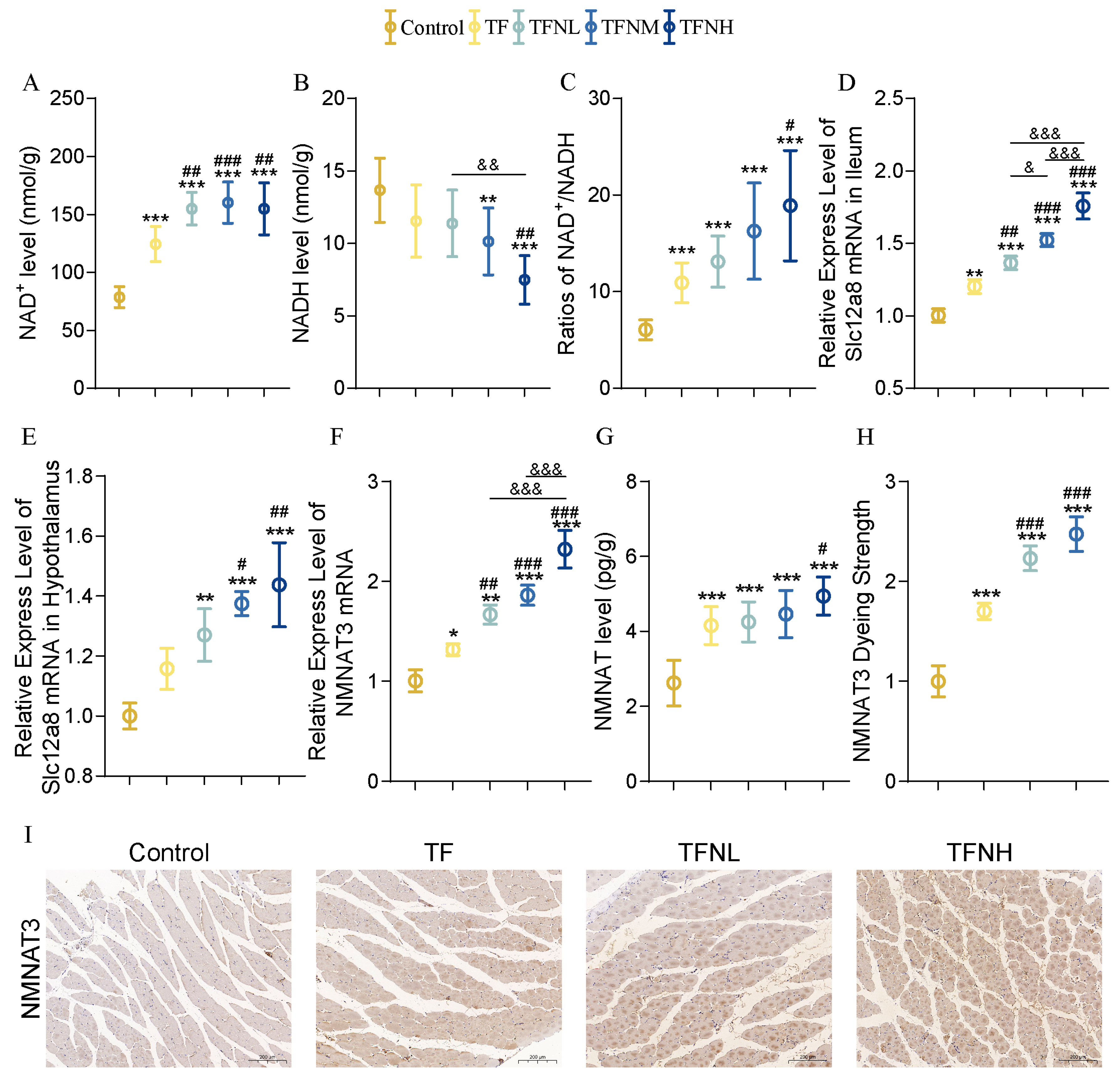
3.6. Effect of TF and TFN on NMN Metabolism of Mice
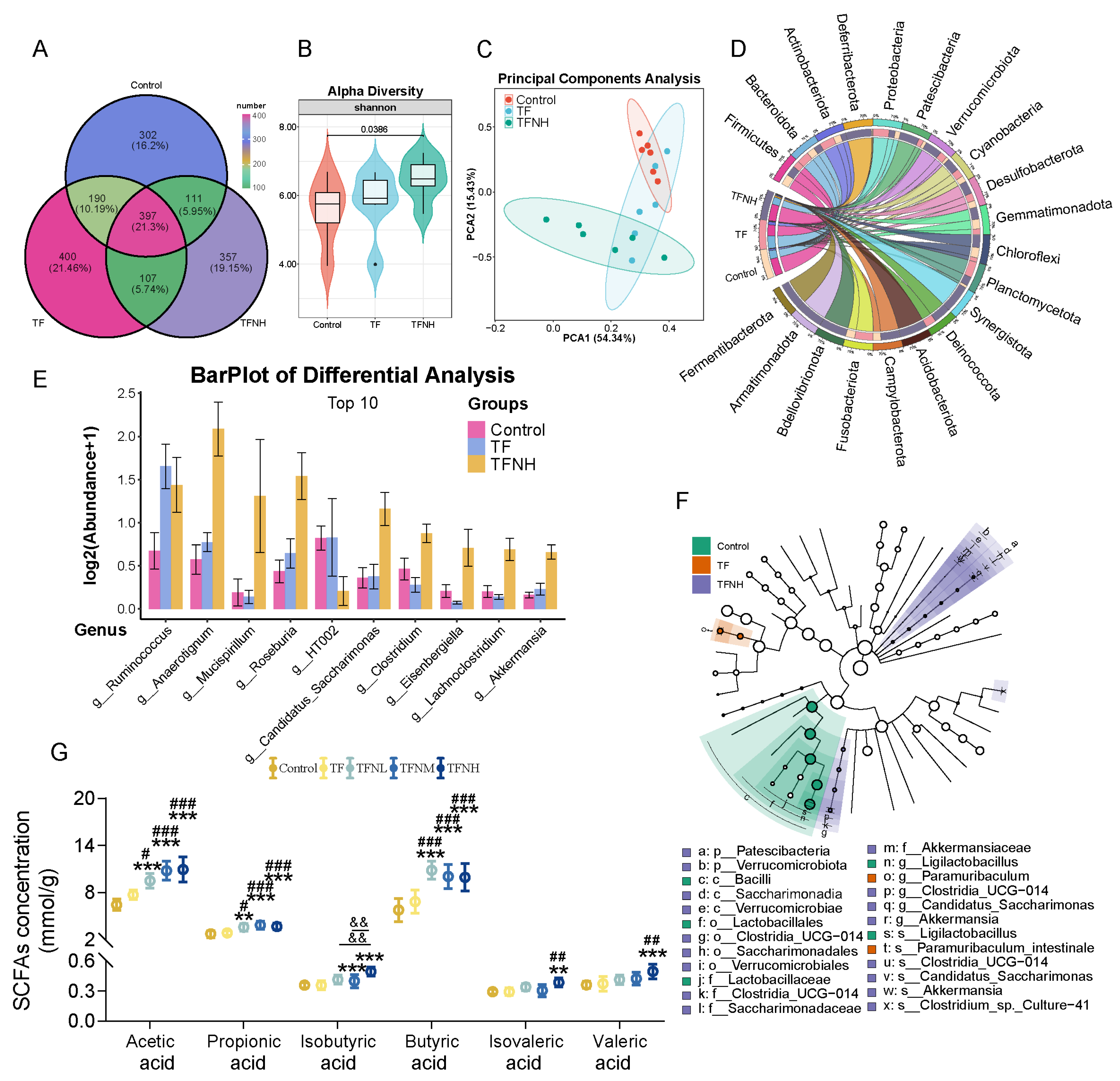
3.7. Effects of TF and TFN on Gut Microbiota and SCFAs Concentrations of Mice
3.8. Effects of TF and TFN on Mitochondrial Energy Metabolism in C2C12 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OXPHOS | Oxidative phosphorylation |
| TF | Time-restricted fasting |
| IF | Intermittent fasting |
| TCA cycle | Tricarboxylic acid cycle |
| NAD+ | Nicotinamide adenine dinucleotide |
| NMN | Nicotinamide mononucleotide |
| TFN | TF combined with NMN |
| NMNAT | Nicotinamide mononucleotide adenylyltransferase |
| LG | Low glucose |
| PCO | Protein carbonyl |
| MDA | Malondialdehyde |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| LDH | Lactate dehydrogenase |
| PDH | Pyruvate dehydrogenase |
| Cx | Mitochondrial respiratory chain complex enzymes |
| OCR | Oxygen consumption rate |
| mtDNA | Mitochondrial DNA |
References
- Mootha, V.K.; Bunkenborg, J.; Olsen, J.V.; Hjerrild, M.; Wisniewski, J.R.; Stahl, E.; Bolouri, M.S.; Ray, H.N.; Sihag, S.; Kamal, M.; et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 2003, 115, 629–640. [Google Scholar] [CrossRef]
- Kappler, L.; Hoene, M.; Hu, C.; von Toerne, C.; Li, J.; Bleher, D.; Hoffmann, C.; Bohm, A.; Kollipara, L.; Zischka, H.; et al. Linking bioenergetic function of mitochondria to tissue-specific molecular fingerprints. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E374–E387. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Gay, N.R.; Jimenez-Morales, D.; Jean, B.P.; Ramaker, M.E.; Raja, A.N.; Zhao, B.; Sun, Y.; Marwaha, S.; Gaul, D.A.; et al. The mitochondrial multi-omic response to exercise training across rat tissues. Cell Metab. 2024, 36, 1411–1429. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. The molecular bases of training adaptation. Sports Med. 2007, 37, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef]
- Hernandez-Lougedo, J.; Mate-Munoz, J.L.; Garcia-Fernandez, P.; Ubeda-D’Ocasar, E.; Hervas-Perez, J.P.; Pedauye-Rueda, B. The Relationship between Vegetarian Diet and Sports Performance: A Systematic Review. Nutrients 2023, 15, 4703. [Google Scholar] [CrossRef]
