A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Base Case
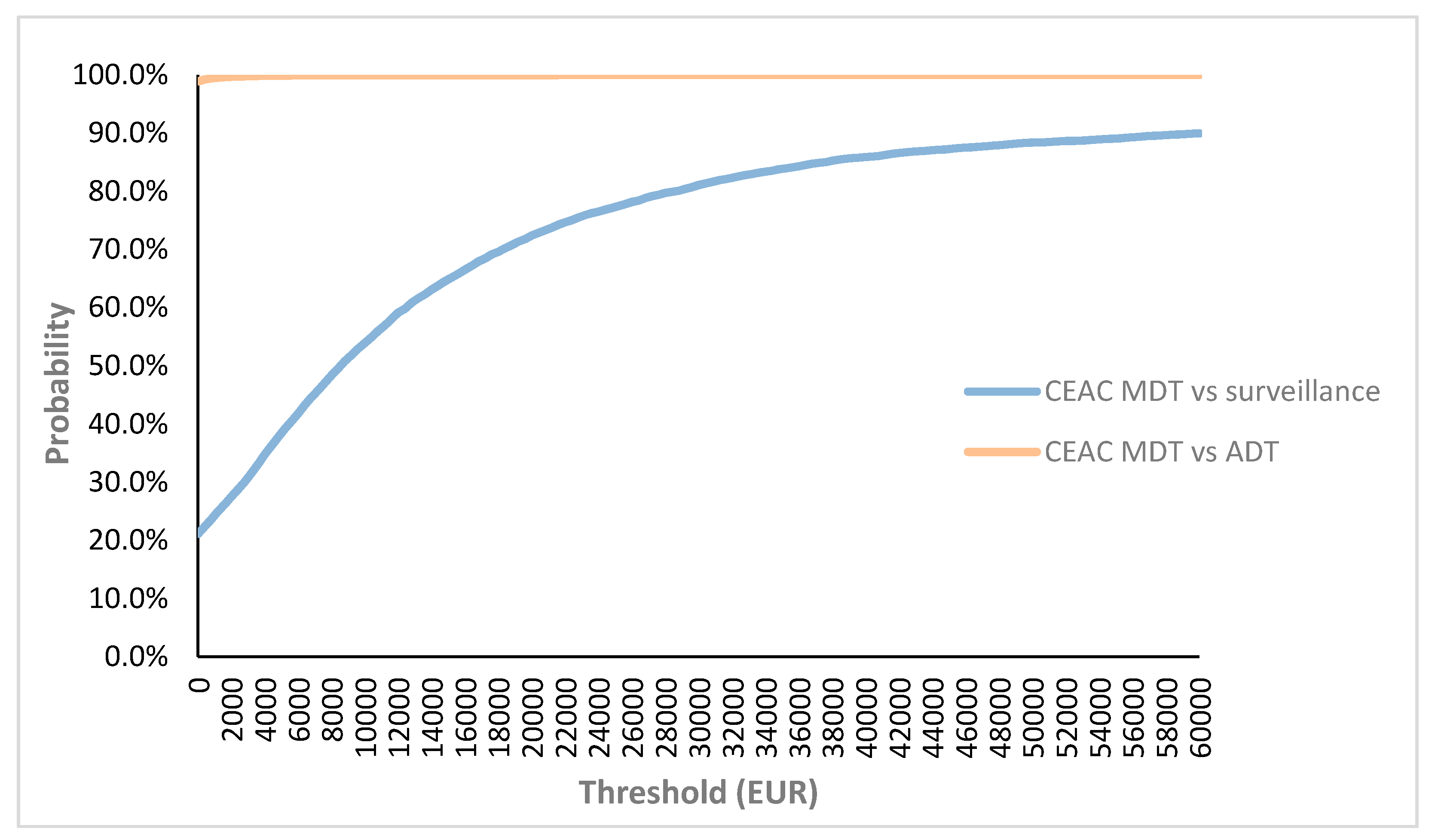
2.2. Probabilistic Sensitivity Analyses for Base Case
2.3. One-Way Sensitivity Analysis
2.4. Scenario Analyses
3. Discussion
Limitations
4. Material and Methods
4.1. Patients and Procedures
4.2. Model Structure
4.3. Time Horizon
4.4. Discounting
4.5. Model Inputs
4.5.1. Health State Transition Probabilities
4.5.2. Non-Prostate Cancer-Specific Mortality
4.6. Toxicities
4.7. Utilities
4.8. Costs
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Inputparameter | Deterministic Value | Standard Error | Distribution | Source |
|---|---|---|---|---|
| Mean age (years) | 68.15 | NA | NA | [5] |
| Discount rate costs (%) | 3% | NA | NA | [22] |
| Discount rate utilities (%) | 1.5% | NA | NA | [22] |
| Disease-related probabilities (%) | ||||
| ADT-free survival in MDT | 6 months: 94% | NA | NA | [5] |
| 12 months: 67% | ||||
| 18 months: 46% | ||||
| 24 months: 46% | ||||
| ADT-free survival in surveillance | 6 months: 85% | NA | NA | [5] |
| 12 months: 56% | ||||
| 18 months: 32% | ||||
| 24 months: 23% | ||||
| Transition to CRPC state from ADT state | 6 months: 1% | NA | NA | [9] |
| 12 months: 7% | ||||
| 18 months: 12% | ||||
| 24 months: 20% | ||||
| 30 months: 26% | ||||
| 36 months: 28% | ||||
| 42 months: 32% | ||||
| 48 months: 35% | ||||
| Mortality risk | ||||
| Death from other causes | 68 year: 0.018123 | NA | NA | [23] |
| 69 year 0.019817 | ||||
| 70 years: 0.020578 | ||||
| 71 years 0.023583 | ||||
| 72 years 0.026009 | ||||
| PCa death in CPRC state | 5.7143% | NA | NA | [9] |
| Utilities * | ||||
| MDT during the first month | 0.72 | 0.17 | Beta | [15] |
| ADT-free state | 0.92 | 0.23 | Beta | [16,17] |
| ADT state | 0.78 | 0.19 | Beta | [17] |
| CRPC state (chemotherapy accounted for 30% in this state) | 0.6 | 0.15 | Beta | [16,18] |
| Costs intervention (€) | ||||
| MDT cost month 1 (ADT-free state) | 4549 | 1137 | Gamma | [20,21] |
| MDT cost other months (ADT-free state) | 47 | 12 | Gamma | [20] |
| Surveillance cost month 1 (ADT-free state) | 865 | 216.3 | Gamma | [20] |
| Surveillance cost other months (ADT free state) | 17 | 4.4 | Gamma | [20] |
| Cost diagnostics and start ADT (ADT state) | 1266 | 317 | Gamma | [20,21] |
| Cost ADT in follow-up months ADT (ADT state) | 298 | 74.7 | Gamma | [20,21] |
| CRPC costs (combination of diagnostics, treatment and follow-up) (distribution of abiraterone acetate 35%, enzalutamide 35% and docetaxel 30%) | 775 | 193 | Gamma | [20,21] |
| ADT toxicities probabilities | ||||
| Gynecomastia | 13% | NA | Beta | [28] |
| Osteoporosis | 10% | NA | Beta | [28] |
| Diabetes | 9% | NA | Beta | [28] |
| Fatigue | 80% | NA | Beta | [28] |
| Sexual dysfunction | 95% | NA | Beta | [28] |
| Reduced penile/testis size | 93% | NA | Beta | [28] |
