Unveiling the Link Between Vitamin D, Hashimoto’s Thyroiditis, and Thyroid Functions: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Parameters
2.3. Hashimoto’s Thyroiditis and Group Classification
2.4. Thyroid Function Status and Subgroups
2.5. Vitamin D Level and Classification
2.6. Statistical Analyses
3. Results
3.1. Patient Demographics and Laboratory Characteristics
3.2. Hashimoto’s Thyroiditis Status
3.3. Vitamin D Levels and Relationship with Hashimoto’s Thyroiditis
3.4. Thyroid Functional Status and Vitamin D Levels
3.5. Adjusted Analyses for Demographic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HT | Hashimoto’s thyroiditis |
| TSH | Thyroid-stimulating hormone |
| fT4 | Free thyroxine |
| fT3 | Free triiodothyronine |
| 25(OH)D | 25-hydroxyvitamin D |
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| VDR | Vitamin D receptor |
| anti-TPO | Antithyroid peroxidase |
| anti-TG | Antithyroglobulin |
| AITD | Autoimmune thyroid disease |
References
- Zhao, R.; Zhang, W.; Ma, C.; Zhao, Y.; Xiong, R.; Wang, H.; Chen, W.; Zheng, S.G. Immunomodulatory function of vitamin D and its role in autoimmune thyroid disease. Front. Immunol. 2021, 12, 574967. [Google Scholar] [CrossRef] [PubMed]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2021, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef]
- Lehmann, B.; Meurer, M. Vitamin D metabolism. Dermatol. Ther. 2010, 23, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef]
- Verlinden, L.; Carmeliet, G. Integrated view on the role of vitamin D actions on bone and growth plate homeostasis. J. Bone Miner. Res. Plus. 2021, 5, e10577. [Google Scholar] [CrossRef]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and risk for type 2 diabetes in people with prediabetes: A systematic review and meta-analysis of individual participant data from 3 randomized clinical trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of vitamin D on cardiovascular risk and oxidative stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D intake and the risk of colorectal cancer: An updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef]
- Pang, B.; Li, L.; Liu, X.; Cao, Z.; Pang, T.; Wang, Q.; Wei, J. Association between serum vitamin D level and Graves’ disease: A systematic review and meta-analysis. Nutr. J. 2024, 23, 60. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Bima, A.; Eldakhakhny, B.; Nuwaylati, D.; Alnami, A.; Ajabnoor, M.; Elsamanoudy, A. The interplay of vitamin D deficiency and cellular senescence in the pathogenesis of obesity-related co-morbidities. Nutrients 2021, 13, 4127. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Miteva, M.Z.; Nonchev, B.I.; Orbetzova, M.M.; Stoencheva, S.D. Vitamin D and autoimmune thyroid diseases-a review. Folia Med. 2020, 62, 223–229. [Google Scholar] [CrossRef]
