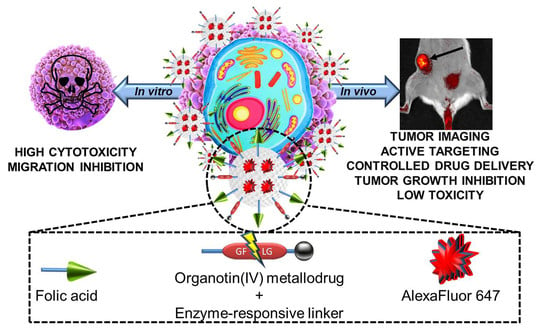
Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer
Abstract
:1. Introduction
2. Results and Discussion
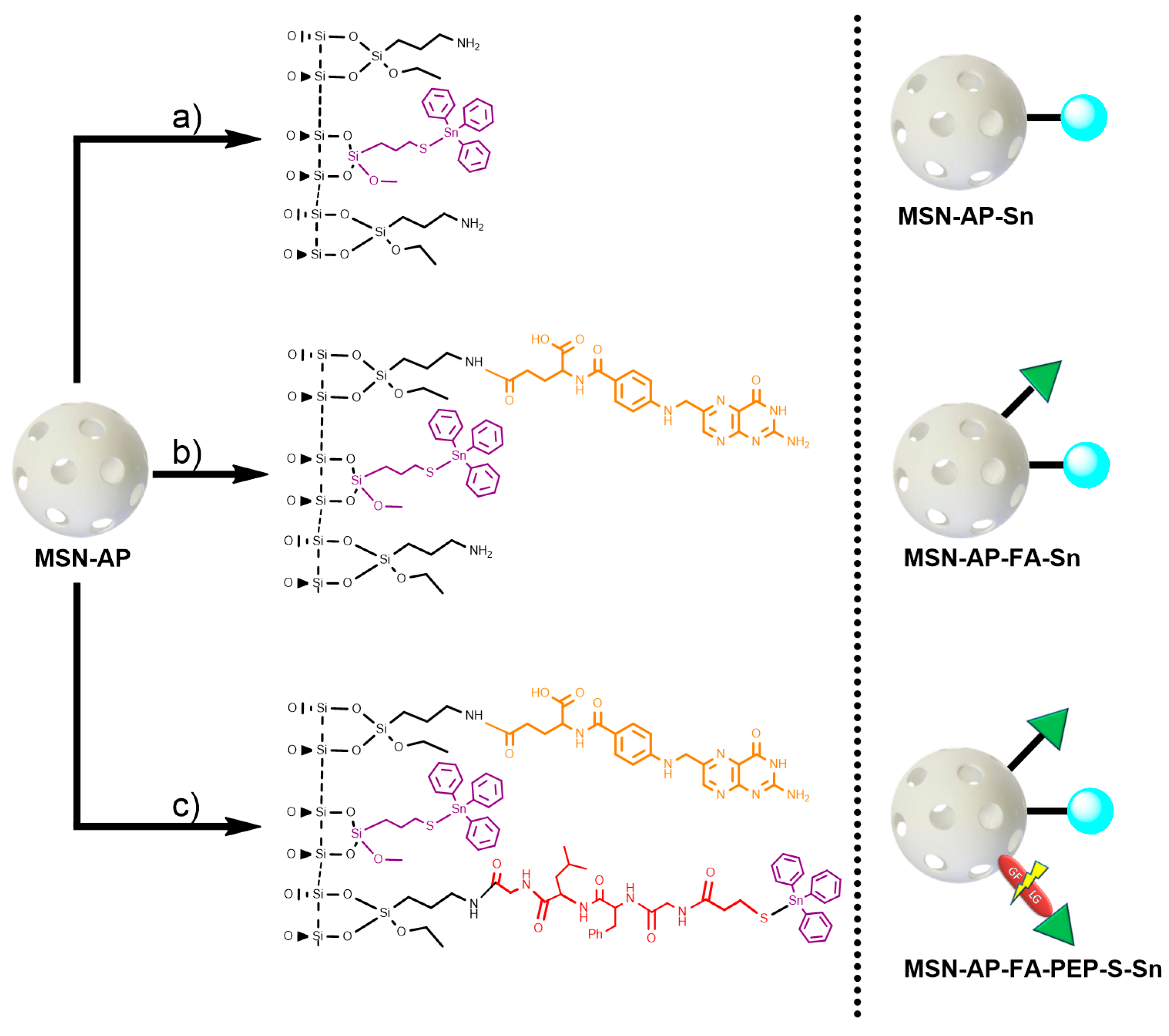
2.1. Synthesis of the Different MSN-Based Multifunctional Nanomaterials
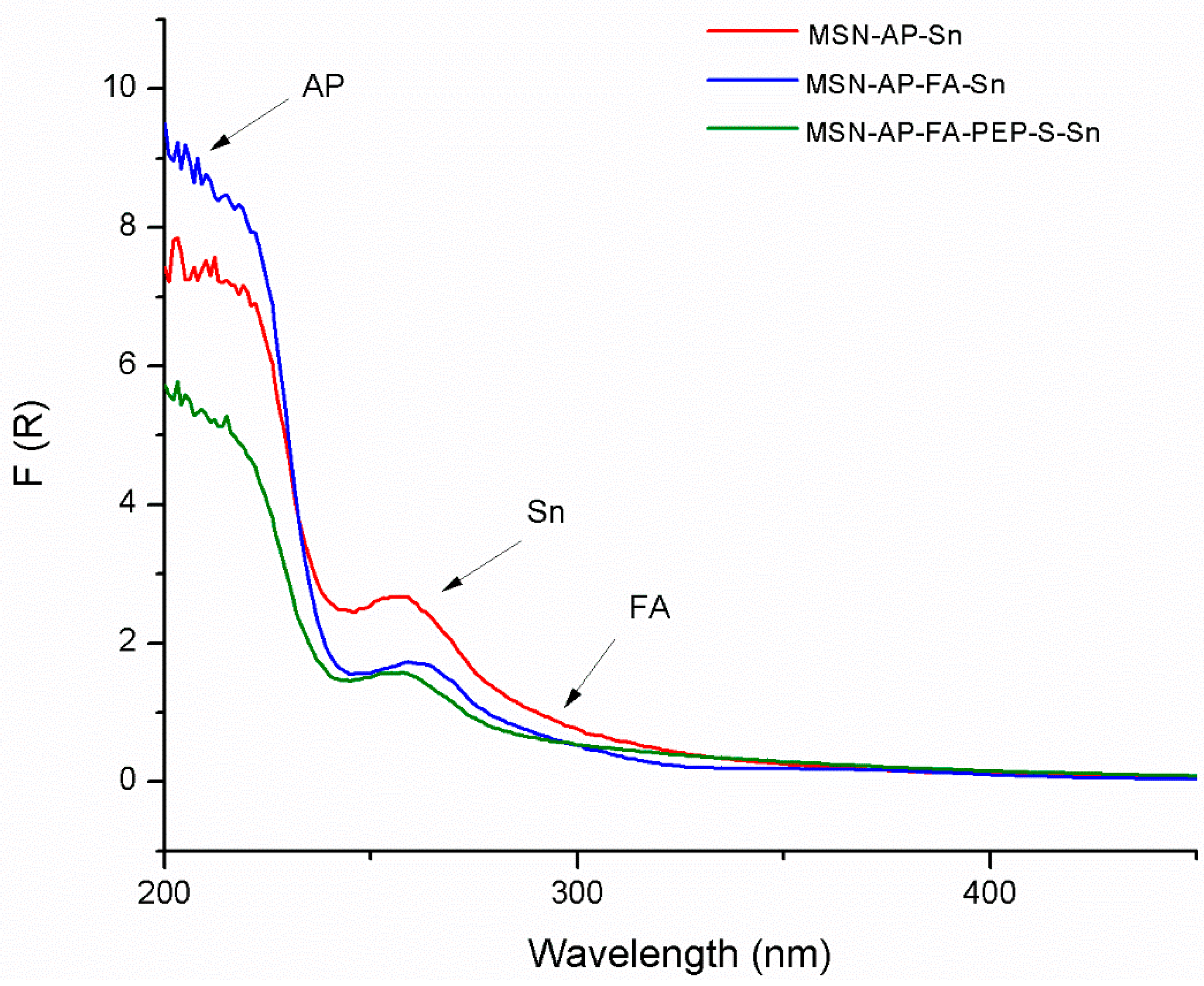
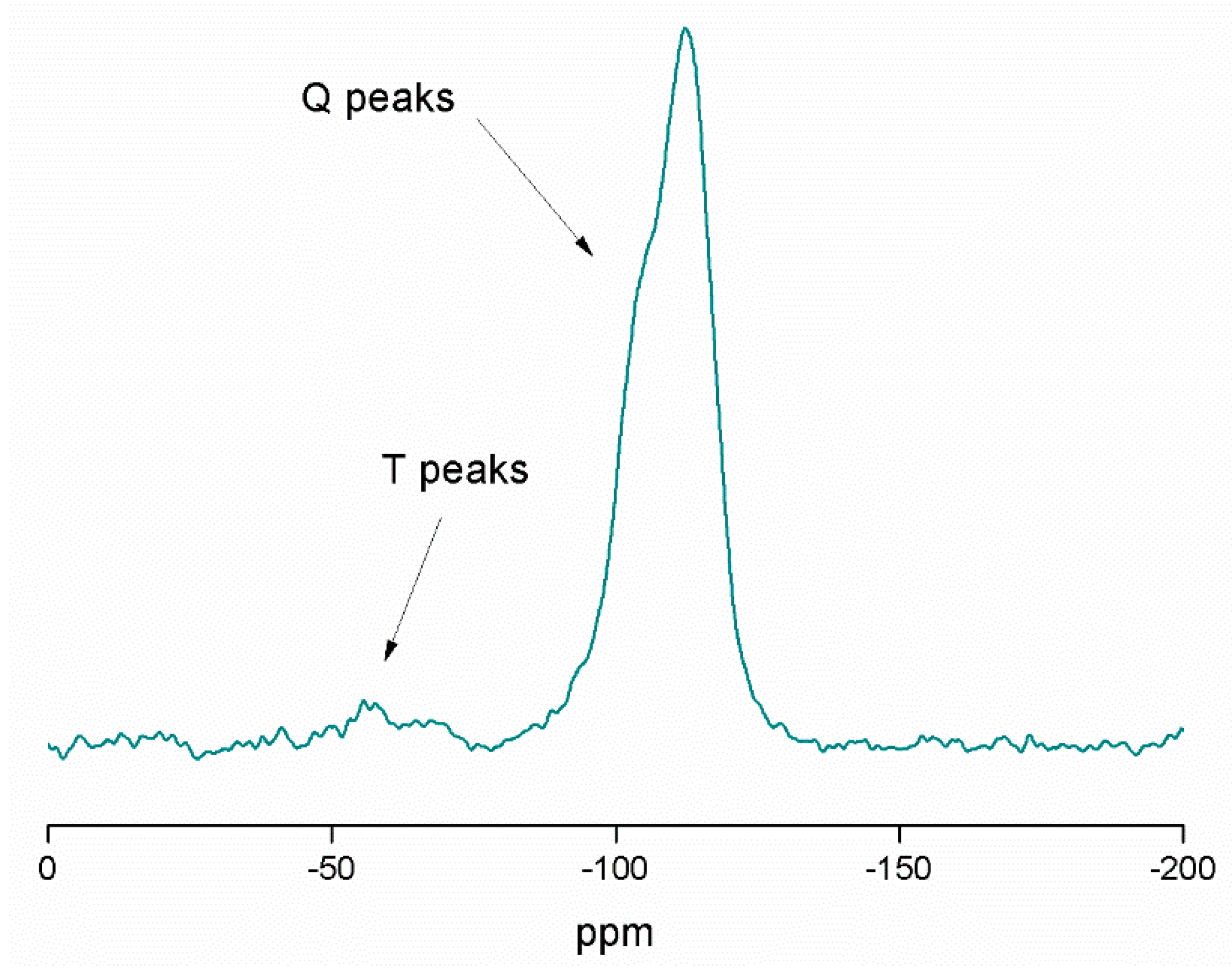
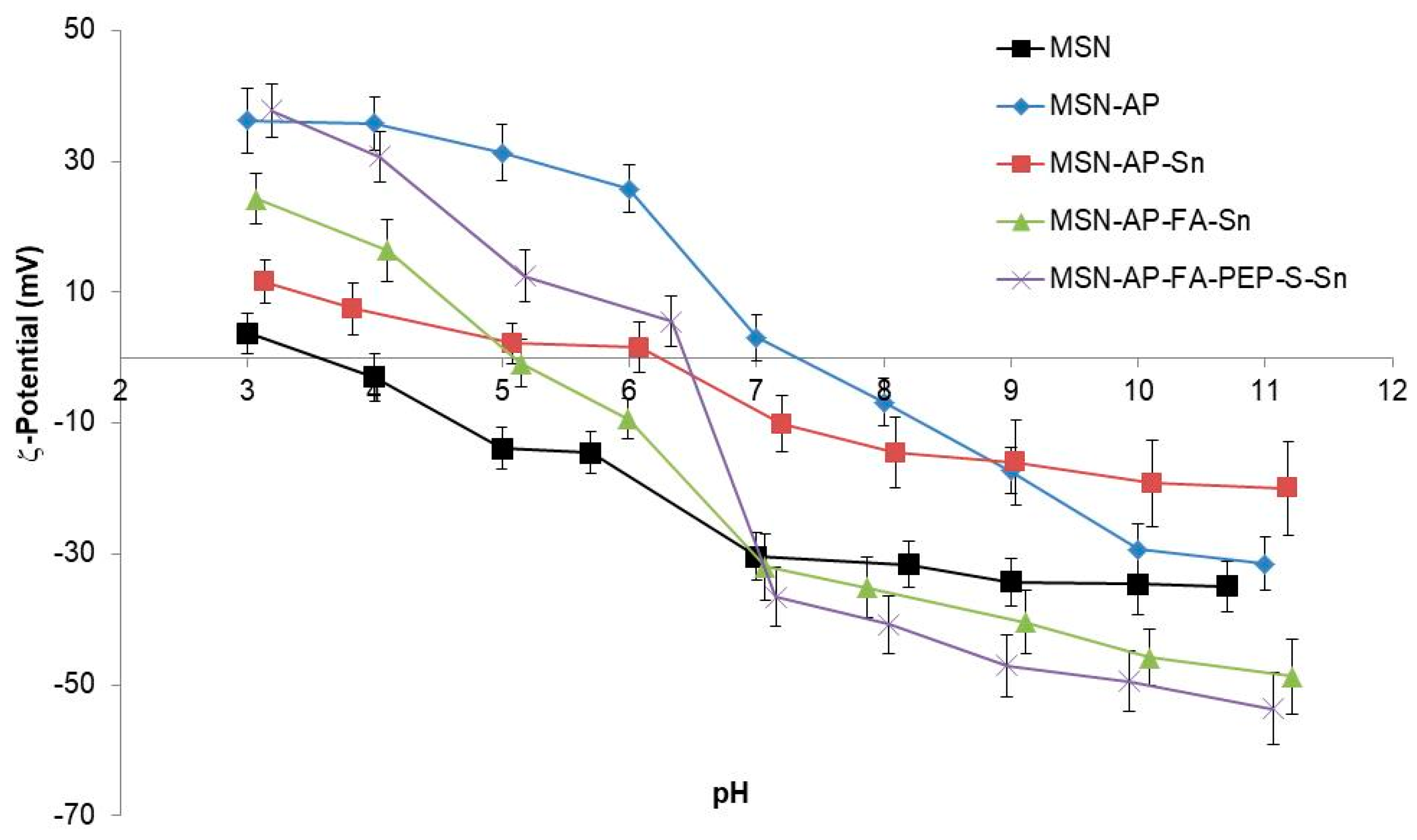
2.2. Physical and Chemical Characterization of the Different MSN-Based Multifunctional Nanomaterials
