Film-Shaped Self-Powered Electro-Osmotic Micropump Array
Abstract
:1. Introduction
2. Materials and Methods
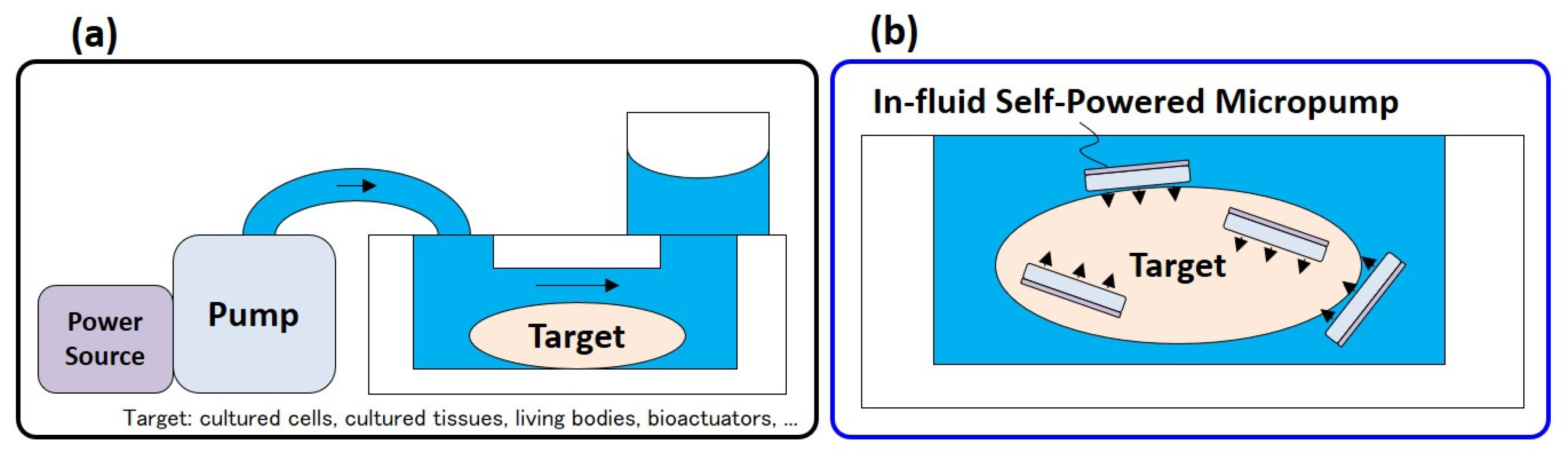
2.1. Concept
2.2. Configuration and Design
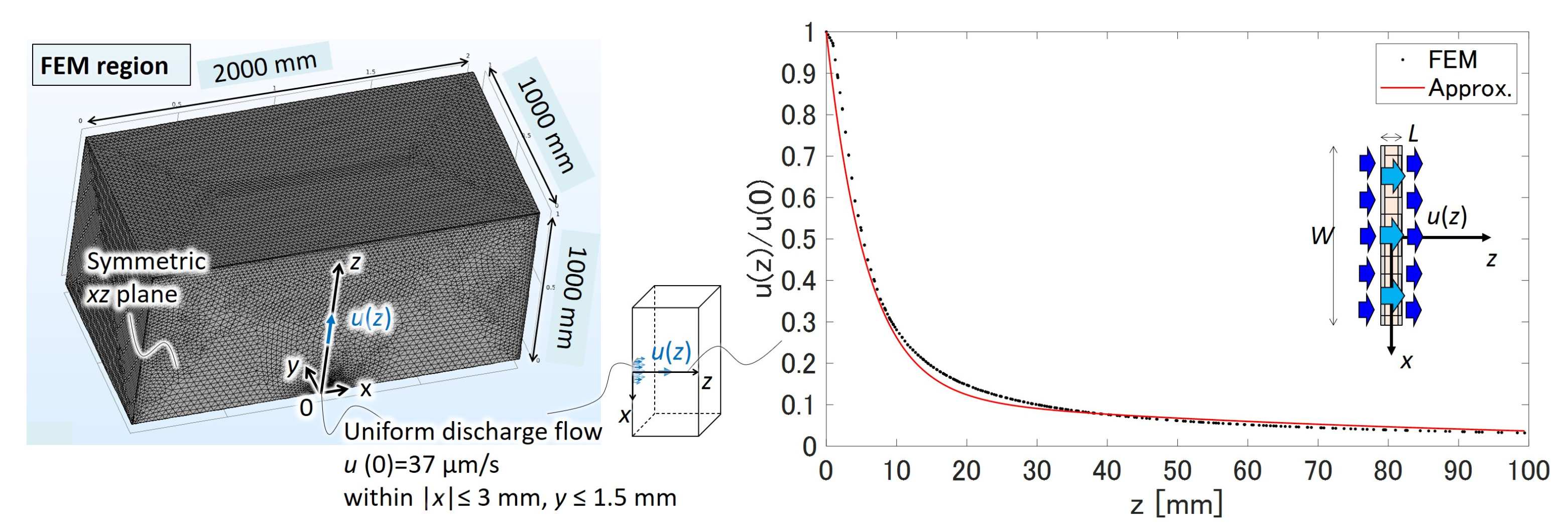
2.3. Theoretical Model
| Meaning | Part | Symbol | Value (Units) | Comment |
|---|---|---|---|---|
| Permittivity | Glucose solution | 6.580 × 10 (F/m) | Water (310 K) | |
| Viscosity | Glucose solution | 0.692 × 10 (Pa s) | Water (310 K) | |
| Mass density | Glucose solution | 993 (kg/m) | Water (310 K) | |
| OCP | Electrode layers | ≥100 (mV) | [23] | |
| Zeta potential | Insulating layer | −30 (mV) | SU-8 [27] | |
| Thickness | Insulating layer | L | 30 (m) | – |
| Inner diameter | Through-hole | D | 70 (m) | – |
| Array pitch | Through-hole | P | 100 (m) | – |
| Lateral size | Micropump array | W | 3 (mm) | – |
| EOF velocity | Micropump array | ≥95 (m/s) | – | |
| Mean planar flow velocity | Micropump array | u | ≥37 (m/s) | – |
| Mean flow rate | Micropump array | Q | ≥20 (L/min) | – |
2.4. Theoretical Performance
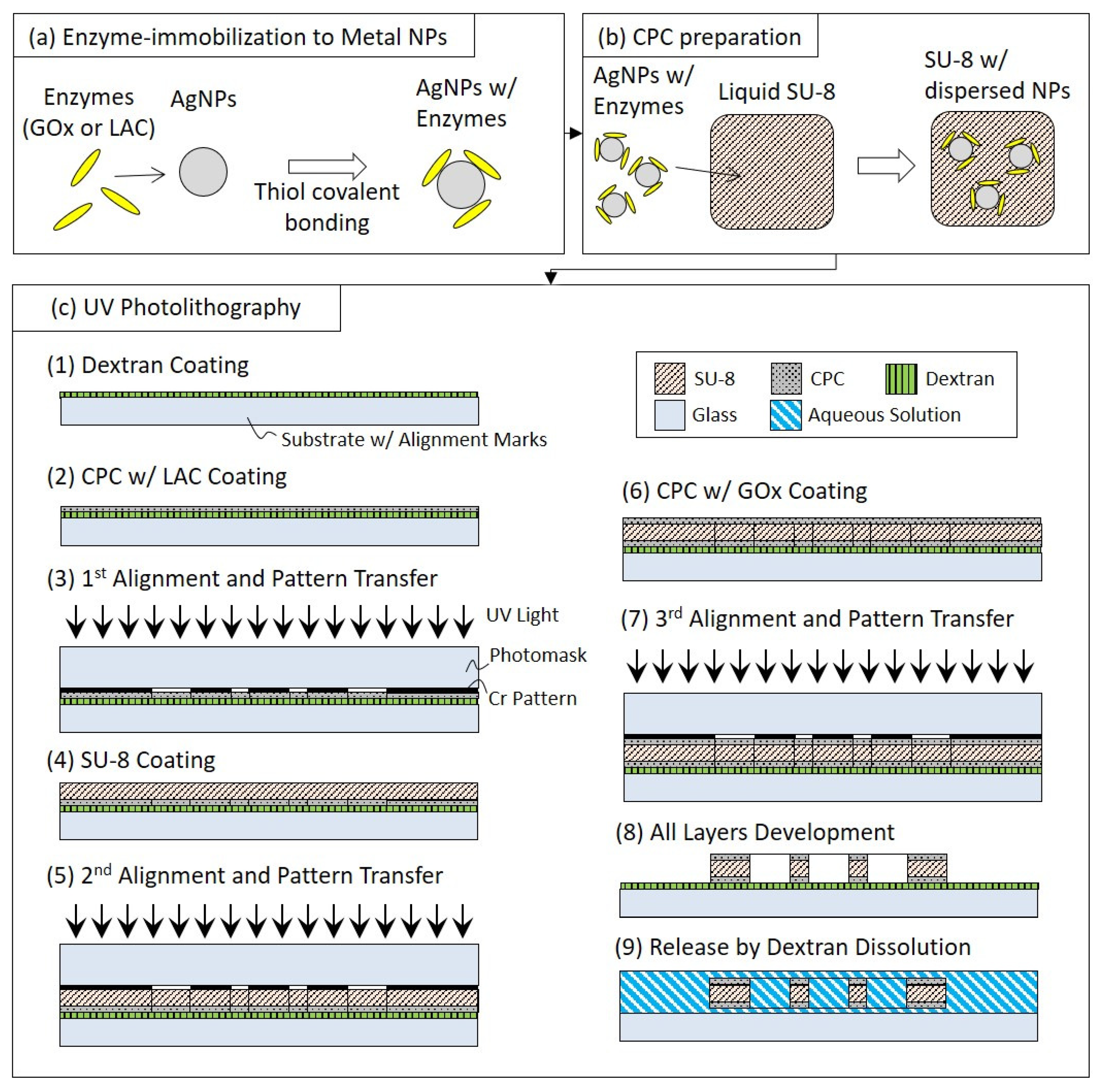
2.5. Fabrication Process
2.5.1. Enzyme-Immobilization to Metal NPs
2.5.2. CPC Preparation
