SOI MEMS Electro-Thermal Actuators for Biomedical Applications: Operation in 0.9% NaCl Solution
Abstract
:1. Introduction
- Thermal Criteria: As shall be seen later in this paper, MEMS electro-thermal actuators (ETAs) may be required to operate whilst fully submerged in aqueous media. For this reason, an elementary specification is that the device must not generate temperatures higher than the boiling temperature of the aqueous medium. In the current work, a maximum temperature of 100 °C was set as the temperature specification, as also implemented in [19]. This allows for bubble-free procedures that, in turn, allow for more accurate optical measurements. Secondly, human RBCs have a predefined temperature limit within which they maintain their original integrity. A previous work by Singh et al. [20] deduced that the decrease in deformability of a RBC is “highly significant” at test temperatures below 21.5 °C. In another publication by Xu et al. [21], the authors recalled that, below 22 °C, phosphatidylcholine lipids transition from a liquid-like state to a near-crystalline configuration. This implies that RBC stiffness increases at temperatures below 22 °C, therefore confirming the observations by Singh et al. [20]. Moreover, in [21], it was observed that stiffness alterations in RBCs in whole-blood when tested between 23 °C and 37 °C were negligible. More recent studies by Marai et al. [22] determined that human RBC stiffness in whole-blood samples was barely altered when tested between 37 °C and 39 °C. At temperatures above 40 °C, however, RBC stiffness begins to decay gradually in whole-blood samples. When human RBCs are suspended in aqueous