Heat Transfer Performance of Gel Foam Layer with Nanoparticles Doping under a Radiative Heat Flux
Abstract
:1. Introduction
2. Numerical Model
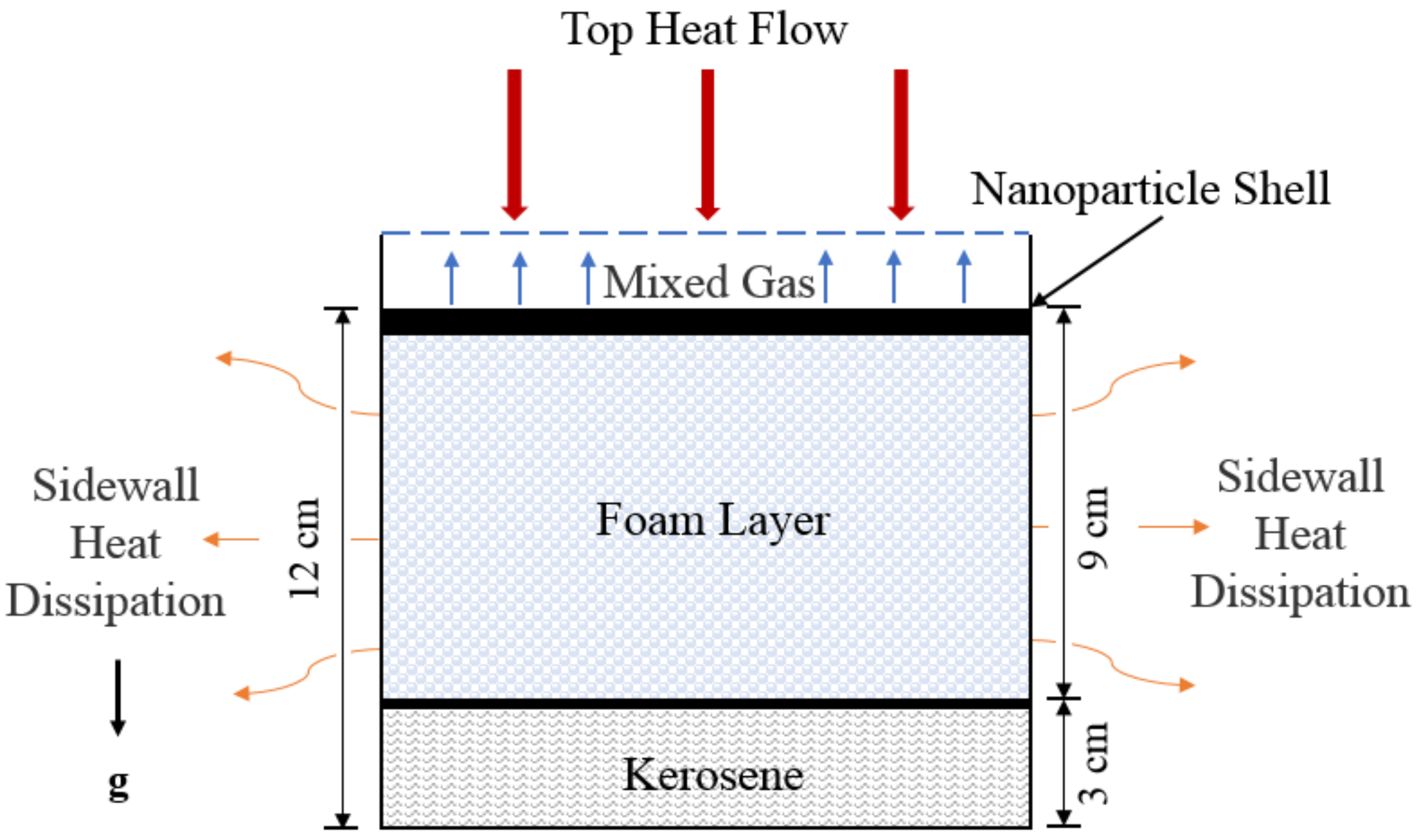
2.1. Simulation Domain and Boundary Conditions
2.2. Numerical Model of the Nanoparticle Shell
2.3. Numerical Model of Three-Phase Gel Foam Layer
- (a)
- Heat conduction equation
- (b)
- Equation of radiation heat transfer
- (c)
- Equation for the surface movement of the foam layer
2.4. Numerical Model of the Kerosene Layer
2.5. Numerical Model of the Mixed Gas Layer
2.6. Model Validation

3. Results and Discussion
3.1. Characteristics of Foam Thickness and Heat Flux Distribution in the Gel Foam Layer
3.1.1. Parameters from Typical Conditions
3.1.2. Thickness Change of the Foam Layer
3.1.3. Temperature in the Gel Foam Layer
3.1.4. Volume Fraction of the Gas Phase
3.1.5. Thermal Diffusivity
3.2. Effect of Foam Composition and Operating Conditions
3.2.1. Effect of the Top Heating Temperature
3.2.2. Effect of the Initial Gas Volume Fraction in Foam
3.2.3. Effect of the Concentration of the Silica Nanoparticles
3.2.4. Effect of Size of the Silica Nanoparticles
4. Conclusions
- During the heating progress, the foam layer thickness decreases gradually with the increase in the kerosene layer surface temperature.