- Arnason, T.G.; Bowen, M.W.; Mansell, K.D. Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World J. Diabetes 2017, 8, 154–164. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.W.; Dai, Z.H.; Ho, R.S.; Wendy, Y.H.; Wong, S.H. Comparative effects of time-restricted feeding versus normal diet on physical performance and body composition in healthy adults with regular exercise habits: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2024, 10, e001831. [Google Scholar] [CrossRef] [PubMed]
- Klempel, M.C.; Kroeger, C.M.; Bhutani, S.; Trepanowski, J.F.; Varady, K.A. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr. J. 2012, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.C.; Kuo, H.Y.; Hong, H.K.; Cedernaes, J.; Hepler, C.; Wright, A.G.; Sommars, M.A.; Kobayashi, Y.; Marcheva, B.; Gao, P.; et al. NADH inhibition of SIRT1 links energy state to transcription during time-restricted feeding. Nat. Metab. 2021, 3, 1621–1632. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, A.; Rubio-Arias, J.A.; Garcia-De, F.J.; Vicente-Martinez, M.; Gunnarsson, T.P. Effect of High-Intensity Interval Training and Intermittent Fasting on Body Composition and Physical Performance in Active Women. Int. J. Environ. Res. Public Health 2021, 18, 6431. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, C.; Li, T.; Zhang, W.; Zong, Z.; Liu, X.; Deng, H. Reduced Nicotinamide Mononucleotide (NMNH) Potently Enhances NAD(+) and Suppresses Glycolysis, the TCA Cycle, and Cell Growth. J. Proteome Res. 2021, 20, 2596–2606. [Google Scholar] [CrossRef]
- Berger, F.; Lau, C.; Dahlmann, M.; Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005, 280, 36334–36341. [Google Scholar] [CrossRef]
- Soma, M.; Lalam, S.K. The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Mol. Biol. Rep. 2022, 49, 9737–9748. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhao, Y.; Wang, D.; Zhang, X.; Hao, X.; Hu, M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: A randomized, double-blind study. J. Int. Soc. Sport. Nutr. 2021, 18, 54. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Janice, S.B.; Tremblay, A.K.; Leduc-Gaudet, J.P.; Hall, D.T.; Kovacs, E.; Ma, J.F.; Mubaid, S.; Hallauer, P.L.; Phillips, B.L.; Vest, K.E.; et al. Depletion of HuR in murine skeletal muscle enhances exercise endurance and prevents cancer-induced muscle atrophy. Nat. Commun. 2019, 10, 4171. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Wu, M.F.; Lee, M.C.; Huang, C.C. Exercise training combined with Bifidobacterium longum OLP-01 treatment regulates insulin resistance and physical performance in db/db mice. Food Funct. 2021, 12, 7728–7740. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Zhan, X.; Li, B.; Zhang, Z.; Li, S.; Xie, Y.; Song, X.; Shen, Y.; Liu, J.; et al. R13 preserves motor performance in SOD1(G93A) mice by improving mitochondrial function. Theranostics 2021, 11, 7294–7307. [Google Scholar] [CrossRef]
- Ueno, H.; Takahashi, Y.; Murakami, S.; Wani, K.; Miyazaki, T.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. The prevention of home-cage grid climbing affects muscle strength in mice. Sci. Rep. 2022, 12, 15263. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Ojano-Dirain, C.; Iqbal, M.; Wing, T.; Cooper, M.; Bottje, W. Glutathione and respiratory chain complex activity in duodenal mitochondria of broilers with low and high feed efficiency. Poult. Sci. 2005, 84, 782–788. [Google Scholar] [CrossRef]
- Uddin, G.M.; Youngson, N.A.; Sinclair, D.A.; Morris, M.J. Head to Head Comparison of Short-Term Treatment with the NAD(+) Precursor Nicotinamide Mononucleotide (NMN) and 6 Weeks of Exercise in Obese Female Mice. Front. Pharmacol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Rang, Y.; Liu, H.; Cheng, X.; Li, W.; Shi, J.; Ou, G.; Huang, H.; Chen, C.; Xiao, X.; Liu, C. Structural characterization of pectic polysaccharides from Amaranth caudatus leaves and the promotion effect on hippocampal glucagon-like peptide-1 level. Int. J. Biol. Macromol. 2023, 242, 124967. [Google Scholar] [CrossRef] [PubMed]
- Bramble, D.M.; Lieberman, D.E. Endurance running and the evolution of Homo. Nature 2004, 432, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Morton, J.P.; Burke, L.M. A Framework for Periodized Nutrition for Athletics. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 141–151. [Google Scholar] [CrossRef]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3beta-MCL-1 Axis. Cancer Cell 2019, 35, 798–815. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, J.; Liu, W.; Jing, W.; Lyu, B.; Yu, H.; Zhang, Z. Insoluble Dietary Fiber from Okara Combined with Intermittent Fasting Treatment Synergistically Confers Antiobesity Effects by Regulating Gut Microbiota and Its Metabolites. J. Agric. Food Chem. 2023, 71, 13346–13362. [Google Scholar] [CrossRef] [PubMed]