saline solutions (phosphate-buffered saline, PBS) rather than whole-blood, the rate of stiffness decay is even more exaggerated. The target fluid for cell manipulation in this study was identified as a 0.9 wt% NaCl solution rather than whole-blood. Considering the above criteria, the temperature range for cell manipulation and characterisation in this work was defined as no less than 22 °C and no more than 40 °C [20,23,24]. This requirement implies that the designated characterisation or manipulation region on the device (specifically the end-effector tips) must not experience temperature increases beyond these set limits [15].
- Physical Attributes: A device must be able to physically compress a human RBC by at least 7 µm along its largest diameter. This is because human RBCs are typically characterised by their major diameter, in the order of 10 µm, and must travel through capillaries with a 3 µm diameter [25].
- Operational Media: In order for a human RBC to maintain its original integrity, it must be kept in an isotonic fluid [26,27]. A 0.9% NaCl solution is considered an isotonic solution [28]. Given the micron-size footprint of MEMS devices, partial immersion may be a challenge in itself. In the current work, it was specified that the device must be able to operate in fully submerged conditions. In such conditions, a number of issues may arise. Among them are electrolysis [29] and aqueous corrosion [29]. Similarly to why boiling ought to be avoided, the electrolysis of a fluid must also be avoided since gas evolution may impede displacement measurements and also affect corrosion kinetics. The authors of [30] proposed using high-frequency AC pulses with a mean voltage of 0 V to avoid the occurrence of electrolysis and corrosion phenomena. This strategy was successfully implemented in [15,19], and a similar methodology was adopted in our study.