- On the premise of ensuring the foaming expansion ratio, decreasing the initial gas content could enhance the thermal stability and heat insulation performance of the gel foam layer.
- Small size and high concentration nanoparticles are preferred to the thermal stability and heat insulation performance of the three-phase gel foam.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| c | Specific heat capacity, J·kg−1·K−1 |
| d | Size parameter |
| E | Foam expansion ratio |
| f | Volume fraction of particle |
| H | Thickness of the foam layer, cm |
| mf | Total mass of the gel foam layer, kg |
| Q | Efficiency factor |
| qr | Radiant heating flux, kW·m−2 |
| T | Temperature, K |
| V | Volume, m3 |
| v | Velocity, m·s−1 |
| Greek symbols | |
| γ | Structure coefficient |
| θ | Mass fraction of the gas, liquid, or solid phase, % |
| κ | Scattering or absorption coefficient, m−1 |
| Mean free path of phonon, m | |
| λ | Thermal conductivity, W·m−1·K−1 |
| ρ | Density, kg·m−3 |
| Heating time, s | |
| Volume fraction of gas, liquid, or solid phase | |
| Subscripts | |
| bulk | Body phase |
| Foam layer | |
| g | Gas phase |
| l | Liquid phase |
| p | Particle phase |
| r | Radiation |
| s | Solid phase |
| sh,lo | Lower surface of shell |
| sh,up | Upper surface of shell |
| strut | Plateau border of foam cell |
| wall | Wall of foam cell |
References
- Wu, G.; Wang, X.; Xie, B.; Ji, C.; Gao, Z.; Zhao, C. Temperature distribution characteristics of steel circular tube members under oil pool fire conditions: Experiment and numerical simulation. Int. J. Thermophys. 2021, 42, 114. [Google Scholar] [CrossRef]
- Yi, H.; Feng, Y.; Wang, Q. Computational fluid dynamics (CFD) study of heat radiation from large liquefied petroleum gas (LPG) pool fires. J. Loss Prevent. Proc. 2019, 61, 262–274. [Google Scholar] [CrossRef]
- Boyd, C.; Marzo, M. The behaviour of a fire-protection foam exposed to radiant heating. Int. J. Heat Mass Tran. 1998, 41, 1719–1728. [Google Scholar] [CrossRef]
- Aksoy, M.; Alhosani, M.; Bayazitoglu, Y. Thermal resistance for Au-Water and Ag-Water interfaces: Molecular dynamics simulations. Int. J. Thermophys. 2021, 42, 87. [Google Scholar] [CrossRef]
- Poureslami, P.; Siavashi, M.; Moghimi, H.; Hosseini, M. Pore-scale convection-conduction heat transfer and fluid flow in open-cell metal foams: A three-dimensional multiple-relaxation time lattice Boltzmann (MRT-LBM) solution. Int. Commun. Heat Mass 2021, 126, 105465. [Google Scholar] [CrossRef]
- Sheng, Y.; Peng, Y.; Zhang, S.; Guo, Y.; Ma, L.; Wang, Q.; Zhang, H. Study on thermal stability of Gel Foam Co-Stabilized by Hydrophilic Silica Nanoparticles and Surfactants. Gels 2022, 8, 123. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, X.; Gu, Y.; Dai, C.; Yun, S.; Mao, P.; Guan, G.; Chen, J. Multifunctional Melamine Formaldehyde Composite Foam for High-Temperature Insulation, Flame Retardancy, and Oil-Water Separation. Ind. Eng. Chem. Res. 2022, 61, 6458–6467. [Google Scholar] [CrossRef]
- Xu, H.J.; Xing, Z.B.; Wang, F.Q.; Cheng, Z.M. Review on heat conduction, heat convection, thermal radiation and phase change heat transfer of nanofluids in porous media: Fundamentals and applications. Chem. Eng. Sci. 2019, 195, 462–483. [Google Scholar] [CrossRef]