2.5.3. UV Photolithography with Mask Pattern Transfers
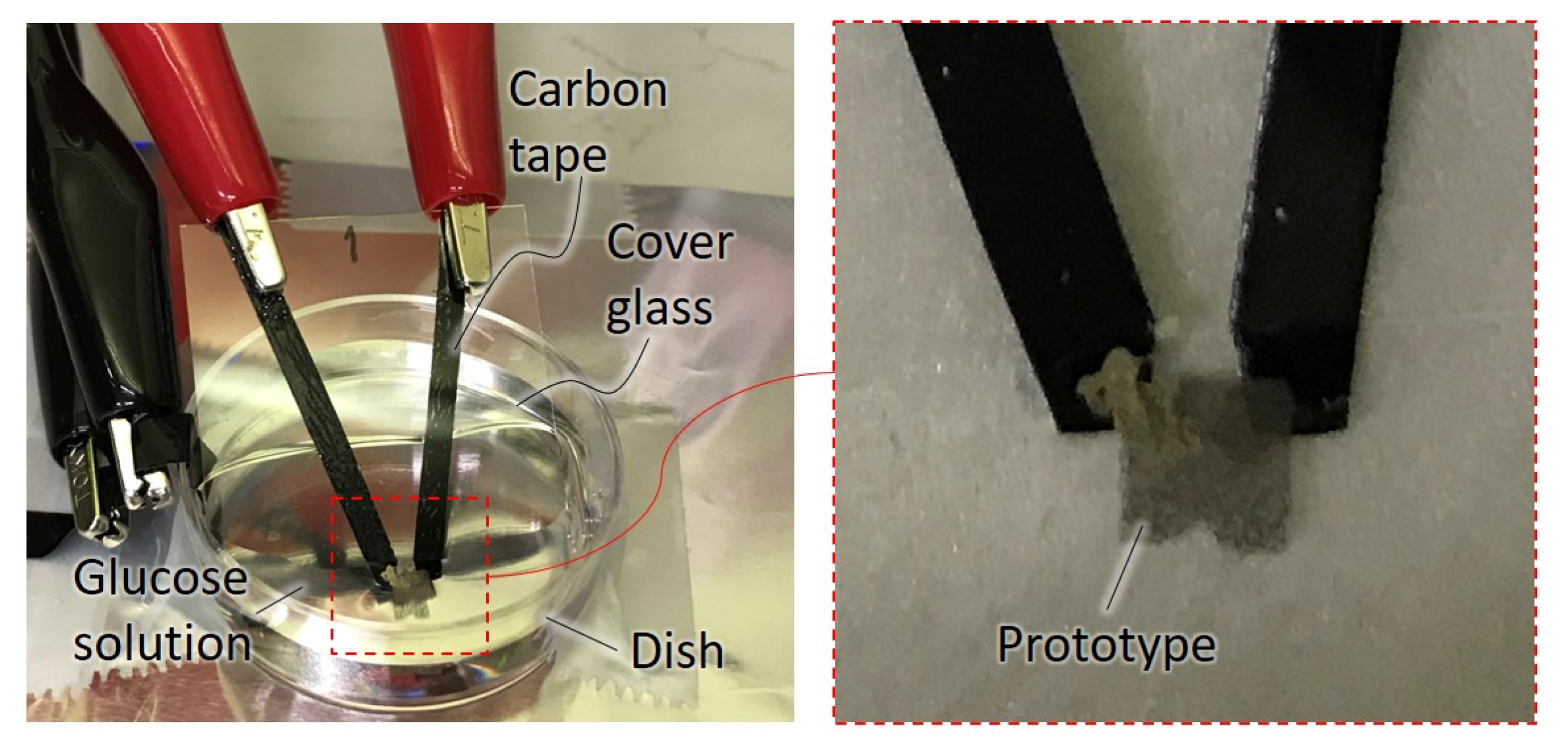
2.6. Fabricated Prototype
2.7. Experimental Methods
3. Results and Discussion
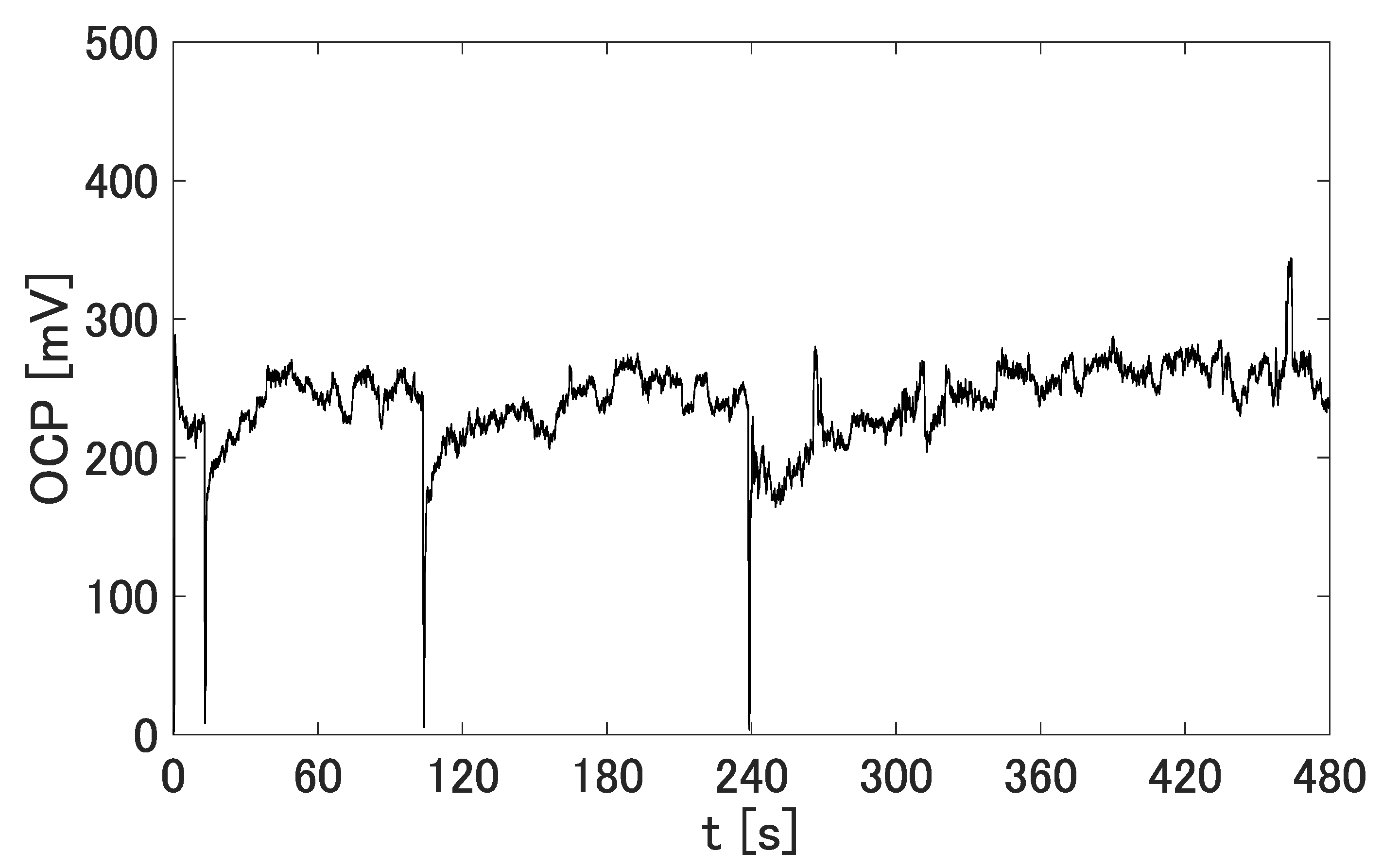
3.1. OCP Generated by the Prototypes
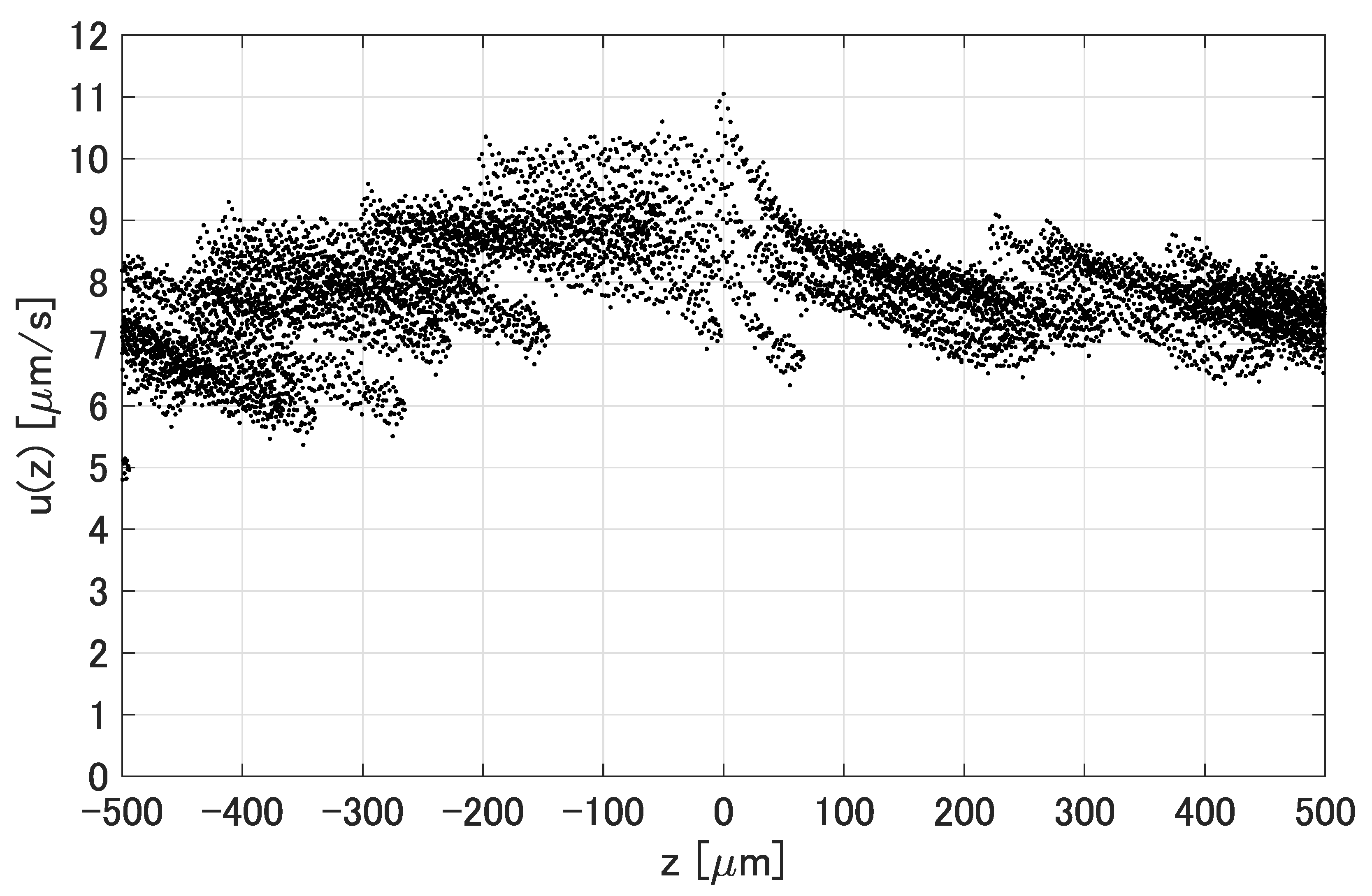
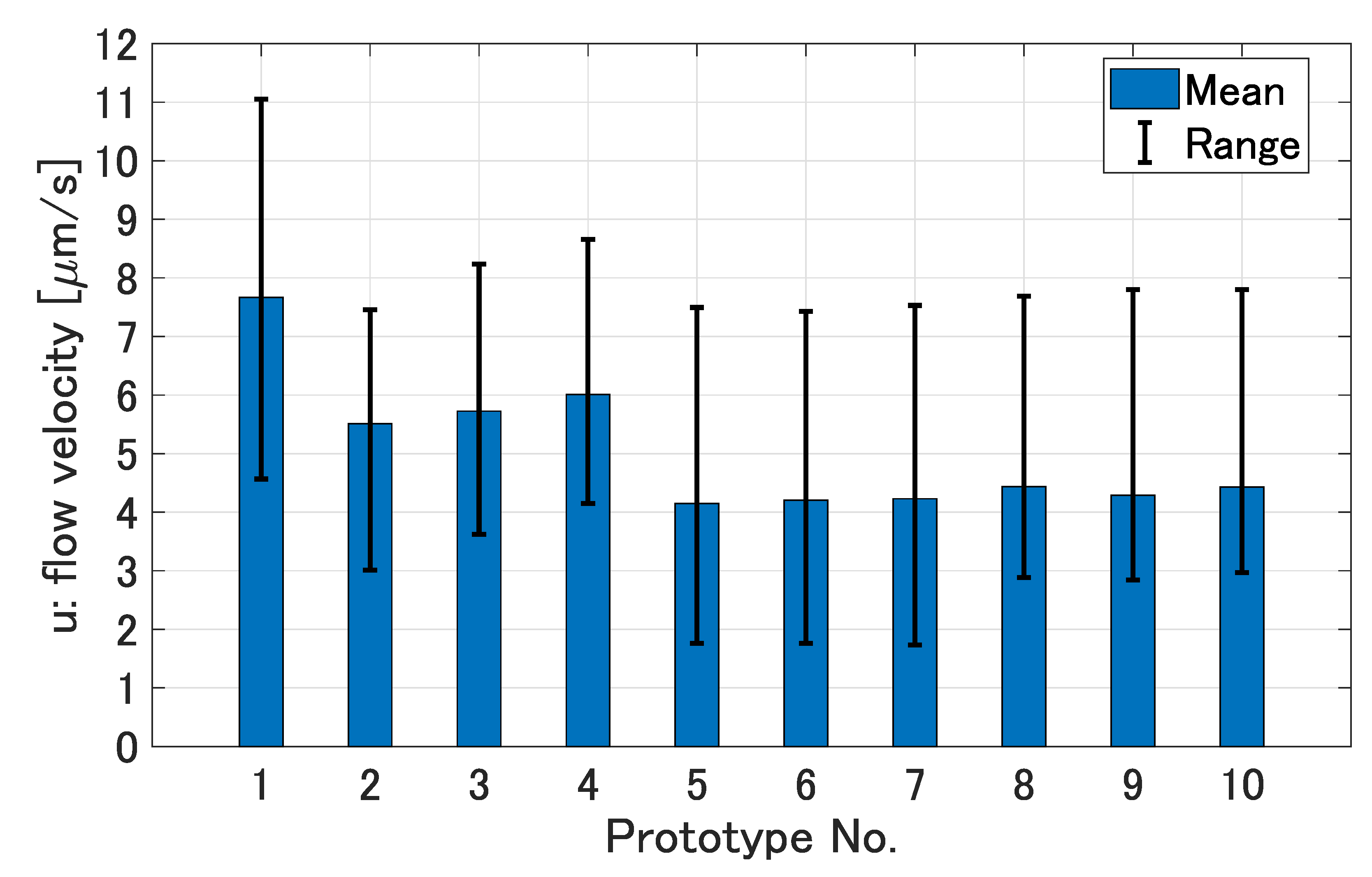
3.2. Generated Flows around the Prototypes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 11-MUA | 11-mercaptoundecanoic acid |
| AgNP | Silver nanoparticle |
| BFC | Biofuel cell |
| CPC | Conductive polymer composite |
| DCC | N,N’-dicyclohexylcarbodiimide |
| EO | Electro-osmotic |
| EOF | Electro-osmotic flow |
| GOx | Glucose oxidase |
| LAC | Laccase |
| NHS | N-hydroxysuccinimide |
| NP | Nanoparticle |
| OCP | Open-circuit potential |
| SAM | Self-assembled monolayer |
| SEM | Scanning electron microscope |
| UV | Ultraviolet |
References
- Chan, V.; Asada, H.H.; Bashir, R. Utilization and control of bioactuators across multiple length scales. Lab Chip 2014, 14, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chu, L.Y.; Chueh, B.H.; Shen, M.; Hazarika, B.; Phadke, N.; Takayama, S. Arrays of horizontally-oriented mini-reservoirs generate steady