2. Materials and Fabrication Process Overview
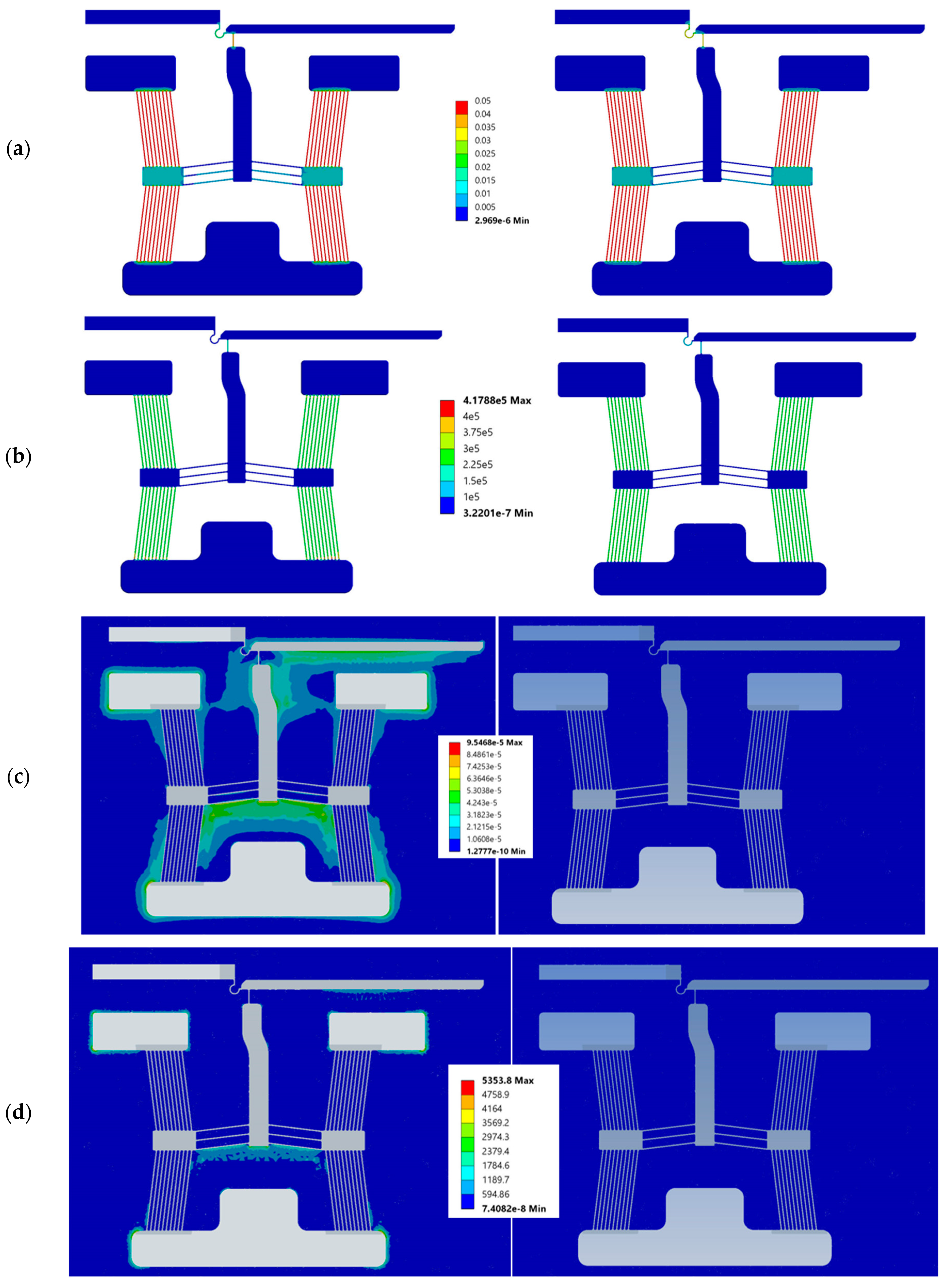
3. Numerical Modelling
| Property | Value |
|---|---|
| Thermal conductivity [34], k (W/m.K) | 0.594 |
| Specific heat capacity [35], c (J/kg.K) | 4182 |
| Electrical resistivity (Ω.m) | 0.621 |
| Density [36], (g/(cm)3) | 0.1004 |
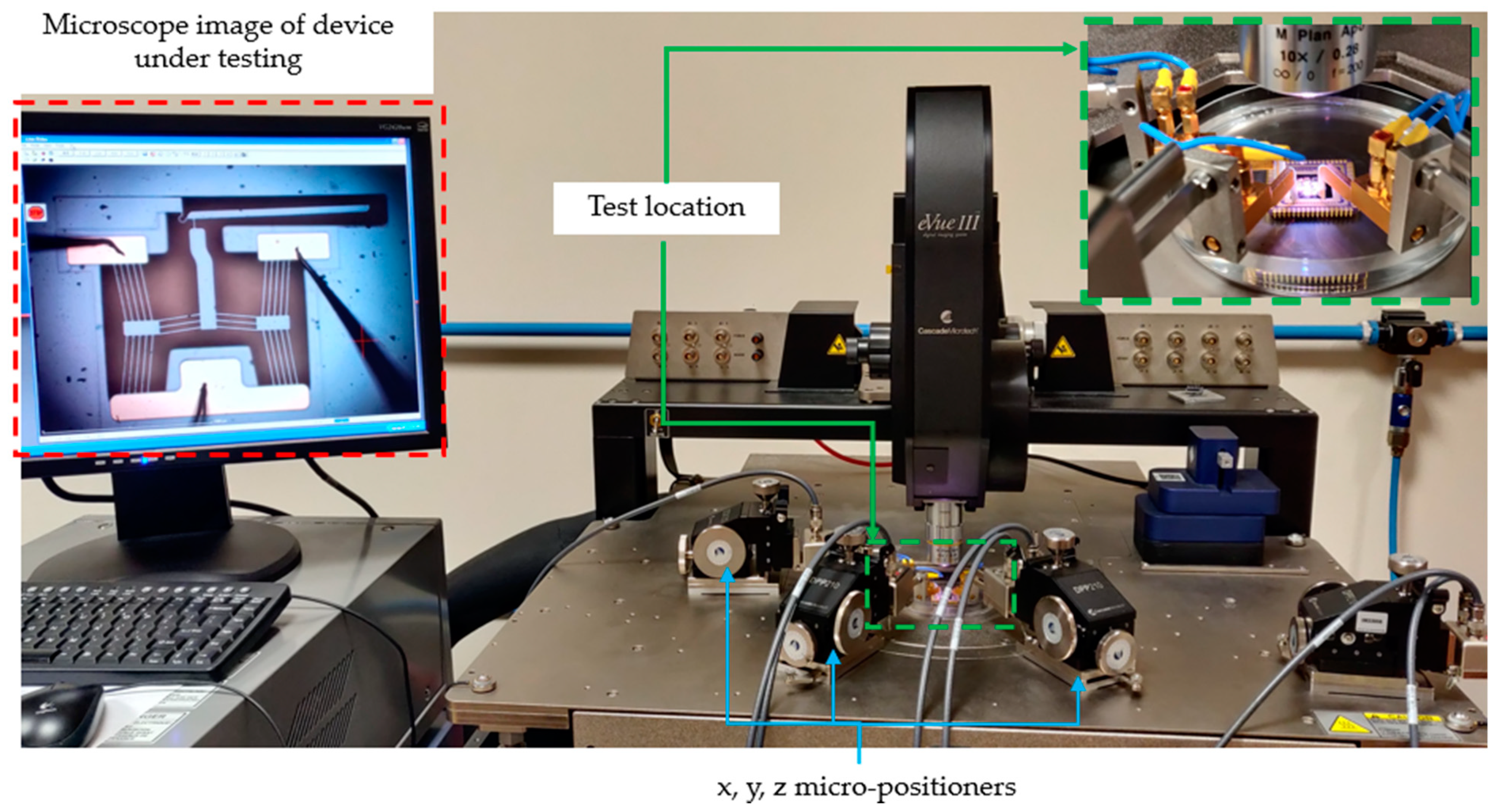
4. Experimental Testing
4.1. Frequency Independence Study
4.2. Testing in Aqueous Solutions
- The devices were first soaked in high-purity isopropyl alcohol (IPA) for 10 min;
- The devices were then removed from the IPA and rinsed for three consecutive times in the aqueous medium in which they were to be tested;
- Finally, they were submerged in their respective medium, ready for testing.
5. Results and Discussion
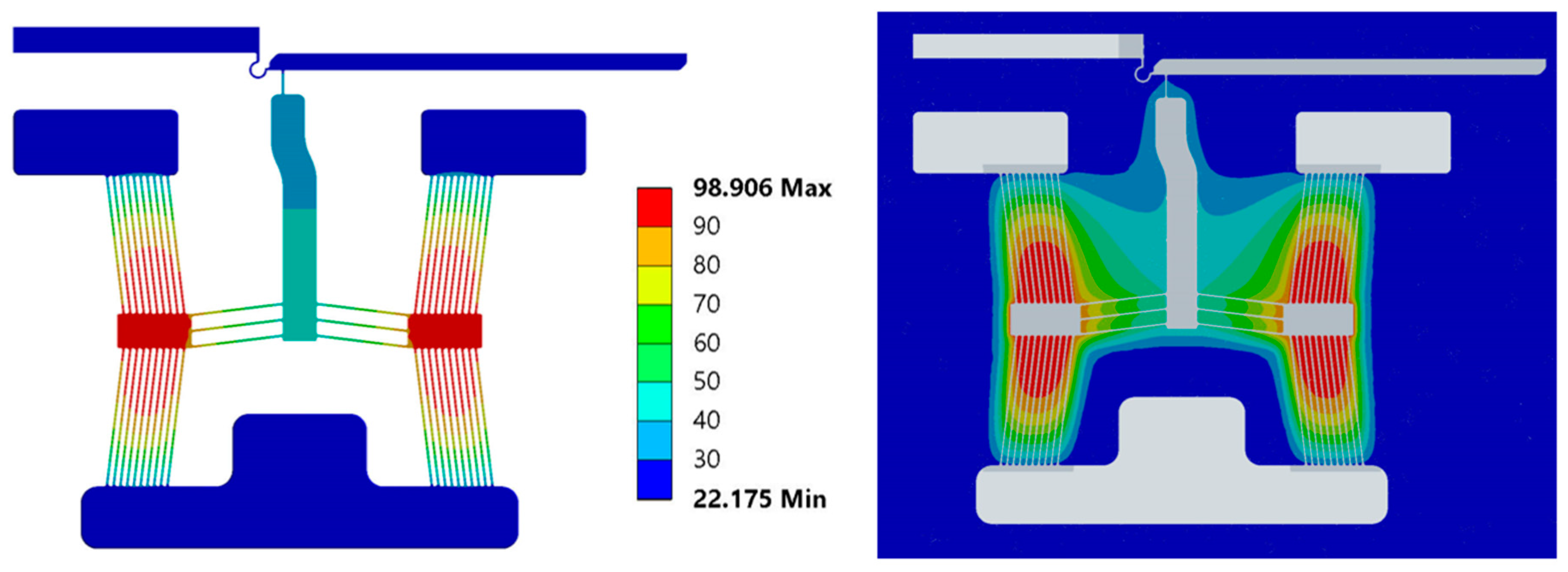
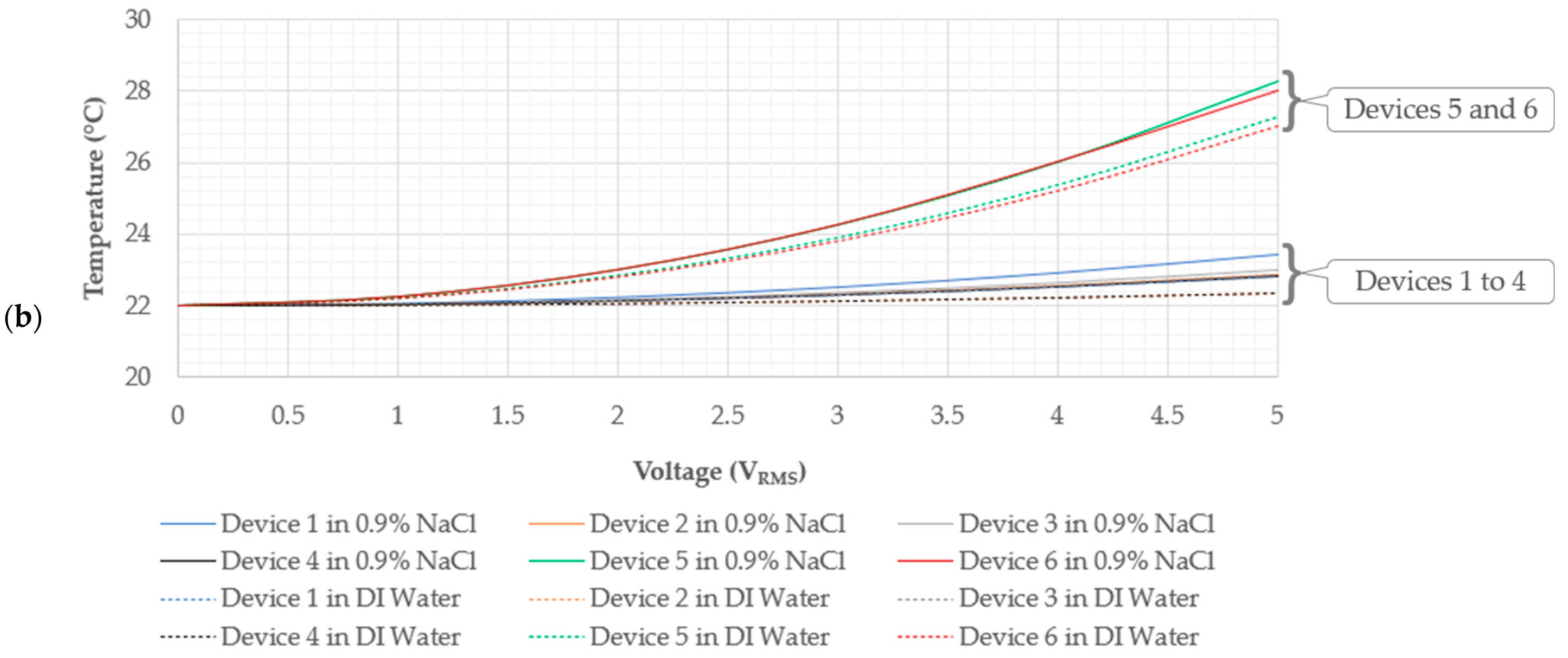
5.1. Electro-Thermal Characterisation
5.2. Structural Characterisation
5.2.1. Frequency Study
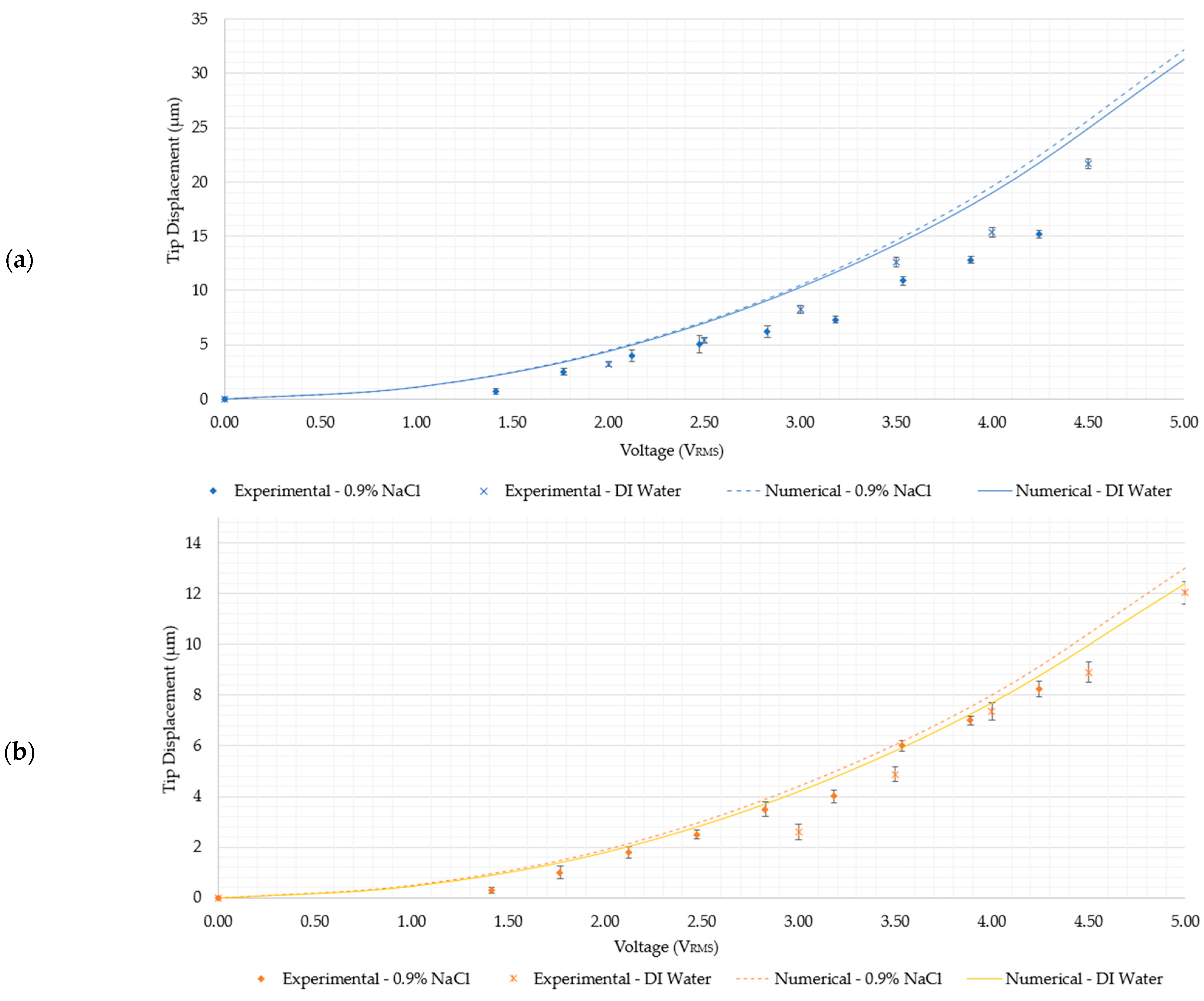
5.2.2. Structural Performance in 0.9% NaCl Solution
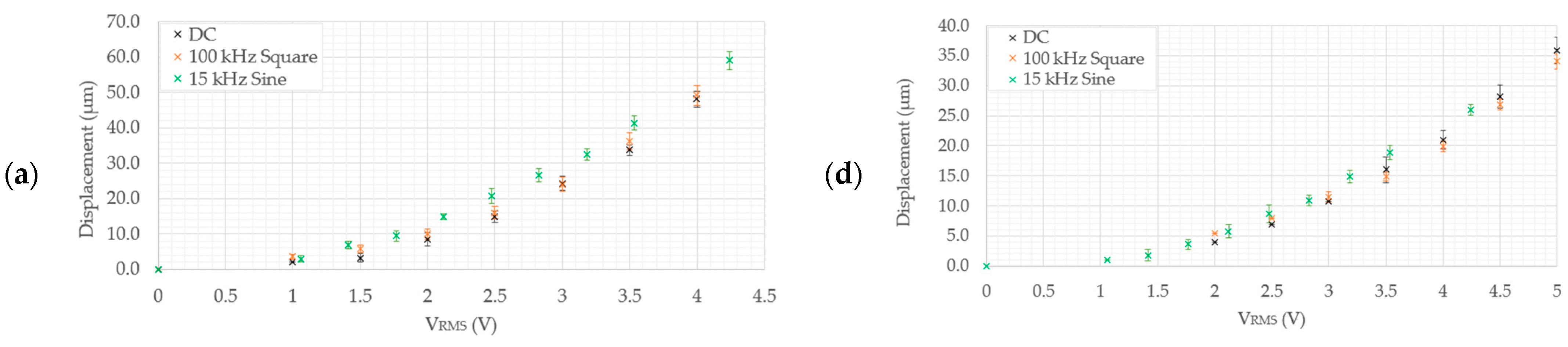
- Devices 1 and 3 generate the highest tip displacements in both media. The experimental tip displacements of devices 1 and 3 were measured at 21.7 µm and 15 µm, respectively, at an input voltage of 4.5 VRMS in DI water. The numerically calculated tip displacements of devices 1 and 3 in DI water at the same input voltage were 25 µm and 16.6 µm, respectively. The experimental