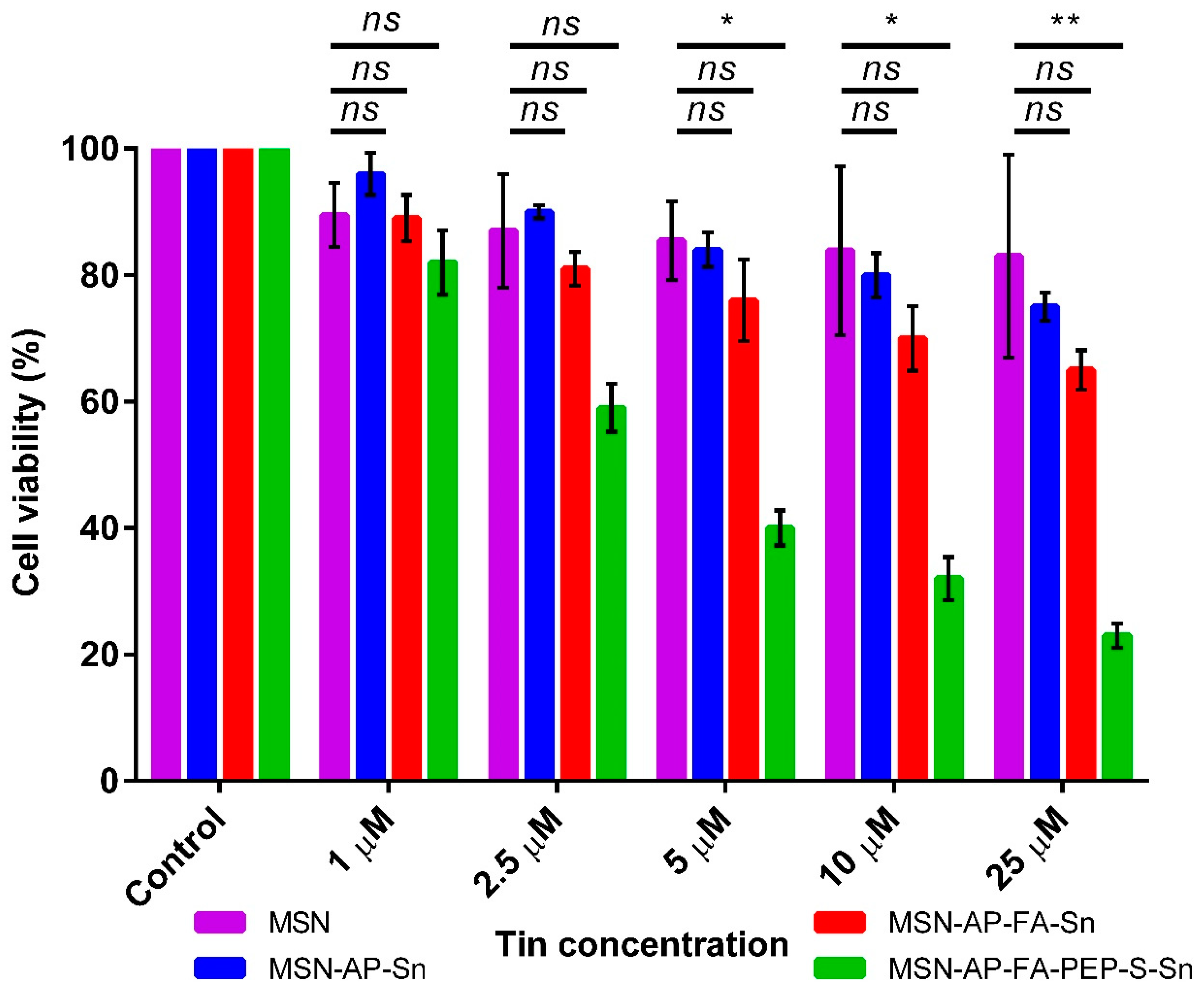
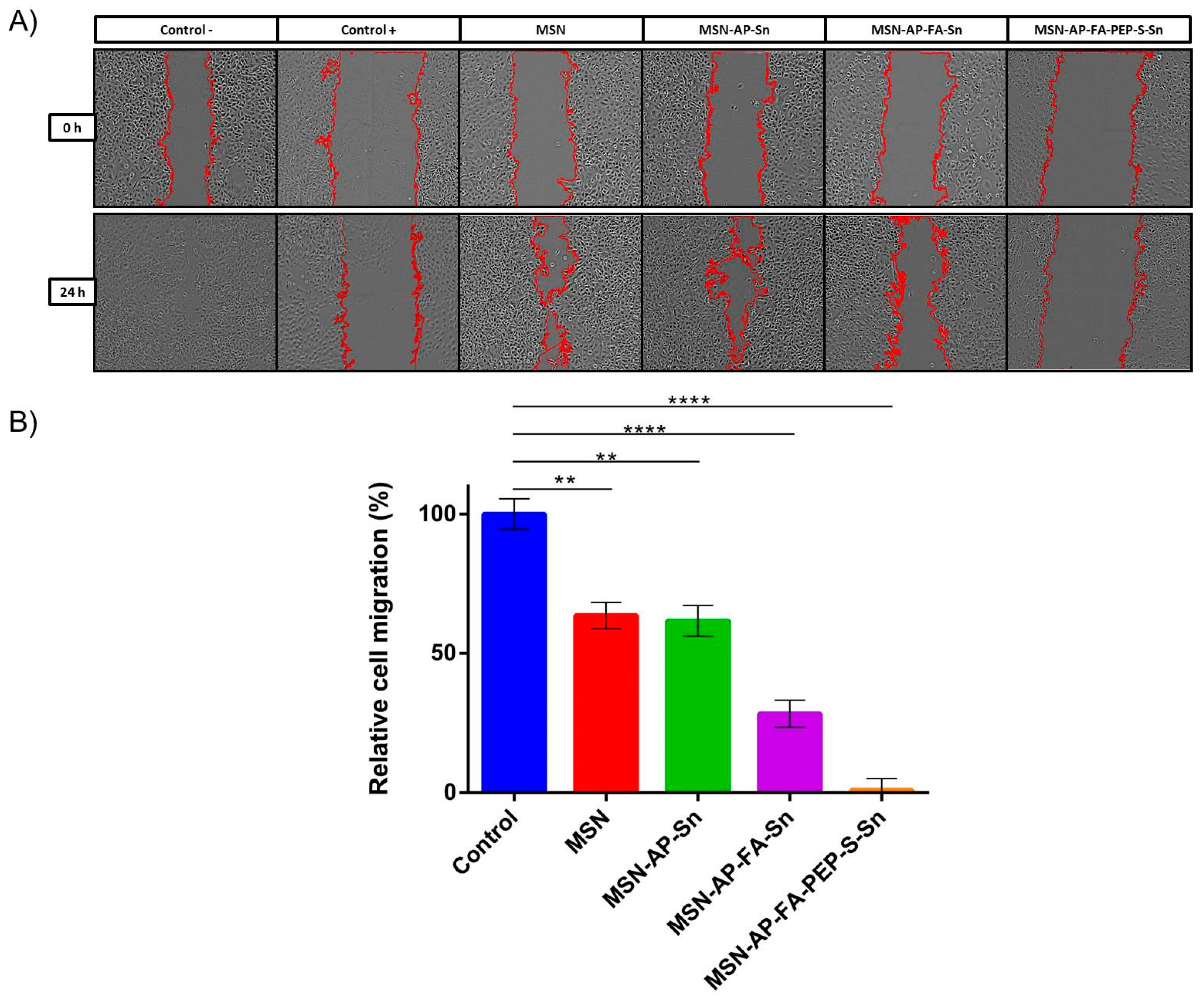
2.3. In Vitro Characterization of the Different MSN-Based Multifunctional Nanomaterials
2.4. In Vivo Evaluation of Theranostic Properties of Metallodrug Based MSN Nanomaterials on Breast Adenocarcinoma Mice Models
3. Materials and Methods
3.1. Synthesis of Nanomaterials
3.1.1. General Conditions
3.1.2. Synthesis of the Starting Material: Mesoporous Silica Nanoparticles (MSN)