microfluidic flows for continuous perfusion cell culture and gradient generation. Analyst 2004, 129, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Goral, V.N.; Zhou, C.; Lai, F.; Yuen, P.K. A continuous perfusion microplate for cell culture. Lab Chip 2013, 13, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Furutani, S.; Nagai, H. The activity determination of single cell by isolation and cultivation on a centrifugal flow disk. ECS Trans. 2009, 16, 1. [Google Scholar] [CrossRef]
- Ren, Y.; Chow, L.M.C.; Leung, W.W.F. Cell culture using centrifugal microfluidic platform with demonstration on Pichia pastoris. Biomed. Microdevices 2013, 15, 321–337. [Google Scholar] [CrossRef]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Aghdam, N.; Lin, R.; Lee, L.P. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab Chip 2005, 5, 44–48. [Google Scholar] [CrossRef]
- Park, J.; Kerner, A.; Burns, M.A.; Lin, X.N. Microdroplet-enabled highly parallel co-cultivation of microbial communities. PLoS ONE 2011, 6, e17019. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.B.; Wang, S.S.; Hsieh, C.H.; Lin, Y.C.; Lai, C.S.; Wu, M.H. An integrated microfluidic cell culture system for high-throughput perfusion three-dimensional cell culture-based assays: Effect of cell culture model on the results of chemosensitivity assays. Lab Chip 2013, 13, 1133–1143. