A Continuous Microfluidic Concentrator for High-Sensitivity Detection of Bacteria in Water Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication
2.2. Sample Preparation
2.3. Experimental Procedure and Post Analysis
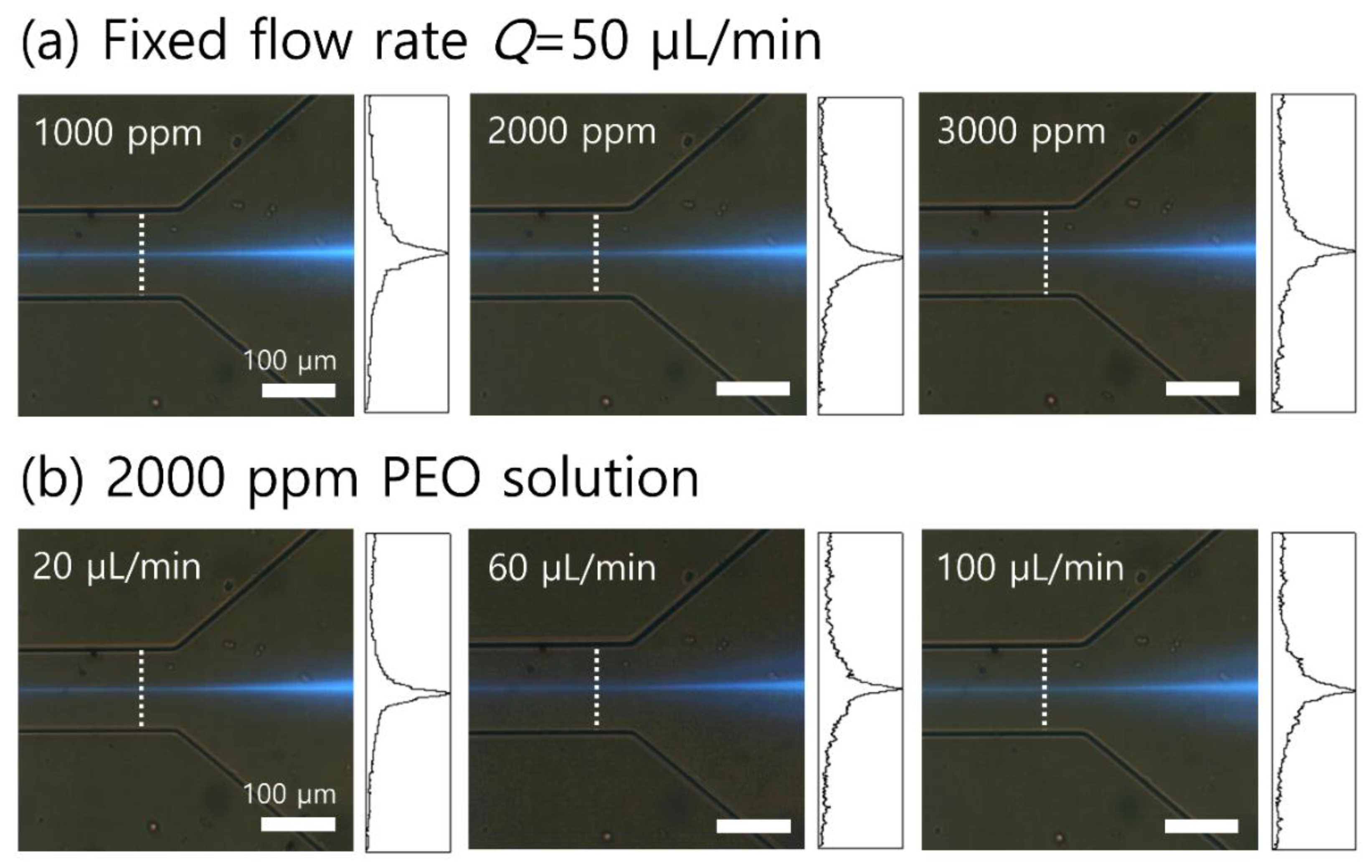
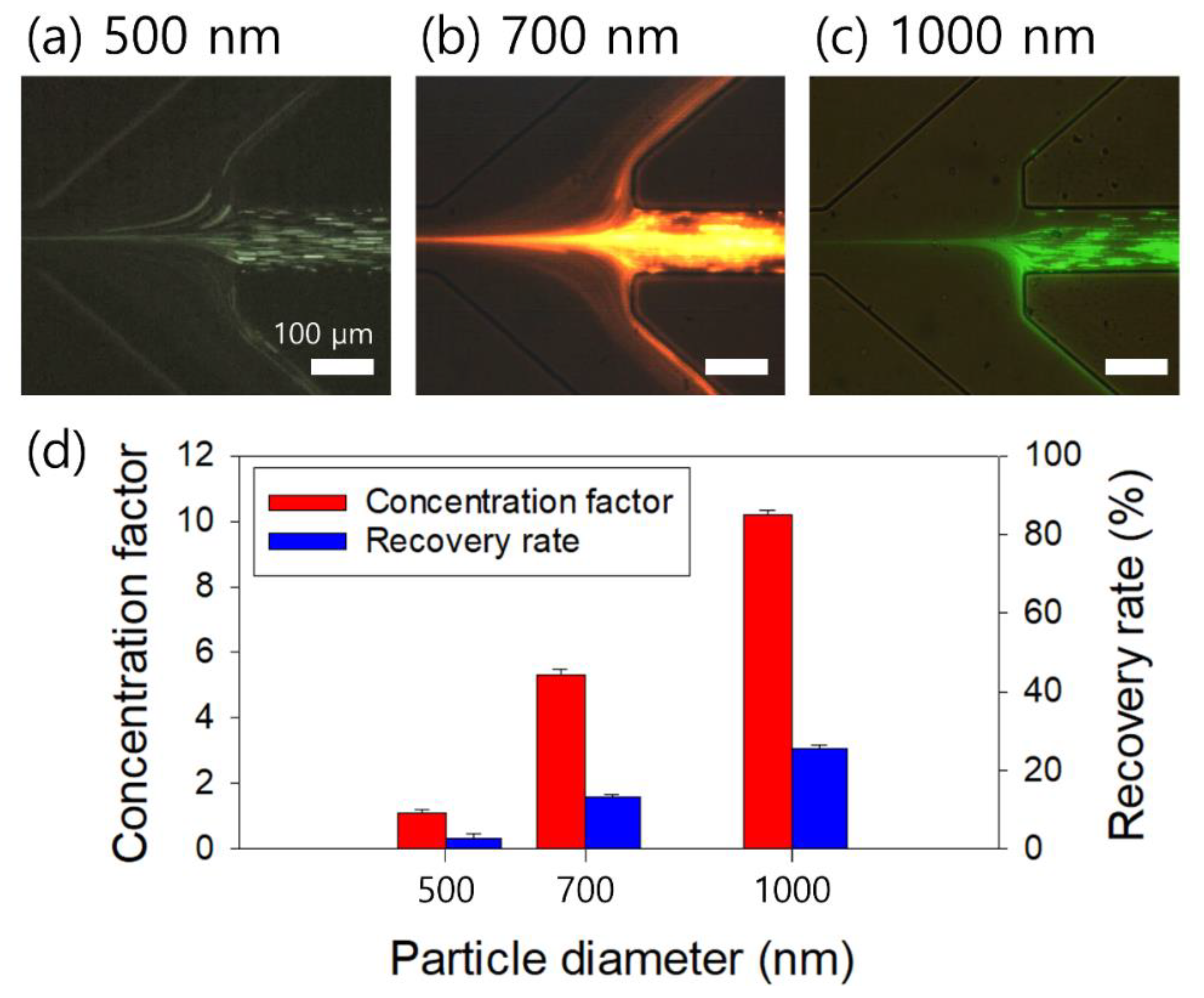
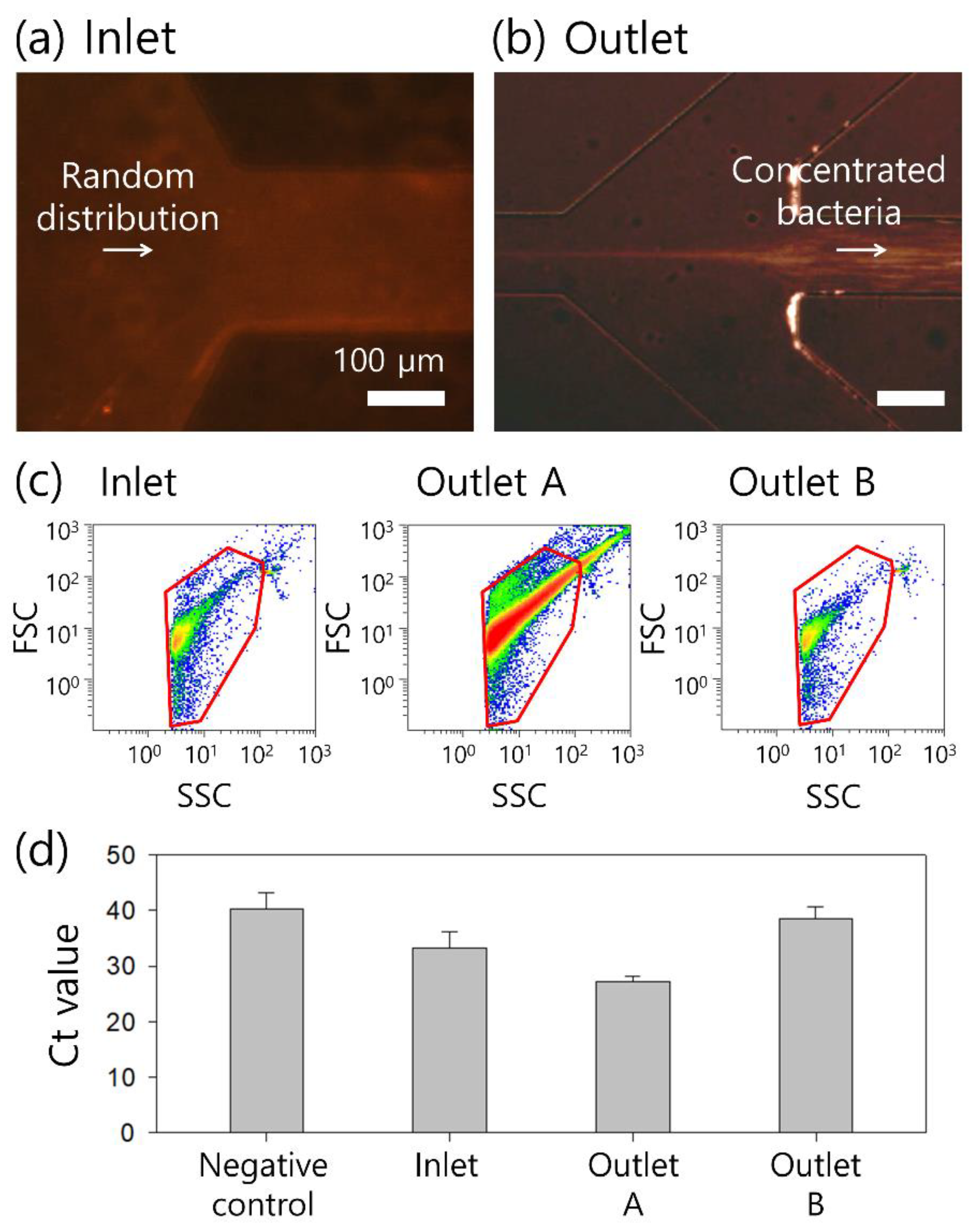
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Council, C.C. Drinking Water Chlorination: A Review of Disinfection Practices and Issues; Chlorine Chemistry Council/American Chemistry Council: Arlington, VA, USA, 2003; p. 68. [Google Scholar]
- Harakeh, S.; Yassine, H.; Hajjar, S.; El-Fadel, M. Isolates of Staphylococcus aureus and saprophyticus resistant to antimicrobials isolated from the Lebanese aquatic environment. Mar. Pollut. Bull. 2006, 52, 912–919. [Google Scholar] [CrossRef]
- Kessie, G.; Ettayebi, M.; Haddad, A.M.; Shibl, A.M.; Al-Shammary, F.J.; Tawfik, A.F.; Al-Ahdal, M.N. Plasmid profile and antibiotic resistance in coagulase-negative staphylococci isolated from polluted water. J. Appl. Microbiol. 1998, 84, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Seyedmonir, E.; Yilmaz, F.; Icgen, B.J. mecA gene dissemination among staphylococcal and non-staphylococcal isolates shed in surface waters. Bull. Environ. Contam. Toxicol. 2015, 95, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Capitanio, B.; Ascenzioni, F.; Pimpinelli, F.; Morrone, A.; Ensoli, F. Staphylococcus aureus and the cutaneous microbiota biofilms in the pathogenesis of atopic dermatitis. Microorganisms 2019, 7, 301. [Google Scholar] [CrossRef] [Green Version]