3.1.3. Functionalization with Amino Ligands. Preparation of MSN-AP Material
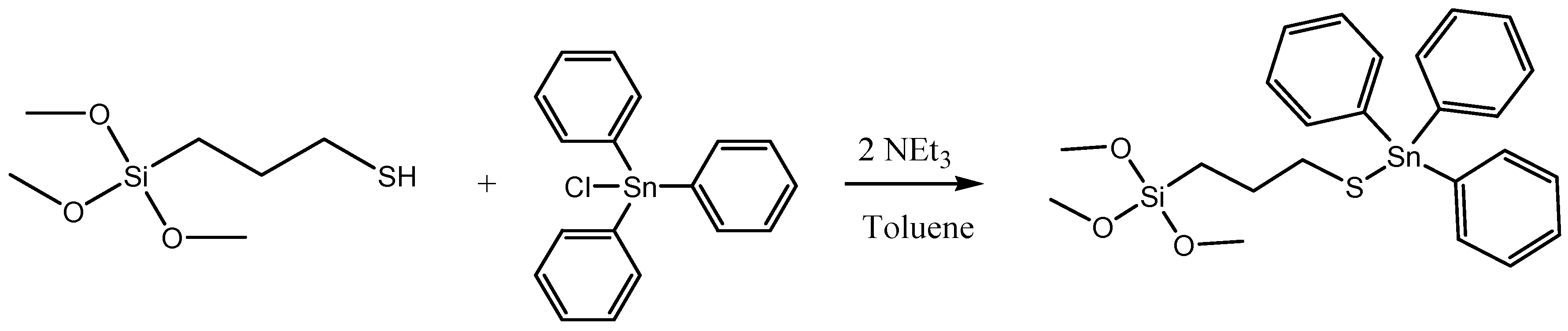
3.1.4. Functionalization with the Cytotoxic Agent (Sn). Synthesis of MSN-AP-Sn
3.1.5. Incorporation of Folic Acid (FA). Synthesis of MSN-AP-FA
3.1.6. Functionalization with the Cytotoxic Agent (Sn). Synthesis of MSN-AP-FA-Sn
3.1.7. Simultaneous Incorporation of Folic Acid (FA) and Targeting Peptide (Fmoc-Gly-Phe-Leu-Gly-COOH). Synthesis of MSN-AP-FA-Pep