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. A micro cell culture analog (μCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 2009, 9, 1385–1394. [Google Scholar] [CrossRef]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Sjöberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 2007, 79, 8557–8563. [Google Scholar] [CrossRef] [PubMed]
- Meyvantsson, I.; Warrick, J.W.; Hayes, S.; Skoien, A.; Beebe, D.J. Automated cell culture in high density tubeless microfluidic device arrays. Lab Chip 2008, 8, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Ko, J.M.; Cha, H.C.; Park, J.Y.; Im, C.H.; Lee, S.H. An electrofusion chip with a cell delivery system driven by surface tension. J. Micromech. Microeng. 2008, 19, 015004. [Google Scholar] [CrossRef]
- Li, P.C.; Harrison, D.J. Transport, manipulation, and reaction of biological cells on-chip using electrokinetic effects. Anal. Chem. 1997, 69, 1564–1568. [Google Scholar] [CrossRef]
- Xuan, X.; Li, D. Focused electrophoretic motion and selected electrokinetic dispensing of particles and cells in cross-microchannels. Electrophoresis 2005, 26, 3552–3560. [Google Scholar] [CrossRef]
- Park, J.Y.; Hwang, C.M.; Lee, S.H.; Lee, S.H. Gradient generation by an osmotic pump and the behavior of human mesenchymal stem cells under the fetal bovine serum concentration gradient. Lab Chip 2007, 7, 1673–1680. [Google Scholar] [CrossRef]
- Xu, Z.R.; Yang, C.G.; Liu, C.H.; Zhou, Z.; Fang, J.; Wang, J.H. An osmotic micro-pump integrated on a microfluidic chip for perfusion cell culture. Talanta 2010, 80, 1088–1093. [Google Scholar] [CrossRef]
- Glawdel, T.; Ren, C.L. Electro-osmotic flow control for living cell analysis in microfluidic PDMS chips. Mech. Res. Commun. 2009, 36, 75–81. [Google Scholar] [CrossRef]
- Glawdel, T.; Elbuken, C.; Lee, L.E.; Ren, C.L. Microfluidic system with integrated electroosmotic pumps, concentration gradient generator and fish cell line (RTgill-W1)—Towards water toxicity testing. Lab Chip 2009, 9, 3243–3250. [Google Scholar] [CrossRef]
- Byun, C.K.; Abi-Samra, K.; Cho, Y.K.; Takayama, S. Pumps for microfluidic cell culture. Electrophoresis 2014, 35, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Katz, E.; MacVittie, K. Implanted biofuel cells operating in vivo–methods, applications and perspectives–feature article. Energy Environ. Sci. 2013, 6, 2791–2803. [Google Scholar] [CrossRef]