- Iannetta, D.; Weavil, J.C.; Laginestra, F.G.; Thurston, T.S.; Broxterman, R.M.; Jenkinson, R.H.; Curtis, M.C.; Chang, J.; Wan, H.Y.; Amann, M. Control of hyperpnoea and pulmonary gas exchange during prolonged exercise: The role of group III/IV muscle afferent feedback. J. Physiol. 2024, 602, 5375–5389. [Google Scholar] [CrossRef]
- Stuart, C.A.; Stone, W.L.; Howell, M.E.; Brannon, M.F.; Hall, H.K.; Gibson, A.L.; Stone, M.H. Myosin content of individual human muscle fibers isolated by laser capture microdissection. Am. J. Physiol.-Cell Physiol. 2016, 310, C381–C389. [Google Scholar] [CrossRef]
- Ramirez-Martinez, A.; Zhang, Y.; van den Boogaard, M.J.; McAnally, J.R.; Rodriguez-Caycedo, C.; Chai, A.C.; Chemello, F.; Massink, M.P.; Cuppen, I.; Elferink, M.G.; et al. Impaired activity of the fusogenic micropeptide Myomixer causes myopathy resembling Carey-Fineman-Ziter syndrome. J. Clin. Investig. 2022, 132, 11. [Google Scholar] [CrossRef]
- Barone, V.; Del, R.V.; Gamberucci, A.; Polverino, V.; Galli, L.; Rossi, D.; Costanzi, E.; Toniolo, L.; Berti, G.; Malandrini, A.; et al. Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum. Mutat. 2017, 38, 1761–1773. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance exercise-induced histone methylation modification involved in skeletal muscle fiber type transition and mitochondrial biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Liu, J.; Sun, J.; Fan, B.; Lu, C.; Wang, F. Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice. Nutrients 2024, 16, 2783. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Karamanlidis, G.; Lee, C.F.; Garcia-Menendez, L.; Kolwicz, S.J.; Suthammarak, W.; Gong, G.; Sedensky, M.M.; Morgan, P.G.; Wang, W.; Tian, R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013, 18, 239–250. [Google Scholar] [CrossRef]
- Lu, L.; Tang, L.; Wei, W.; Hong, Y.; Chen, H.; Ying, W.; Chen, S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp. Ther. Med. 2014, 8, 943–950. [Google Scholar] [CrossRef]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Nomiyama, T.; Setoyama, D.; Yasukawa, T.; Kang, D. Mitochondria metabolomics reveals a role of beta-nicotinamide mononucleotide metabolism in mitochondrial DNA replication. J. Biochem. 2022, 171, 325–338. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Mattar, P.; Toledo, M.; Bains, H.; Kalyani, M.; Aoun, M.L.; Sharma, M.; McIntire, L.; Gunther-Cummins, L.; Macaluso, F.P.; et al. mTORC2-NDRG1-CDC42 axis couples fasting to mitochondrial fission. Nat. Cell Biol. 2023, 25, 989–1003. [Google Scholar] [CrossRef]