tip displacements of devices 1 and 3 in the 0.9% NaCl solution were measured at 15.18 µm and 11.1 µm at an input voltage of 4.24 VRMS. Their numerically calculated counterparts were 22.5 µm for device 1 and 10.9 µm for device 3. This implies that both devices are well-suited for biomedical applications requiring devices to function whilst fully submerged in aqueous solutions up to a maximum input voltage of 4.5 VRMS.
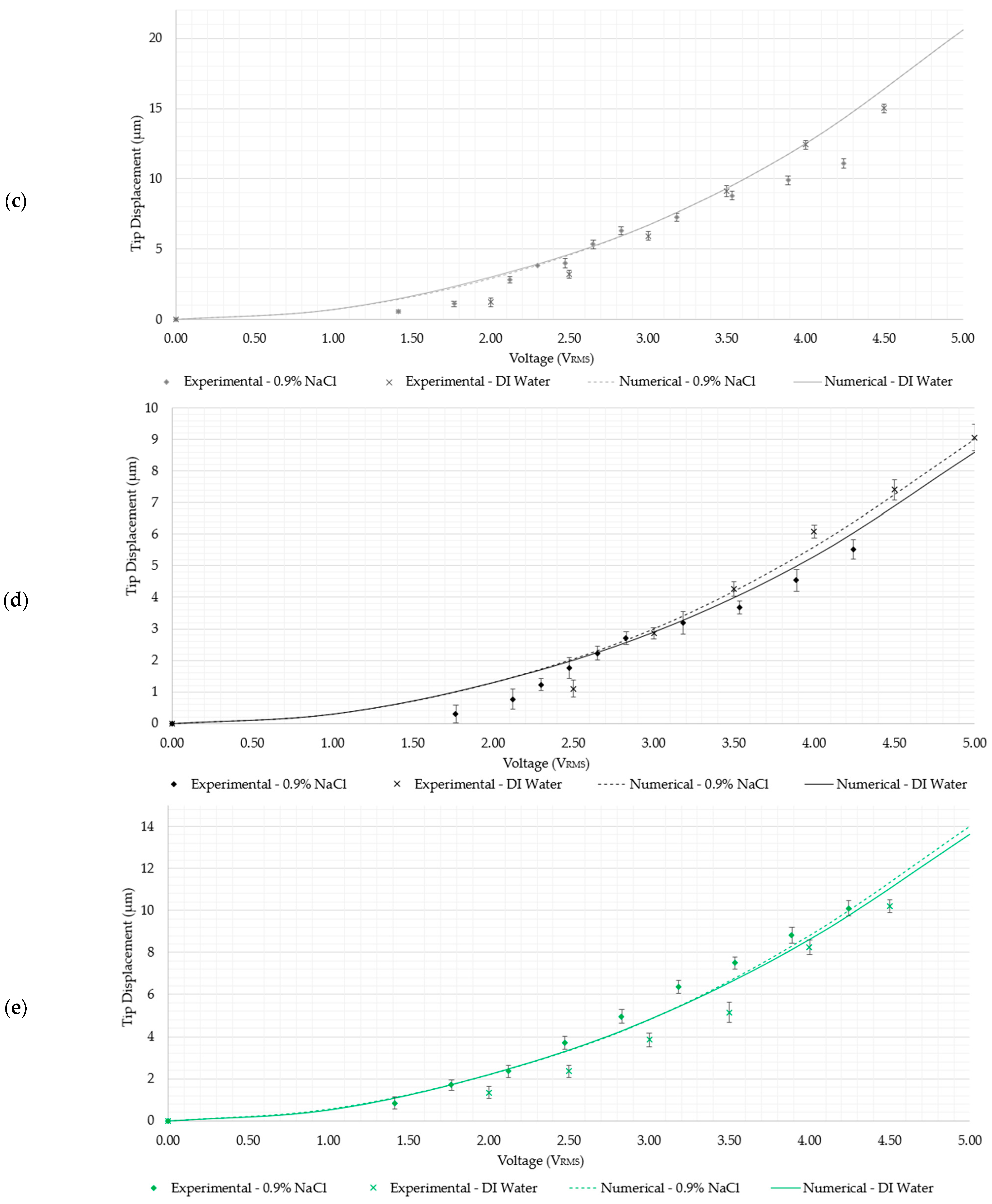
- Devices 2 and 4 exhibited tip displacements of 12.0 µm and 9.1 µm, respectively, at an input voltage of 5 VRMS in DI water when measured experimentally. Also experimentally, in the 0.9% NaCl solution, devices 2 and 4 exhibited tip displacements of 8.3 µm and 5.5 µm, respectively, at an input voltage of 4.24 VRMS. The equivalent numerically calculated values for device 2 were 12.4 µm and 9.2 µm in DI water and the 0.9% NaCl solution, respectively. Meanwhile, the numerically calculated values of device 4 were 8.6 µm and 6.4 µm in DI water and the 0.9% NaCl solution, respectively. Similar to devices 1 and 3, device 2 is also a potential candidate for human RBC characterisation in aqueous solutions. While device 4 exceeds the 7 µm threshold in DI water, unfortunately, it does not exceed it in the 0.9% NaCl solution with the maximum available voltage supply from the available signal generator. Note, however, that by extrapolating the trend as exhibited in Figure 8e, the device may produce a 7 µm tip displacement with a 5 VRMS input. With reference to Figure 5, devices 2 and 4 may be operated up to 5 VRMS from an electro-thermal standpoint, since they do not reach a maximum temperature of 100 °C.