- Gamella, M.; Koushanpour, A.; Katz, E. Biofuel cells–activation of micro-and macro-electronic devices. Bioelectrochemistry 2018, 119, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Arai, F. Self-propelled swimming microrobot using electroosmotic propulsion and biofuel cell. IEEE Robot. Autom. Lett. 2018, 3, 1787–1792. [Google Scholar] [CrossRef]
- Yamanaka, T.; Arai, F. Miniaturization effect of electroosmotic self-propulsive microswimmer powered by biofuel cell. Robomech J. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Yamanaka, T.; Arai, F. Electroosmotic Micropump Array Film Using Biofuel Cell. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1032–1035. [Google Scholar]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2007; Volume 18. [Google Scholar]
- Sikanen, T.; Tuomikoski, S.; Ketola, R.A.; Kostiainen, R.; Franssila, S.; Kotiaho, T. Characterization of SU-8 for electrokinetic microfluidic applications. Lab Chip 2005, 5, 888–896. [Google Scholar] [CrossRef]
- Soukharev, V.; Mano, N.; Heller, A. A four-electron O2-electroreduction biocatalyst superior to platinum and a biofuel cell operating at 0.88 V. J. Am. Chem. Soc. 2004, 126, 8368–8369. [Google Scholar] [CrossRef]
- Zebda, A.; Gondran, C.; Le Goff, A.; Holzinger, M.; Cinquin, P.; Cosnier, S. Mediatorless high-power glucose biofuel cells based on compressed carbon nanotube-enzyme electrodes. Nat. Commun. 2011, 2, 370. [Google Scholar] [CrossRef] [Green Version]
- Luckarift, H.R.; Atanassov, P.B.; Johnson, G.R. Enzymatic Fuel Cells: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Patel, N.; Davies, M.C.; Hartshorne, M.; Heaton, R.J.; Roberts, C.J.; Tendler, S.J.; Williams, P.M. Immobilization of protein molecules onto homogeneous and mixed carboxylate-terminated self-assembled monolayers. Langmuir 1997, 13, 6485–6490. [Google Scholar] [CrossRef]
- Li, D.; He, Q.; Cui, Y.; Duan, L.; Li, J. Immobilization of glucose oxidase onto gold nanoparticles with enhanced thermostability. Biochem. Biophys. Res. Commun. 2007, 355, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Karabchevsky, A.; Tsapovsky, L.; Marks, R.S.; Abdulhalim, I. Study of immobilization procedure on silver nanolayers and detection of estrone with diverged