- Bizzaro, G.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases: Facts and unresolved questions. Immunol. Res. 2015, 61, 46–52. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, L.; He, Y.; Yuan, S.; Xu, C. The association between vitamin D and Hashimoto Thyroiditis: An up-to-date systematic review and meta-analysis. Food Mater. Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Cvek, M.; Kaličanin, D.; Barić, A.; Vuletić, M.; Gunjača, I.; Torlak Lovrić, V.; Škrabić, V.; Punda, A.; Perica, V.B. Vitamin D and Hashimoto’s thyroiditis: Observations from CROHT biobank. Nutrients 2021, 13, 2793. [Google Scholar] [CrossRef]
- Turashvili, N.; Javashvili, L.; Giorgadze, E. Vitamin D deficiency is more common in women with autoimmune thyroiditis: A retrospective study. Int. J. Endocrinol. 2021, 2021, 4465563. [Google Scholar] [CrossRef]
- Yasmeh, J.; Farpour, F.; Rizzo, V.; Kheradnam, S.; Sachmechi, I. Hashimoto thyroiditis not associated with vitamin D deficiency. Endocr. Pract. 2016, 22, 809–813. [Google Scholar] [CrossRef]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The role of the immune system in the course of Hashimoto’s Thyroiditis: The current state of knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef]
- Piticchio, T.; Savarino, F.; Volpe, S.; Prinzi, A.; Costanzo, G.; Gamarra, E.; Frasca, F.; Trimboli, P. Inflammatory Profile Assessment in a Highly Selected Athyreotic Population Undergoing Controlled and Standardized Hypothyroidism. Biomedicines 2024, 12, 239. [Google Scholar] [CrossRef]
- da Silva, G.B.; Yamauchi, M.A.; Bagatini, M.D. Oxidative stress in Hashimoto’s thyroiditis: Possible adjuvant therapies to attenuate deleterious effects. Mol. Cell. Biochem. 2023, 478, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic characteristics of hashimoto’s thyroiditis patients and the role of microelements and diet in the disease management—An overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, Y.; Li, Y.; Xia, Y.; Liu, L.; Lin, F.; Shi, Y. Therapeutic effect of vitamin D in Hashimoto’s thyroiditis: A prospective, randomized and controlled clinical trial in China. Am. J. Transl. Res. 2023, 15, 6234. [Google Scholar] [PubMed]