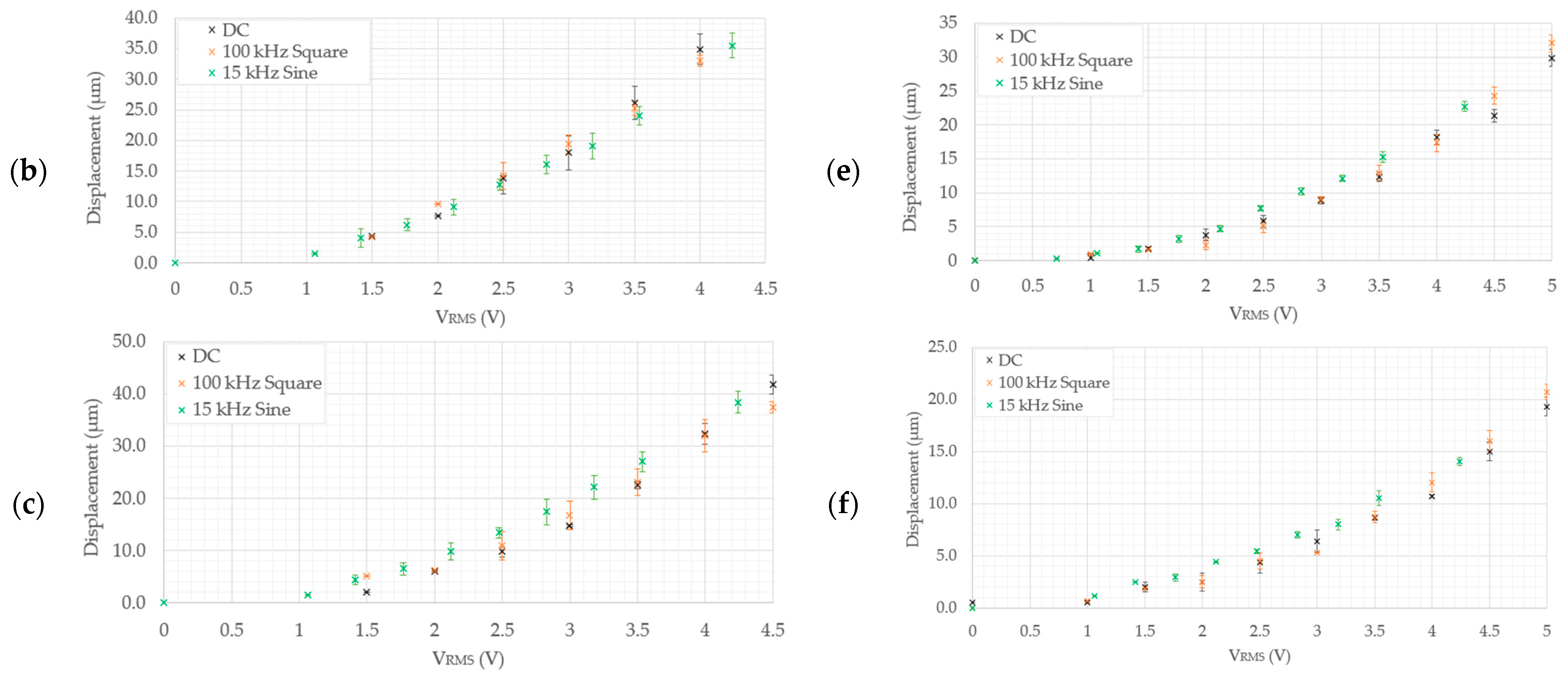
- As measured experimentally, device 5 exhibits a total gap opening of 10.2 µm at 4.5 VRMS and 10.1 µm at 4.24 VRMS in DI water and the 0.9% NaCl solution, respectively. The corresponding numerically calculated values are 10.1 µm and 11.3 µm in the 0.9% NaCl solution and DI water, respectively. This implies that device 5 is also a suitable candidate for submerged, aqueous biomedical applications.