| Hot flashes | 80% | NA | Beta | [28] |
| Cognitive changes | 48% | NA | Beta | [28] |
| Anemia | 13% | NA | Beta | [28] |
| Metabolic syndrome | 55% | NA | Beta | [28] |
| MDT toxicities probabilities | ||||
| Lymph oedema | 0.4% | 0.001 | Beta | [3] |
| Anemia needing blood transfusion | 0.2% | 0.0005 | Beta | [3] |
| Symptomatic lymphocoele | 5% | 0.0125 | Beta | [3] |
| Neuropraxia | 0.4% | 0.001 | Beta | [3] |
| Pain | 1% | 0.0025 | Beta | Expert opinion |
| Diarrhea | 4% | 0.01 | Beta | [26] |
| Cost | Specification | Cost Estimates (€) |
|---|---|---|
| Cost at diagnosis | Imaging (choline PET CT, MR soft tissue and MR total spine), consultation, laboratory monitoring and multidisciplinary oncological consultation (MOC) | Choline PET-CT: 747.72 MR: 207.48 Consultation at urologist/radiation oncologist: 25.43 PSA laboratory: 30.5 MOC: 61.54 |
| Initial treatment-SBRT | Physician fees, CT-simulation, planning, treatment, drugs, laboratory monitoring (with calculated possibility of new SBRT round) | SBRT: 3782.24 |
| Initial treatment-ADT | We investigated the different sorts of ADT (Luteinizing hormone releasing hormone (LHRH)- agonist and antagonist), taken into account the frequency of injection, the associated consultation, etc. and decided to use the drug associated with the lowest cost | Range of costs of different ADT: 63.40–141.5 per month |
| Initial treatment-surgery | Physician fees, anesthetic drugs, hospital admission, medication and laboratory monitoring | Robot-assisted PLND: 3109.35 |
| Cost of surveillance group | Diagnostics cost, follow-up visit and laboratory monitoring | Choline PET-CT: 747.72 MR: 207.48 Consultation at urologist/radiation oncologist: 25.43 PSA laboratory: 30.5 |
| Treatment CRPC state | Diagnostics costs (imaging, consultation, laboratory and MOC), three possible treatment strategies (abiraterone acetate (AA), enzalutamide and docetaxel *), monitoring costs depending on the treatment. | CRPC first month inclusive diagnostic costs and ADT: 1165.76 Overall mean cost of CRPC state per month: 775.66 |

References
- Weichselbaum, R.R.; Hellman, S. Oligometastases Revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Gandaglia, G.; Borgmann, H.; de Visschere, P.; Heidegger, I.; Kretschmer, A.; Mathieu, R.; Surcel, C.; Tilki, D.; Tsaur, I.; et al. Salvage Lymph Node Dissection for Nodal Recurrent Prostate Cancer: A Systematic Review. Eur. Urol. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Bleser, E.; Tran, P.T.; Ost, P. Radiotherapy as Metastasis-Directed Therapy for Oligometastatic Prostate Cancer. Curr. Opin. Urol. 2017, 27, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- OECD Data: Selected indicators for Belgium. Available online: https://data.oecd.org/belgium.htm (accessed on 8 October 2018).