- Kane, T.L.; Carothers, K.E.; Lee, S.W. Virulence factor targeting of the bacterial pathogen Staphylococcus aureus for vaccine and therapeutics. Curr. Drug Targets 2018, 19, 111–127. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Seidler, R.J. Staphylococcus aureus in rural drinking water. Appl. Environ. Microbiol. 1980, 39, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mairi, A.; Touati, A.; Lavigne, J.P. Methicillin-resistant Staphylococcus aureus ST80 clone: A systematic review. Toxins 2020, 12, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlin, I.; Rokney, A.; Onn, A.; Glikman, D.; Peretz, A. Hospital clones of methicillin-resistant Staphylococcus aureus are carried by medical students even before healthcare exposure. Antimicrob. Resist. Infect. Control 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Associatio: Washington, DC, USA, 2005; Volume 21. [Google Scholar]
- Hao, X.; Yeh, P.; Qin, Y.; Jiang, Y.; Qiu, Z.; Li, S.; Le, T.; Cao, X. Aptamer surface functionalization of microfluidic devices using dendrimers as multi-handled templates and its application in sensitive detections of foodborne pathogenic bacteria. Anal. Chim. Acta 2019, 1056, 96–107. [Google Scholar] [CrossRef]
- Li, N.; Lu, Y.; Cheng, J.; Xu, Y. A self-contained and fully integrated fluidic cassette system for multiplex nucleic acid detection of bacteriuria. Lab Chip 2020, 20, 384–393. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Yagupsky, P.; Nolte, F. Quantitative aspects of septicemia. Clin. Microbiol. Rev. 1990, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.W.; Sharma, P.K.; van der Mei, H.C.; Busscher, H.J. Bacterial cell surface damage due to centrifugal compaction. Appl. Environ. Microbiol. 2012, 78, 120–125. [Google Scholar] [CrossRef] [Green Version]
- D’Avino, G.; Maffettone, P.; Greco, F.; Hulsen, M. Viscoelasticity-induced migration of a rigid sphere in confined shear flow. J. Non-Newton. Fluid Mech. 2010, 165, 466–474. [Google Scholar] [CrossRef]