- Device 6 is the least-performing device, in that it was seen to produce experimental tip displacements of 6.5 µm at 4.5 VRMS and 5.19 µm at 4.24 VRMS in DI water and the 0.9% NaCl solution, respectively. The numerically derived values were 4.3 µm and 4.7 µm in 0.9% NaCl and DI water, respectively. Although marginally below specification, this device needs a design intervention prior to being a potential candidate for underwater biomedical applications. The amplification mechanism is the target element requiring optimisation here, since the electro-thermal driver section has been successfully utilised in all the other devices.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Boundary condition |
| ETA | Electro-thermal actuator |
| MEMS | Microelectromechanical system |
| SOI | Silicon-on-insulator |
| RBC | Red blood cell |
| DI | Deionised |
References
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Perret, G.; Kumemura, M.; Ataka, M.; Meignan, S.; Karsten, S.L.; Fujita, H.; Collard, D.; Lagadec, C.; Tarhan, M.C. Developing a MEMS Device with Built-in Microfluidics for Biophysical Single Cell Characterization. Micromachines 2018, 9, 275. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Faivre, M.; Bessaa, A.; Da Costa, L.; Joly, P.; Gauthier, A.; Connes, P. Impact of Surface-Area-to-Volume Ratio, Internal Viscosity and Membrane Viscoelasticity on Red Blood Cell Deformability Measured in Isotonic Condition. Sci. Rep. 2019, 9, 6771. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Violet, P.-C.; Tu, H.; Nichols, J.; Pittman, C.A.; Fitzhugh, C.; Fleming, R.E.; Mohandas, N.; Tisdale, J.F.; Levine, M. Measuring Deformability and Red Cell Heterogeneity in Blood by Ektacytometry. J. Vis. Exp. 2018, 131, e56910. [Google Scholar] [CrossRef]
- Chen, X.; Feng, L.; Jin, H.; Feng, S.; Yu, Y. Quantification of the Erythrocyte Deformability Using Atomic Force Microscopy: Correlation Study of the Erythrocyte Deformability with Atomic Force Microscopy and Hemorheology. Clin. Hemorheol. Microcirc. 2009, 43, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Fornal, M.; Lekka, M.; Pyka-Fościak, G.; Lebed, K.; Grodzicki, T.; Wizner, B.; Styczeń, J. Erythrocyte Stiffness in Diabetes Mellitus Studied with Atomic Force Microscope. Clin. Hemorheol. Microcirc. 2006, 35, 273–276. [Google Scholar] [PubMed]
- Shakoor, A.; Gao, W.; Zhao, L.; Jiang, Z.; Sun, D. Advanced Tools and Methods for Single-Cell Surgery. Microsystems Nanoeng. 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.; Suner, L.; Galimand, J.; Bonnel, A.; Pascreau, T.; Couque, N.; Fenneteau, O.; Mohandas, N. Diagnostic Tool for Red Blood Cell Membrane Disorders: Assessment of a New Generation Ektacytometer. Blood Cells. Mol. Dis. 2016, 56, 9–22. [Google Scholar] [CrossRef]
- Bustamante, C.J.; Chemla, Y.R.; Liu, S.; Wang, M.D. Optical Tweezers in Single-Molecule Biophysics. Nat. Rev. Methods Prim. 2021, 1, 25. [Google Scholar] [CrossRef]
- Musielak, M. Red Blood Cell-Deformability Measurement: Review of Techniques. Clin. Hemorheol. Microcirc. 2009, 42, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Cauchi, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. The Effects of Structure Thickness, Air Gap Thickness and Silicon Type on the Performance of a Horizontal Electrothermal MEMS Microgripper. Actuators 2018, 7, 38. [Google Scholar] [CrossRef]
- Kim, K.; Cheng, J.; Liu, Q.; Wu, X.Y.; Sun, Y. Investigation of Mechanical Properties of Soft Hydrogel Microcapsules in Relation to Protein Delivery Using a MEMS Force Sensor. J. Biomed. Mater. Res. A 2010, 92, 103–113. [Google Scholar] [CrossRef]
- Yallew, T.S.; Pantano, M.F.; Bagolini, A. Design and Finite Element Analysis of an Electrothermally Actuated Microgripper for Biomedical Applications. In Proceedings of the 2021 Symposium on Design, Test, Integration & Packaging of MEMS and MOEMS (DTIP), Paris, France, 25–27 August 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Sciberras, T.; Demicoli, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. Thermo-Mechanical Fluid–Structure Interaction Numerical Modelling and Experimental Validation of MEMS Electrothermal Actuators for Aqueous Biomedical Applications. Micromachines 2023, 14, 1264. [Google Scholar] [CrossRef]
- Nemani, K.V.; Moodie, K.L.; Brennick, J.B.; Su, A.; Gimi, B. In Vitro and in Vivo Evaluation of SU-8 Biocompatibility. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Potrich, C.; Lunelli, L.; Bagolini, A.; Bellutti, P.; Pederzolli, C.; Verotti, M.; Belfiore, N.P. Innovative Silicon Microgrippers for Biomedical Applications: Design, Mechanical Simulation and Evaluation of Protein Fouling. Actuators 2018, 7, 12. [Google Scholar] [CrossRef]
- Joshitha, C.; Ragavyshnavi, D.; Priyanka, D.S.; Nagarjuna, P.; Sreeja, B.S. Electrothermal Micro Tweezer for Biomedical Applications. {IOP} Conf. Ser. Mater. Sci. Eng. 2021, 1070, 12072. [Google Scholar] [CrossRef]
- Sciberras, T.; Mollicone, P.; Demicoli, M.; Grech, I.; Sammut, N.; Mallia, B. MEMS Electrothermal Actuators for Underwater Manipulation and Mechanical Characterisation of Human Red Blood Cells. In Proceedings of the 2023 Symposium on Design, Test, Integration & Packaging of MEMS/MOEMS (DTIP), Valetta, Malta, 28–31 May 2023; pp. 1–4. [Google Scholar] [CrossRef]
- Singh, M.; Stoltz, J. Influence of Temperature Variation from 5 Degrees C to 37 Degrees C on Aggregation and Deformability of Erythrocytes. Clin. Hemorheol. Microcirc. 2002, 26, 1–7. [Google Scholar]
- Xu, Z.; Zheng, Y.; Wang, X.; Shehata, N.; Wang, C.; Sun, Y. Stiffness Increase of Red Blood Cells during Storage. Microsystems Nanoeng. 2018, 4, 17103. [Google Scholar] [CrossRef]
- Matrai, A.A.; Varga, G.; Tanczos, B.; Barath, B.; Varga, A.; Horvath, L.; Bereczky, Z.; Deak, A.; Nemeth, N. In Vitro Effects of Temperature on Red Blood Cell Deformability and Membrane Stability in Human and Various Vertebrate Species. Clin. Hemorheol. Microcirc. 2021, 78, 291–300. [Google Scholar] [CrossRef]
- Lecklin, T.; Egginton, S.; Nash, G.B. Effect of Temperature on the Resistance of Individual Red Blood Cells to Flow through Capillary-Sized Apertures. Pflugers Arch. 1996, 432, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Fildes, J.; Fisher, S.; Sheaff, C.M.; Barrett, J.A. Effects of Short Heat Exposure on Human Red and White Blood Cells. J. Trauma Acute Care Surg. 1998, 45, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.F.; Groom, A.C. Capillary Diameter and Geometry in Cardiac and Skeletal Muscle Studied by Means of Corrosion Casts. Microvasc. Res. 1983, 25, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Attaway, J. Osmosis and Tonicity. Available online: https://www.khanacademy.org/science/biology/membranes-and-transport/diffusion-and-osmosis/a/osmosis (accessed on 25 March 2023).