- Bozkurt, N.C.; Karbek, B.; Ucan, B.; Sahin, M.; Cakal, E.; Ozbek, M.; Delibasi, T. The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 2013, 19, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D status and thyroid autoantibodies in autoimmune thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef]
- Safabakhsh, M.; Tangestani, H.; Saeidifard, N.; Ebaditabar, M.; Akbarzade, Z.; Shabbidar, S. Does vitamin D improve the serum level of anti-TPO and anti-TG in Autoimmune thyroid diseases? A systematic review and meta-analysis. J. Nutr. Sci. Diet. 2018, 4, 50–58. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Quade, D. Using weighted rankings in the analysis of complete blocks with additive block effects. J. Am. Stat. Assoc. 1979, 74, 680–683. [Google Scholar] [CrossRef]
- Yazici, D.; Yavuz, D.; Tarcin, O.; Sancak, S.; Deyneli, O.; Akalin, S. Vitamin D receptor gene Apal, Taql, FokI and BsmI polymorphisms in a group of Turkish patients with Hashimoto’s thyroiditis. Minerva Endocrinol. 2013, 38, 195–201. [Google Scholar]
- Feng, M.; Li, H.; Chen, S.F.; Li, W.F.; Zhang, F.B. Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: A meta-analysis. Endocrine 2013, 43, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K. Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Kim, D. Low vitamin D status is associated with hypothyroid Hashimoto’s thyroiditis. Hormones 2016, 15, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 03000605211060675. [Google Scholar] [CrossRef]
- Effraimidis, G.; Badenhoop, K.; Tijssen, J.G.; Wiersinga, W.M. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 2012, 167, 43–48. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, F.; Liu, E.M.; Zhu, M.; Lei, P.Y. Effects of 1,25-dihydroxyvitamin D3 in rats with experimental autoimmune thyroiditis. J. South. Med. Univ. 2010, 30, 1573–1576. [Google Scholar]
- Yasuda, T.; Okamato, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine 2012, 42, 739–741. [Google Scholar] [CrossRef]
- Albert, P.J.; Proal, A.D.; Marshall, T.G. Vitamin D: The alternative hypothesis. Autoimmun. Rev. 2009, 8, 639–644. [Google Scholar] [CrossRef]
- Ahi, S.; Dehdar, M.R.; Hatami, N. Vitamin D deficiency in non-autoimmune hypothyroidism: A case-control study. BMC Endocr. Disord. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Mackawy, A.M.H.; Al-Ayed, B.M.; Al-Rashidi, B.M. Vitamin D deficiency and its association with thyroid disease. Int. J. Health Sci. 2013, 7, 267. [Google Scholar] [CrossRef]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.J.; Palmieri, T.; Young, A.R. Physical determinants of vitamin D photosynthesis: A review. J. Bone Miner. Res. Plus 2021, 5, e10460. [Google Scholar] [CrossRef]