- Leshansky, A.M.; Bransky, A.; Korin, N.; Dinnar, U. Tunable nonlinear viscoelastic “focusing” in a microfluidic device. Phys. Rev. Lett. 2007, 98, 234501. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D. Inertial microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Kim, J.M. Elasto-inertial particle focusing under the viscoelastic flow of DNA solution in a square channel. Biomicrofluidics 2016, 10, 024111. [Google Scholar] [CrossRef]
- Liu, C.; Xue, C.; Hu, G. Sheathless separation of particles and cells by viscoelastic effects in straight rectangular microchannels. Procedia Eng. 2015, 126, 721–724. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guo, J.; Tian, F.; Yang, N.; Yan, F.; Ding, Y.; Wei, J.; Hu, G.; Nie, G.; Sun, J. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 2017, 11, 6968–6976. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.; Jee, H.; Jang, W.S.; Yoon, J.; Park, B.G.; Lee, S.J.; Lim, C.S. Sheathless shape-based separation of Candida albicans using a Viscoelastic non-Newtonian fluid. Micromachines 2019, 10, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Lim, H.; Kim, D.; Jung, H.; Shin, S. Continuous separation of microparticles in a microfluidic channel via the elasto-inertial effect of non-Newtonian fluid. Lab Chip 2012, 12, 1347–1354. [Google Scholar] [CrossRef]
- Nam, J.; Namgung, B.; Lim, C.T.; Bae, J.E.; Leo, H.L.; Cho, K.S.; Kim, S. Microfluidic device for sheathless particle focusing and separation using a viscoelastic fluid. J. Chromatogr. A 2015, 1406, 244–250. [Google Scholar] [CrossRef]
- Nam, J.; Tan, J.K.S.; Khoo, B.L.; Namgung, B.; Leo, H.L.; Lim, C.T.; Kim, S. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics 2015, 9, 064117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Jang, W.S.; Lim, C.S. Non-electrical powered continuous cell concentration for enumeration of residual white blood cells in WBC-depleted blood using a viscoelastic fluid. Talanta 2019, 197, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, H.; Yuan, D.; Jang, D.; Yan, S.; Li, M. Separation and enrichment of yeast saccharomyces cerevisiae by shape using viscoelastic microfluidics. Anal. Chem. 2020, 93, 1586–1595. [Google Scholar] [CrossRef]
- Kalashnikov, M.; Lee, J.C.; Campbell, J.; Sharon, A.; Sauer-Budge, A.F. A microfluidic platform for rapid, stress-induced antibiotic susceptibility testing of Staphylococcus aureus. Lab Chip 2012, 12, 4523–4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Yoon, J.; Jee, H.; Jang, W.S.; Lim, C.S. High-Throughput Separation of Microvesicles from Whole Blood Components Using Viscoelastic Fluid. Adv. Mater. Technol. 2020, 5, 2000612. [Google Scholar] [CrossRef]
- Seo, K.W.; Byeon, H.J.; Huh, H.K.; Lee, S.J. Particle migration and single-line particle focusing in microscale pipe flow of viscoelastic fluids. RSC Adv. 2014, 4, 3512–3520. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, M. An experimental study of particle migration in pipe flow of viscoelastic fluids. J. Rheol. 1996, 40, 1057–1077. [Google Scholar] [CrossRef]
- Jeon, H.; Kwon, T.; Yoon, J.; Han, J. Engineering a deformation-free plastic spiral inertial microfluidic system for CHO cell clarification in biomanufacturing. Lab Chip 2022, 22, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, Z.; Lin, M.; Dai, Z.; Cao, X. Development of a highly effective multi-stage surface acoustic wave SU-8 microfluidic concentrator. Sens. Actuators B Chem. 2015, 215, 77–85. [Google Scholar] [CrossRef]