- Cheng, Z.M.; Wang, F.Q.; Xie, Y.M.; Ma, L.X.; Xu, H.J.; Tan, J.Y.; Bai, F.W. Investigation of optical properties and radiative transfer of sea water-based nanofluids for photocatalysis with different salt concentrations. Int. J. Hydrogen Energ. 2017, 42, 26626–26638. [Google Scholar]
- Rambabu, S.; Parthasarathy, P.; Kishore, V.R. A numerical study of forced convection in ideal and randomized reticulated porous structures and a propoasl for a new correlation. Int. J. Heat Mass Tran. 2022, 184, 122292. [Google Scholar] [CrossRef]
- He, Y.; Xie, T. A review of heat transfer models of nanoporous silica aerogel insulation material. Chin. Sci. Bull. 2015, 60, 137–163. (In Chinese) [Google Scholar]
- French, R.J. The resistance of fire-fighting foams to destruction by radiant heat. J. App. Chem. 1952, 2, 60–64. [Google Scholar] [CrossRef]
- Xie, T.; He, Y.; Hu, Z. Theoretical study on thermal conductivities of silica aerogel composite insulating material. Int. J. Heat Mass Tran. 2013, 58, 540–552. [Google Scholar] [CrossRef]
- Zhou, R.F.; Lang, X.Q.; Zhang, X.; Tao, B.; He, L.M. Thermal stability and insulation characteristics of three-phase fire-fight foam exposed to radiation heating. Process Saf. Environ. 2021, 146, 360–368. [Google Scholar] [CrossRef]
- Perrson, H. Fire extinguishing foams resistance against heat radiation. In 1st International Conference on Fire Suppression Research; Swedish National Testing and Research Institute: Stockholm, Sweden, 1992; pp. 359–376. [Google Scholar]
- Ahern, A.; Verbist, G.; Weaire, D.; Phelan, R.; Fleurent, H. The conductivity of foams: A generalisation of the electrical to the thermal case. Colloid Surf. A Physicochem. Eng. Asp. 2005, 263, 275–279. [Google Scholar] [CrossRef]
- Godoy, W.F.; DesJardin, P.E. Efficient transmission calculations for polydisperse water sprays using spectral scaling. J. Quant. Spectrosc. Ra. 2007, 108, 440–453. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Dembele, S.; Wen, J.X. A simplified model for the shielding of fire thermal radiation by water mists. Int. J. Heat Mass Tran. 2016, 96, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qiao, Y.; Zhang, X.; Fang, S. Structural and thermal study of highly porous nanocomposite SiO2-based aerogels. J. Non-Cryst. Solids 2010, 356, 879–883. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; He, F. Microstructural characterization and properties of ambient-dried SiO2 matrix aerogel doped with opacified TiO2 powder. J. Alloys Compd. 2009, 469, 366–369. [Google Scholar] [CrossRef]
- Kwon, Y.G.; Choi, S.Y.; Kang, E.S.; Baek, S.S. Ambient-dried silica aerogel doped with TiO2 powder for thermal insulation. J. Mater. Sci. 2000, 35, 6075–6079. [Google Scholar] [CrossRef]
- Kuhn, J.; Gleissner, T.; Arduini-Schuster, M.C.; Korder, S.; Fricke, J. Integration of mineral powders into SiO2 aerogels. J. Non-Cryst. Solids 1995, 186, 291–295. [Google Scholar] [CrossRef]
- Zhou, R.; Dou, X.; Lang, X.; He, L.; Liu, J.; Mu, S. Foaming ability and stability of silica nanoparticle-based triple-phase foam for oil fire extinguishing: Experimental. Soft Mater. 2018, 16, 327–338. [Google Scholar] [CrossRef]
- Said, Z.; Sajid, M.H.; Saidur, R.; Mahdiraji, G.A.; Rahim, N.A. Evaluating the optical properties of TiO2 nanofluid for a direct absorption solar collector. Numer. Heat Transf. A-Appl. 2015, 67, 1010–1027. [Google Scholar] [CrossRef] [Green Version]