- Kongsbak, M.; von Essen, M.R.; Levring, T.B.; Schjerling, P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol. 2014, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Tirabassi, G.; Bizzaro, G.; Orio, F.; Paschou, S.A.; Vryonidou, A.; Balercia, G.; Shoenfeld, Y.; Colao, A. Vitamin D and thyroid disease: To D or not to D? Eur. J. Clin. Nutr. 2015, 69, 291–296. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and obesity: Current evidence and controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Allgrove, J. Physiology of calcium, phosphate, magnesium and vitamin D. Endocr. Dev. 2015, 28, 7–32. [Google Scholar]
- Fletcher, J.; Bishop, E.L.; Harrison, S.R.; Swift, A.; Cooper, S.C.; Dimeloe, S.K.; Raza, K.; Hewison, M. Autoimmune disease and interconnections with vitamin D. Endocr. Connect. 2022, 11, e210554. [Google Scholar]
| Total | Non-HT (n = 25,018) | HT (n = 27,800) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | Median (Q1–Q3) | n | Median (Q1–Q3) | n | Median (Q1–Q3) | |||
| Sex | ||||||||
| Male | 22,179 | 27.3% | 6859 | 27.4% | 5307 | 19.1% | <0.001 | |
| Female | 59,001 | 72.7% | 18,159 | 72.6% | 22,493 | 80.9% | ||
| Age (year) | 81,180 | 51 (38–62) | 25,018 | 49 (36–60) | 27,800 | 49 (38–59) | 0.862 | |
| anti-TPO | 53,217 | - | 25,018 | - | 27,069 | - | - | |
| Negative | 36,671 | 68.9% | 25,018 | 100.0% | 10,523 | 38.9% | <0.001 | |
| Positive | 16,546 | 31.1% | 0 | 0.0% | 16,546 | 61.1% | ||
| fT3 (ng/L) | 72,229 | 3.19 (2.89–3.50) | 23,511 | 3.25 (2.96–3.54) | 25,990 | 3.20 (2.91–3.50) | <0.001 | |
| fT4 (ng/dL) | 80,490 | 1.15 (1.04–1.27) | 24,923 | 1.15 (1.05–1.27) | 27,667 | 1.13 (1.02–1.25) | <0.001 | |
| <0.89 | 4449 | 5.5% | 1049 | 4.2% a | 1948 | 7.0% b | <0.001 | |
| 0.89–1.76 | 75,623 | 94.0% | 23,764 | 95.3% a | 25,565 | 92.4% b | ||
| >1.76 | 418 | 0.5% | 110 | 0.4% a | 154 | 0.6% a | ||
| anti-TG | 54,302 | - | 25,018 | - | 27,451 | - | - | |
| Negative | 31,374 | 57.8% | 25,018 | 100.0% | 4523 | 16.5% | <0.001 | |
| Positive | 22,928 | 42.2% | 0 | 0.0% | 22,928 | 83.5% | ||
| TSH (mU/L) | 81,180 | 1.97 (1.28–3.13) | 25,018 | 1.78 (1.18–2.69) | 27,800 | 2.37 (1.47–3.90) | <0.001 | |
| 0.55–4.78 | 72,044 | 88.7% | 23,442 | 93.7% | 22,930 | 82.5% | <0.001 | |
| >4.78 | 9136 | 11.3% | 1576 | 6.3% | 4870 | 17.5% | ||
| fT4–TSH | ||||||||
| Euthyroid | 68,475 | 88.2% | 22,455 | 93.4% | 21,818 | 81.8% | <0.001 | |