- Nam, J.; Jang, W.S.; Hong, D.H.; Lim, C.S. Viscoelastic separation and concentration of fungi from blood for highly sensitive molecular diagnostics. Sci. Rep. 2019, 9, 3067. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Shao, X.; Bostwick, J.B.; Xuan, X. Particle separation in xanthan gum solutions. Microfluid. Nanofluidics 2019, 23, 125. [Google Scholar] [CrossRef]
- Al-Halhouli, A.; Albagdady, A.; Al-Faqheri, W.; Kottmeier, J.; Meinen, S.; Frey, L.J.; Krull, R.; Dietzel, A. Enhanced inertial focusing of microparticles and cells by integrating trapezoidal microchambers in spiral microfluidic channels. RSC Adv. 2019, 9, 19197–19204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Halhouli, A.; Al-Faqheri, W.; Alhamarneh, B.; Hecht, L.; Dietzel, A. Spiral microchannels with trapezoidal cross section fabricated by femtosecond laser ablation in glass for the inertial separation of microparticles. Micromachines 2018, 9, 171. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Kim, J.; Lee, W. Changes of inertial focusing position in a triangular channel depending on droplet deformability and size. Micromachines 2020, 11, 839. [Google Scholar] [CrossRef]
- Harris, L.G.; Foster, S.; Richards, R.G. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cells Mater. 2002, 4, 100–120. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [Green Version]
- Białas, N.; Kasperkiewicz, K.; Radziejewska-Lebrecht, J.; Skurnik, M. Bacterial cell surface structures in Yersinia enterocolitica. Arch. Immunol. Et Ther. Exp. 2012, 60, 199–209. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30. [Google Scholar] [CrossRef] [PubMed]
- Reshes, G.; Vanounou, S.; Fishov, I.; Feingold, M. Cell shape dynamics in Escherichia coli. Biophys. J. 2008, 94, 251–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, H.W.; Gan, H.Y.; Bhagat, A.A.S.; Li, L.D.; Lim, C.T.; Han, J. A microfluidics approach towards high-throughput pathogen removal from blood using margination. Biomicrofluidics 