- Arslan, N.P.; Taskin, M.; Keles, O.N. Nicotinamide Mononucleotide and Nicotinamide Riboside Reverse Ovarian Aging in Rats Via Rebalancing Mitochondrial Fission and Fusion Mechanisms. Pharm. Res. 2024, 41, 921–935. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, J.; Pan, X.; Meng, D.; Zhu, Y.; Bai, Y.; Shi, C.; Duan, Y.; Wang, T.; Li, X.; et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome 2022, 10, 82. [Google Scholar] [CrossRef]
- Bressa, C.; Bailen-Andrino, M.; Perez-Santiago, J.; Gonzalez-Soltero, R.; Perez, M.; Montalvo-Lominchar, M.G.; Mate-Munoz, J.L.; Dominguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Sun, Y.; Zhang, X. Combined Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat of Metabolic Disease: A Review. Nutrients 2022, 14, 4774. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Zhuang, T.; Li, W.; Wu, X.; Wang, J.; Lyu, R.; Chen, J.; Liu, C. Effects of Time-Restricted Fasting–Nicotinamide Mononucleotide Combination on Exercise Capacity via Mitochondrial Activation and Gut Microbiota Modulation. Nutrients 2025, 17, 1467. https://doi.org/10.3390/nu17091467
Shi J, Zhuang T, Li W, Wu X, Wang J, Lyu R, Chen J, Liu C. Effects of Time-Restricted Fasting–Nicotinamide Mononucleotide Combination on Exercise Capacity via Mitochondrial Activation and Gut Microbiota Modulation. Nutrients. 2025; 17(9):1467. https://doi.org/10.3390/nu17091467
Chicago/Turabian StyleShi, Jian, Tingting Zhuang, Weiye Li, Xueping Wu, Junming Wang, Ruiying Lyu, Jingxin Chen, and Chunhong Liu. 2025. "Effects of Time-Restricted Fasting–Nicotinamide Mononucleotide Combination on Exercise Capacity via Mitochondrial Activation and Gut Microbiota Modulation" Nutrients 17, no. 9: 1467. https://doi.org/10.3390/nu17091467
APA StyleShi, J., Zhuang, T., Li, W., Wu, X., Wang, J., Lyu, R., Chen, J., & Liu, C. (2025). Effects of Time-Restricted Fasting–Nicotinamide Mononucleotide Combination on Exercise Capacity via Mitochondrial Activation and Gut Microbiota Modulation. Nutrients, 17(9), 1467. https://doi.org/10.3390/nu17091467