beam surface plasmon resonance (SPR) imaging. Biosensors 2013, 3, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Jiquet, S.; Bertsch, A.; Hofmann, H.; Renaud, P. Conductive SU8 photoresist for microfabrication. Adv. Funct. Mater. 2005, 15, 1511–1516. [Google Scholar] [CrossRef]
- Linder, V.; Gates, B.D.; Ryan, D.; Parviz, B.A.; Whitesides, G.M. Water-soluble sacrificial layers for surface micromachining. Small 2005, 1, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Psoma, S.D.; van der Wal, P.D.; Frey, O.; de Rooij, N.F.; Turner, A.P. A novel enzyme entrapment in SU-8 microfabricated films for glucose micro-biosensors. Biosens. Bioelectron. 2010, 26, 1582–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, T.; Arai, F. Film-Shaped Self-Powered Electro-Osmotic Micropump Array. Micromachines 2023, 14, 785. https://doi.org/10.3390/mi14040785
Yamanaka T, Arai F. Film-Shaped Self-Powered Electro-Osmotic Micropump Array. Micromachines. 2023; 14(4):785. https://doi.org/10.3390/mi14040785
Chicago/Turabian StyleYamanaka, Toshiro, and Fumihito Arai. 2023. "Film-Shaped Self-Powered Electro-Osmotic Micropump Array" Micromachines 14, no. 4: 785. https://doi.org/10.3390/mi14040785