2012, 6, 024115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, B.L.; Warkiani, M.E.; Tan, D.S.W.; Bhagat, A.A.S.; Irwin, D.; Lau, D.P.; Lim, A.S.T.; Lim, K.H.; Krisna, S.S.; Lim, W.T.; et al. Clinical validation of an ultra high-throughput spiral microfluidics for the detection and enrichment of viable circulating tumor cells. PLoS ONE 2014, 9, e99409. [Google Scholar] [CrossRef] [Green Version]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.W.; Bhagat, A.A.S.; Lim, W.T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245–3255. [Google Scholar] [CrossRef]
- Hupert, M.L.; Jackson, J.M.; Wang, H.; Witck, M.A.; Kamande, J.; Milowsky, M.; Whang, Y.E.; Soper, S.A. Arrays of high-aspect ratio microchannels for high-throughput isolation of circulating tumor cells (CTCs). Microsyst. Technol. 2014, 20, 1815–1825. [Google Scholar] [CrossRef]
- Taly, V.; Viovy, J.-L.; Descroix, S. Microchip Diagnostics: Methods and Protocols; Springer: Berlin, Germany, 2017. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, S.; Lim, H.; Kim, T.E.; Park, J.; Park, K.B.; Park, C.; Lim, C.S.; Nam, J. A Continuous Microfluidic Concentrator for High-Sensitivity Detection of Bacteria in Water Sources. Micromachines 2022, 13, 1093. https://doi.org/10.3390/mi13071093
Choo S, Lim H, Kim TE, Park J, Park KB, Park C, Lim CS, Nam J. A Continuous Microfluidic Concentrator for High-Sensitivity Detection of Bacteria in Water Sources. Micromachines. 2022; 13(7):1093. https://doi.org/10.3390/mi13071093
Chicago/Turabian StyleChoo, Seunghee, Hyunjung Lim, Tae Eun Kim, Jion Park, Kyu Been Park, Chaewon Park, Chae Seung Lim, and Jeonghun Nam. 2022. "A Continuous Microfluidic Concentrator for High-Sensitivity Detection of Bacteria in Water Sources" Micromachines 13, no. 7: 1093. https://doi.org/10.3390/mi13071093