- Siva, S.; Bressel, M.; Murphy, D.G.; Shaw, M.; Chander, S.; Violet, J.; Tai, K.H.; Udovicich, C.; Lim, A.; Selbie, L.; et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur. Urol. 2018, 74, 455–462. [Google Scholar] [CrossRef]
- De Bruycker, A.; Lambert, B.; Claeys, T.; Delrue, L.; Mbah, C.; De Meerleer, G.; Villeirs, G.; De Vos, F.; De Man, K.; Decaestecker, K.; et al. Prevalence and Prognosis of Low-Volume, Oligorecurrent, Hormone-Sensitive Prostate Cancer Amenable to Lesion Ablative Therapy. BJU Int. 2017, 120, 815–821. [Google Scholar] [CrossRef]
- Neumann, P.J.; Cohen, J.T.; Weinstein, M.C. Updating Cost-Effectivenes—The Curious Resilience of the $50,000-per-QALY Threshold. N. Engl. J. Med. 2014, 371, 796–797. [Google Scholar] [CrossRef]
- Duchesne, G.M.; Woo, H.H.; Bassett, J.K.; Bowe, S.J.; D’Este, C.; Frydenberg, M.; King, M.; Ledwich, L.; Loblaw, A.; Malone, S.; et al. Timing of Androgen-Deprivation Therapy in Patients with Prostate Cancer with a Rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A Randomised, Multicentre, Non-Blinded, Phase 3 Trial. Lancet Oncol. 2016, 17, 727–737. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.; Joniau, S.; et al. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Alibhai, S.M.H.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Bultijnck, R.; Surcel, C.; Ploussard, G.; Briganti, A.; De Visschere, P.; Fütterer, J.; Ghadjar, P.; Giannarini, G.; Isbarn, H.; Massard, C.; et al. Practice Patterns Compared with Evidence-Based Strategies for the Management of Androgen Deprivation Therapy–Induced Side Effects in Prostate Cancer Patients: Results of a European Web-Based Survey. Eur. Urol. Focus 2016, 2, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.T.; Lenert, L.; Bhatnagar, V.; Kaplan, R.M. Utilities for Prostate Cancer Health States in Men Aged 60 and Older. Med. Care 2005, 43, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Tengs, T.O.; Wallace, A. One Thousand Health-Related Quality-of-Life Estimates. Med. Care 2000, 38, 583–637. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Ramakrishna, N.R.; Duff, S.B.; Hughes, K.E.; Sadownik, S.; Smith, J.A.; Tewari, A.K. Primary Treatments for Clinically Localised Prostate Cancer: A Comprehensive Lifetime Cost-Utility Analysis. BJU Int. 2013, 111, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Heijnsdijk, E.A.M.; Denham, D.; de Koning, H.J. The Cost-Effectiveness of Prostate Cancer Detection with the Use of Prostate Health Index. Value Health 2016, 19, 153–157. [Google Scholar] [CrossRef]
- Center for the Evaluation of Value and Risk in Health. The Cost-Effectiveness Analysis Registry. 2018. Available online: https://cevr.tuftsmedicalcenter.org/databases/cea-registry (accessed on 2 February 2019).
- RIZIV. Nomensoft. 2018. Available online: https://www.riziv.fgov.be/nl/toepassingen/Paginas/NomenSoft.aspx (accessed on 28 August 2018).