- Manetti, L.L.; Oliveira, A.S.; Moita, H.; Cardoso, E.M. A new pool boiling heat transfer correction for wetting dielectric fluids on meatal foams. Int. J. Heat Mass Transf. 2021, 171, 121070. [Google Scholar] [CrossRef]
- Anderson, O.L. The Debye Temperature of Vitreous Silica. J. Phys. Chem. Solids 1959, 12, 41–52. [Google Scholar] [CrossRef]
- Yang, C.C.; Xiao, M.X.; Li, W.; Jiang, Q. Size effects on Debye temperature, Einstein temperature, and volume thermal expansion coefficient of nanocrystals. Solid State Commun. 2006, 139, 148–152. [Google Scholar] [CrossRef]
- Han, Y.; Xia, X.; Dai, G. Simulation on phonon heat transport of silicon dioxide nanomaterial by Lattice Boltzmann Method. J. Eng. Thermophys. 2011, 32, 1571–1574. (In Chinese) [Google Scholar]
- Dames, C.; Chen, G. Theoretical Phonon Thermal Conductivity of Si/Ge Superlattice Nanowires. In Proceedings of the ASME 2003 Heat Transfer Summer Conference, Las Vegas, NV, USA, 21–23 July 2003; pp. 173–177. [Google Scholar]
- Chen, G. Nanoscale Energy Transport and Conversion: A Parallel Treatment of Electrons, Molecules, Phonons, and Photons; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Han, Y.F.; Xia, X.L.; Tan, H.P.; Liu, H.D. Modeling of phonon heat transfer in spherical segment of silica aerogel grains. Phys. B Condens. Matter 2013, 420, 58–63. [Google Scholar] [CrossRef]
- Goharshadi, E.; Ahmadzadeh, H.; Samiee, S.; Hadadian, M. Nanofluids for heat transfer—A review. Phys. Chem. Res. 2013, 1, 1–33. [Google Scholar]
- Wang, F.Q.; Tan. J.Y.; Jin. H.j.; Leng, Y. Thermochemical performance analysis of solar driven CO2 methane reforming. Energy 2015, 91, 645–654. [Google Scholar]
- Vafai, K. Handbook of Porous Media; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Tang, B. Development and Performances of Three-Phase Foam Extinguishing Agents for Intensifing Resistance to Oil-Fire. Doctoral Thesis, Hebei University of Technology, Tianjin, China, 2016; pp. 37–41. [Google Scholar]








| Parameter | Symbol/Unit | Set Value |
| Heating temperature on top | 500 | |
| Convective heat transfer Coefficient at the wall | 10 | |
| Initial volume fraction of the gas phase in foam | 0.95 | |
| Initial volume fraction of the particle phase in foam | 0.01 | |
| Size of silica nanoparticles | 3 × 10−8 | |
| Average diameter of bubbles in foam | 1 × 10−3 | |
| Liquid volume fraction of the wall in the foam cell | 0.8 | |
| Initial thickness of the foam layer | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Cui, P.; Cheng, Q.; Lang, X.; Zhang, Y.; Sun, Q.; Du, M. Heat Transfer Performance of Gel Foam Layer with Nanoparticles Doping under a Radiative Heat Flux. Micromachines 2022, 13, 2223. https://doi.org/10.3390/mi13122223
Zhou R, Cui P, Cheng Q, Lang X, Zhang Y, Sun Q, Du M. Heat Transfer Performance of Gel Foam Layer with Nanoparticles Doping under a Radiative Heat Flux. Micromachines. 2022; 13(12):2223. https://doi.org/10.3390/mi13122223
Chicago/Turabian StyleZhou, Rifeng, Pengyu Cui, Qingli Cheng, Xuqing Lang, Yong Zhang, Qie Sun, and Mu Du. 2022. "Heat Transfer Performance of Gel Foam Layer with Nanoparticles Doping under a Radiative Heat Flux" Micromachines 13, no. 12: 2223. https://doi.org/10.3390/mi13122223