| Hypothyroid | 9136 | 11.8% | 1576 | 6.6% | 4870 | 18.2% | ||
| Vitamin D (nmol/L) | 15,579 | 40.93 (27.46–59.65) | 4816 | 39.44 (26.71–58.16) | 6586 | 41.43 (27.71–60.40) | <0.001 | |
| Deficient | 9843 | 63.2% | 3153 | 65.5% a | 4087 | 62.1% b | <0.001 | |
| Insufficient | 3762 | 24.1% | 1108 | 23.0% a | 1597 | 24.2% a | ||
| Sufficient | 1974 | 12.7% | 555 | 11.5% a | 902 | 13.7% b | ||
| Vitamin D by sex (nmol/L) | Male | 4069 | 42.68 (30.45–59.40) | 1355 | 42.43 (29.7–59.4) | 1301 | 42.93 (31.2–59.65) | 0.215 |
| Female | 11,510 | 40.06 (26.46–59.65) | 3461 | 38.19 (25.71–57.28) | 5285 | 40.93 (26.71–60.9) | <0.001 | |
| Euthyroid Non-HT n = 4425 | Hypothyroid Non-HT n = 256 | Euthyroid HT n = 5394 | Hypothyroid HT n = 971 | p-Value | ||
|---|---|---|---|---|---|---|
| Vitamin D (nmol/L) | 39.69 a (26.71–58.41) | 36.32 a (23.77–54.35) | 42.18 b (28.2–61.71) | 38.19 a (25.71–55.41) | <0.001 <0.001 adjusted for age and sex * | |
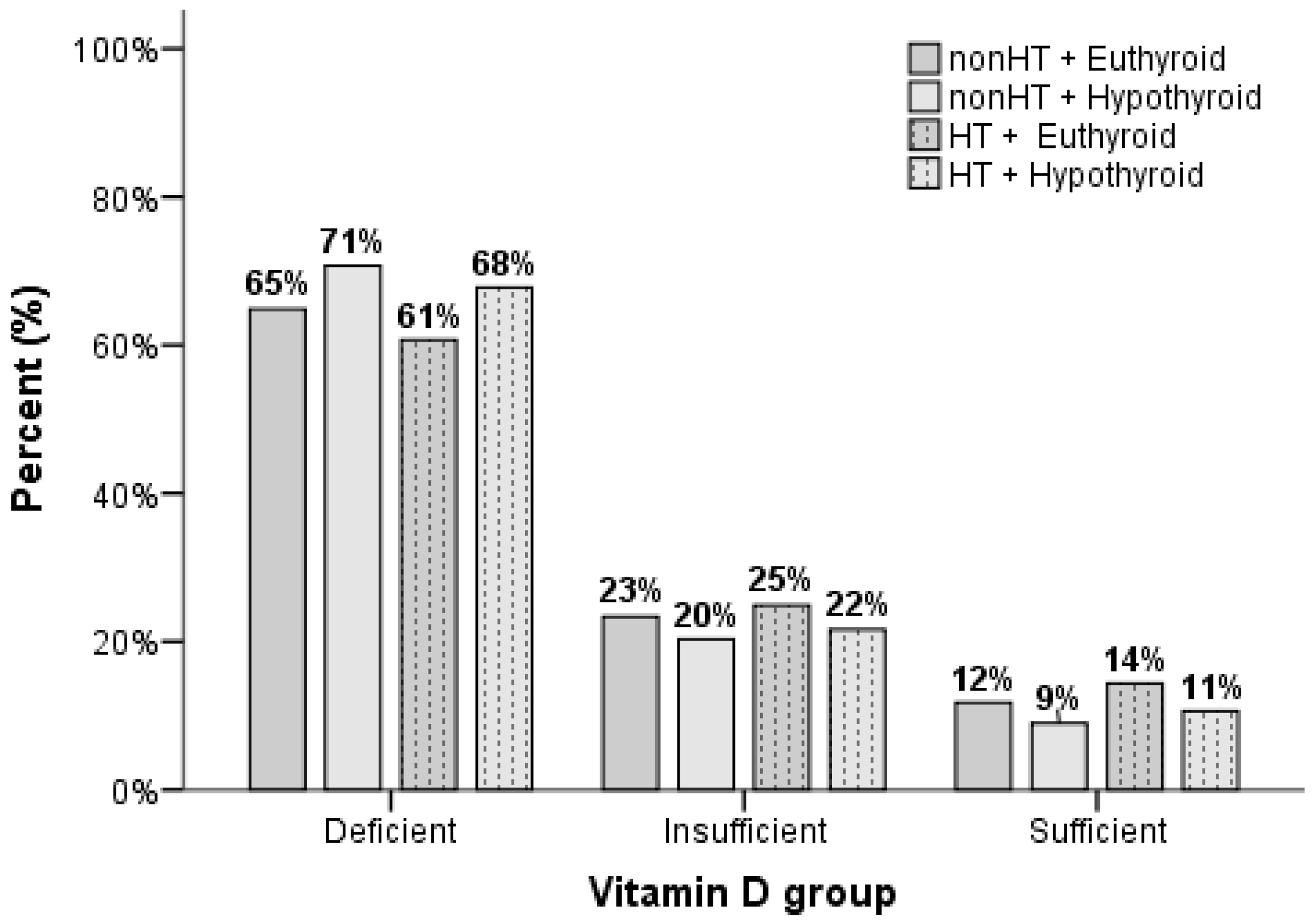
| Deficient | 64.9% a | 70.7% a | 60.7% b | 67.8% a | <0.001 | |
| Insufficient | 23.4% a | 20.3% a | 24.9% a | 21.6% a | ||
| Sufficient | 11.8% a | 9.0% a,b | 14.4% b | 10.6% a | ||
| Vitamin D by sex (nmol/L) | Male | 42.18 (29.7–59.4) | 45.68 (27.21–61.65) | 42.93 (30.83–59.65) | 42.18 (31.45–58.91) | 0.611 |
| Female | 38.44 a (25.96–57.78) | 33.2 b (22.96–51.29) | 41.93 c (27.46–62.28) | 36.69 a,b (24.71–54.72) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakaya, R.E.; Tam, A.A.; Demir, P.; Karaahmetli, G.; Fakı, S.; Topaloğlu, O.; Ersoy, R. Unveiling the Link Between Vitamin D, Hashimoto’s Thyroiditis, and Thyroid Functions: A Retrospective Study. Nutrients 2025, 17, 1474. https://doi.org/10.3390/nu17091474
Karakaya RE, Tam AA, Demir P, Karaahmetli G, Fakı S, Topaloğlu O, Ersoy R. Unveiling the Link Between Vitamin D, Hashimoto’s Thyroiditis, and Thyroid Functions: A Retrospective Study. Nutrients. 2025; 17(9):1474. https://doi.org/10.3390/nu17091474
Chicago/Turabian StyleKarakaya, Rahime Evra, Abbas Ali Tam, Pervin Demir, Gülsüm Karaahmetli, Sevgül Fakı, Oya Topaloğlu, and Reyhan Ersoy. 2025. "Unveiling the Link Between Vitamin D, Hashimoto’s Thyroiditis, and Thyroid Functions: A Retrospective Study" Nutrients 17, no. 9: 1474. https://doi.org/10.3390/nu17091474
APA StyleKarakaya, R. E., Tam, A. A., Demir, P., Karaahmetli, G., Fakı, S., Topaloğlu, O., & Ersoy, R. (2025). Unveiling the Link Between Vitamin D, Hashimoto’s Thyroiditis, and Thyroid Functions: A Retrospective Study. Nutrients, 17(9), 1474. https://doi.org/10.3390/nu17091474