- BCFI. Gecommentarieerd Geneesmiddelenrepertorium 2018. Available online: https://www.bcfi.be/ggr_pdfs/GGR_NL_2018.pdf (accessed on 28 August 2018).
- Cleemput, I.N.M.; Van de Sande, S.; Thiry, N. Belgische Richtlijnen Voor Economische Evaluaties en Budget Impact Analyses: Tweede Editie. 2012. Available online: https://kce.fgov.be/sites/default/files/atoms/files/KCE_183A_economische_evaluaties_tweede_editie_0.pdf (accessed on 28 August 2018). (In Dutch).
- STATBEL. Sterftetafels en Levensverwachting. 2019. Available online: https://statbel.fgov.be/nl/themas/bevolking/sterfte-en-levensverwachting/sterftetafels-en-levensverwachting (accessed on 2 February 2019). (In Dutch).
- Gezondheid Ze. Sterfte door prostaatkanker Brussels 2016. Available online: https://www.zorg-en-gezondheid.be/sterfte-door-prostaatkanker-2016 (accessed on 28 August 2018). (In Dutch).
- Triggiani, L.; Alongi, F.; Buglione, M.; Detti, B.; Santoni, R.; Bruni, A.; Maranzano, E.; Lohr, F.; D’Angelillo, R.; Magli, A.; et al. Efficacy of Stereotactic Body Radiotherapy in Oligorecurrent and in Oligoprogressive Prostate Cancer: New Evidence from a Multicentric Study. Br. J. Cancer 2017, 116, 1520–1525. [Google Scholar] [CrossRef]
- Decaestecker, K.; De Meerleer, G.; Lambert, B.; Delrue, L.; Fonteyne, V.; Claeys, T.; De Vos, F.; Huysse, W.; Hautekiet, A.; Maes, G.; et al. Repeated Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Recurrence. Radiat. Oncol. 2014, 9, 135. [Google Scholar] [CrossRef]
- Fossati, N.; Suardi, N.; Gandaglia, G.; Bravi, C.A.; Soligo, M.; Karnes, R.J.; Shariat, S.; Battaglia, A.; Everaerts, W.; Joniau, S.; et al. Identifying the Optimal Candidate for Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer: Results from a Large, Multi-Institutional Analysis. Eur. Urol. 2019, 75, 176–183. [Google Scholar] [CrossRef]
- Walker, L.M.; Tran, S.; Robinson, J.W. Luteinizing Hormone–Releasing Hormone Agonists: A Quick Reference for Prevalence Rates of Potential Adverse Effects. Clin. Genitourin. Cancer 2013, 11, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.Y.; Lauer, J.A.; De Joncheere, K.; Edejer, T.; Hutubessy, R.; Kieny, M.-P.; Hill, S.R. Cost-Effectiveness Thresholds: Pros and Cons. Bull. World Health Organ. 2016, 94, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Sculpher, M.; Claxton, K. Decision Modelling for Health Economic Evaluation,: 2006. Available online: https://www.herc.ox.ac.uk/downloads/decision-modelling-for-health-economic-evaluation (accessed on 3 October 2018).






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bleser, E.; Willems, R.; Decaestecker, K.; Annemans, L.; De Bruycker, A.; Fonteyne, V.; Lumen, N.; Ameye, F.; Billiet, I.; Joniau, S.; et al. A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer. Cancers 2020, 12, 132. https://doi.org/10.3390/cancers12010132
De Bleser E, Willems R, Decaestecker K, Annemans L, De Bruycker A, Fonteyne V, Lumen N, Ameye F, Billiet I, Joniau S, et al. A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer. Cancers. 2020; 12(1):132. https://doi.org/10.3390/cancers12010132
Chicago/Turabian StyleDe Bleser, Elise, Ruben Willems, Karel Decaestecker, Lieven Annemans, Aurélie De Bruycker, Valérie Fonteyne, Nicolaas Lumen, Filip Ameye, Ignace Billiet, Steven Joniau, and et al. 2020. "A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer" Cancers 12, no. 1: 132. https://doi.org/10.3390/cancers12010132
APA StyleDe Bleser, E., Willems, R., Decaestecker, K., Annemans, L., De Bruycker, A., Fonteyne, V., Lumen, N., Ameye, F., Billiet, I., Joniau, S., De Meerleer, G., Ost, P., & Bultijnck, R. (2020). A Trial-Based Cost-Utility Analysis of Metastasis-Directed Therapy for Oligorecurrent Prostate Cancer. Cancers, 12(1), 132. https://doi.org/10.3390/cancers12010132