- Lukose, J.; Shastry, S.; Mithun, N.; Mohan, G.; Ahmed, A.; Chidangil, S. Red Blood Cells under Varying Extracellular Tonicity Conditions: An Optical Tweezers Combined with Micro-Raman Study. Biomed. Phys. Eng. Express 2020, 6, 15036. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.L. Why 0.9% Saline Is Isotonic: Understanding the Aqueous Phase of Plasma and the Difference between Osmolarity and Osmolality. Pediatr. Nephrol. 2019, 34, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Sameoto, D.; Hubbard, T.; Kujath, M. Operation of Electrothermal and Electrostatic MUMPs Microactuators Underwater. J. Micromechanics Microengineering 2004, 14, 1359. [Google Scholar] [CrossRef]
- Mukundan, V.; Pruitt, B. MEMS Electrostatic Actuation in Conducting Biological Media. Microelectromech. Syst. J. 2009, 18, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Sciberras, T.; Demicoli, M.; Grech, I.; Mallia, B.; Mollicone, P.; Sammut, N. Experimental and Numerical Analysis of MEMS Electrothermal Actuators with Cascaded V-Shaped Mechanisms. In Proceedings of the 2022 Symposium on Design, Test, Integration & Packaging of MEMS/MOEMS, Pont-a-Mousson, France, 11–13 July 2022. [Google Scholar]
- Cowen, A.; Hames, G.; Monk, D.; Wilcenski, S.; Hardy, B. SOIMUMPs Design Handbook; Memscap Inc.: Durham, NC, USA, 2011; Volume 6. [Google Scholar]
- Hickey, R.; Sameoto, D.; Hubbard, T.; Kujath, M. Time and Frequency Response of Two-Arm Micromachined Thermal Actuators. J. Micromechanics Microengineering 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Qasem, N.; Generous, M.M.; Qureshi, B.; Zubair, S. A Comprehensive Review of Saline Water Correlations and Data: Part II—Thermophysical Properties. Arab. J. Sci. Eng. 2021, 46, 1941–1979. [Google Scholar] [CrossRef]
- ANSYS. Academic Research Mechanical, Release 15.0, Help System, ANSYS Fluent User’s Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2013. [Google Scholar]
- Generous, M.M.; Qasem, N.; Qureshi, B.; Zubair, S. A Comprehensive Review of Saline Water Correlations and Data-Part I: Thermodynamic Properties. Arab. J. Sci. Eng. 2020, 45, 8817–8876. [Google Scholar] [CrossRef]
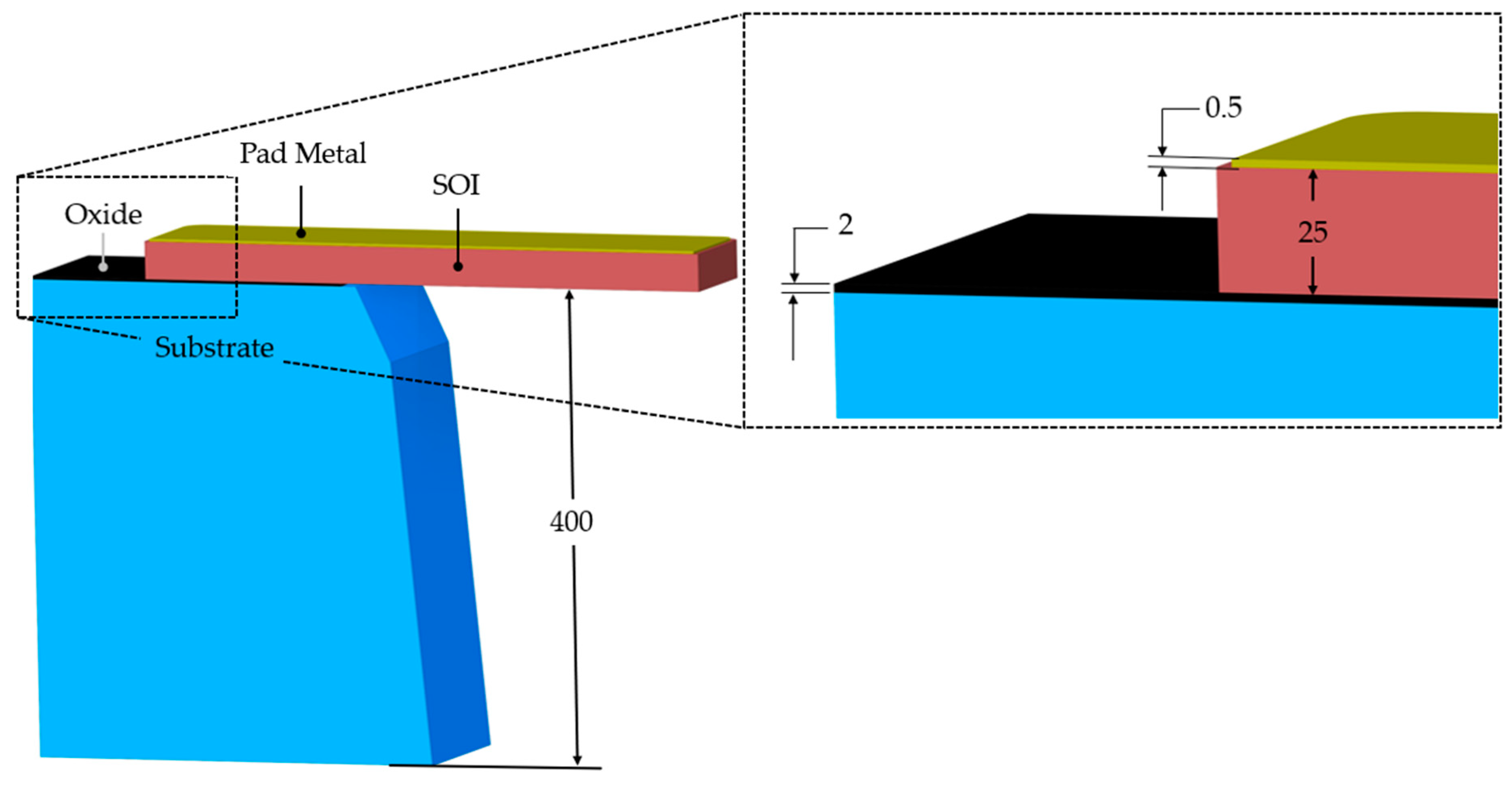
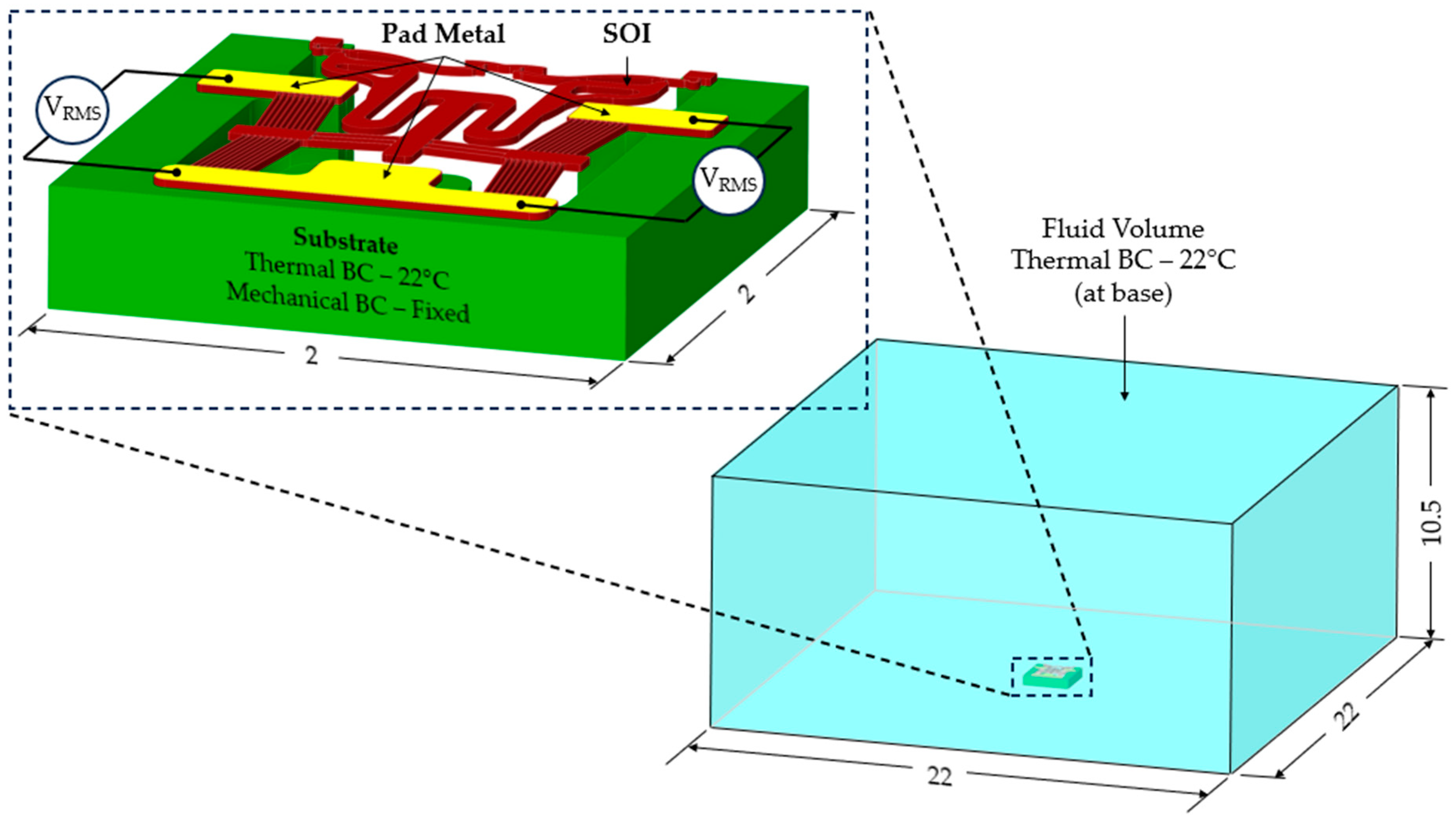


| Property Designation | SOI | Pad Metal (Gold) |
|---|---|---|
| Density [g/(cm)3] | 2.50 | 19.30 |
| Electrical resistivity, ρ [µ.Ω.m] | 125 | 2.86 × 10−2 |
| Coefficient of thermal expansion, α [µm/m.K] | [31,32] | N/A |
| Thermal conductivity, k [W/m.K] | 148 | 297 |
| Specific heat capacity, c [J/kg.K] | 712 | 128.7 |
| Shear modulus, G [GPa] | Gyz = Gzx = 79.6, Gxy = 50.9 | N/A |
| Poisson’s ratio, ν | νyz = 0.36, νzx = 0.29, νxy = 0.064 | 0.35 |
| Young’s modulus, E [GPa] | Ex = Ey = 169, Ez = 130 | 57 |
| Device Configuration | Reference | Designation in Reference | Designation in Current Text |
|---|---|---|---|
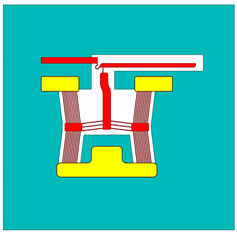 | [31] | Device 1 | Device 1 |
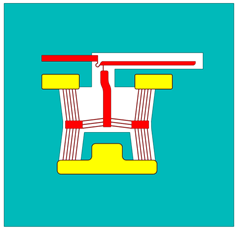 | Device 2 | Device 2 | |
 | Device 3 | Device 3 | |
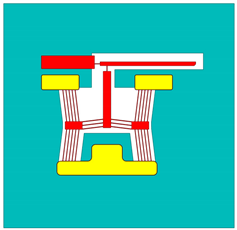 | Device 4 | Device 4 | |
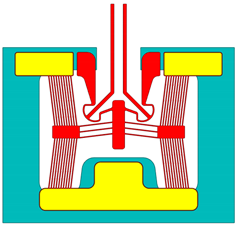 | [19] | Device a | Device 5 |
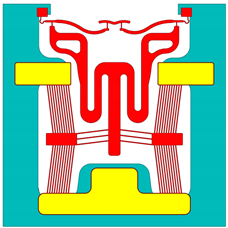 | Device b | Device 6 |
| Voltage (VRMS) | Percentage Difference (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tip Temperature | Maximum Temperature | |||||||||||
| Device Number | Device Number | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 | 0.10 | 0.09 | 0.02 | 0.08 | 0.18 | 0.23 | 0.25 | 0.12 | 0.06 | 0.07 | 0.15 | 0.35 |
| 2 | 0.41 | 0.38 | 0.10 | 0.34 | 0.71 | 0.90 | 0.74 | 0.39 | 0.18 | 0.24 | 0.43 | 1.01 |
| 3 | 0.92 | 0.84 | 0.22 | 0.76 | 1.51 | 1.93 | 1.15 | 0.66 | 0.28 | 0.40 | 0.65 | 1.55 |
| 4 | 1.62 | 1.48 | 0.39 | 1.33 | 2.53 | 3.22 | 1.43 | 0.87 | 0.35 | 0.52 | 0.80 | 1.90 |
| 5 | 2.48 | 2.29 | 0.60 | 2.06 | 3.65 | 3.66 | 1.61 | 1.01 | 0.39 | 0.61 | 0.89 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciberras, T.; Grech, I.; Demicoli, M.; Mallia, B.; Sammut, N.; Mollicone, P. SOI MEMS Electro-Thermal Actuators for Biomedical Applications: Operation in 0.9% NaCl Solution. Micromachines 2024, 15, 881. https://doi.org/10.3390/mi15070881
Sciberras T, Grech I, Demicoli M, Mallia B, Sammut N, Mollicone P. SOI MEMS Electro-Thermal Actuators for Biomedical Applications: Operation in 0.9% NaCl Solution. Micromachines. 2024; 15(7):881. https://doi.org/10.3390/mi15070881
Chicago/Turabian StyleSciberras, Thomas, Ivan Grech, Marija Demicoli, Bertram Mallia, Nicholas Sammut, and Pierluigi Mollicone. 2024. "SOI MEMS Electro-Thermal Actuators for Biomedical Applications: Operation in 0.9% NaCl Solution" Micromachines 15, no. 7: 881. https://doi.org/10.3390/mi15070881
APA StyleSciberras, T., Grech, I., Demicoli, M., Mallia, B., Sammut, N., & Mollicone, P. (2024). SOI MEMS Electro-Thermal Actuators for Biomedical Applications: Operation in 0.9% NaCl Solution. Micromachines, 15(7), 881. https://doi.org/10.3390/mi15070881