3.1.8. Incorporation of 3-Mercaptopropionic Acid (MS). Synthesis of MSN-AP-FA-PEP-S
3.1.9. Functionalization with the Cytotoxic Agent (Sn). Synthesis of MSN-AP-FA-PEP-S-Sn
3.1.10. Incorporation of the Imaging Agent Alexa Fluor 647. Preparation of AX Materials
3.2. General Remarks on Chemico-Physical Characterization of the Materials
3.3. In Vitro Studies
3.3.1. Cell lines and Culture Condition
3.3.2. Nanodrug Preparation
3.3.3. Viability Assay
3.3.4. Cellular Uptake
3.3.5. Wound Healing Assay
3.4. In Vivo Studies
3.4.1. Animals and Ethics
3.4.2. Breast Cancer Mouse Model
3.4.3. Treatment with Nanoparticles and in Vivo Fluorescence Imaging
3.4.4. Tumor Measurement
3.4.5. Statistic and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.L.; Kuo, Y.C.; Ho, Y.S.; Huang, Y.H. Triple-Negative Breast Cancer: Current Understanding and Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers 2019, 11, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Collado, J.; Lopez, J.J.; Jardin, I.; Camello, P.J.; Falcon, D.; Regodon, S.; Salido, G.M.; Smani, T.; Rosado, J.A. Adenylyl Cyclase Type 8 Overexpression Impairs Phosphorylation-Dependent Orai1 Inactivation and Promotes Migration in MDA-MB-231 Breast Cancer Cells. Cancers 2019, 11, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustacchi, G.; De Laurentiis, M. The role of taxanes in triple-negative breast cancer: Literature review. Drug Des. Dev. Ther. 2015, 9, 4303–4318. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Thomas, E.S.; Gomez, H.L.; Li, R.K.; Chung, H.C.; Fein, L.E.; Chan, V.F.; Jassem, J.; Pivot, X.B.; Klimovsky, J.V.; de Mendoza, F.H.; et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J. Clin. Oncol. 2007, 25, 5210–5217. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, W.S.; Vazhappilly, C.G.; Saleh, E.M.; Menon, V.; AlAzawi, A.M.; El-Serafi, A.T.; Mansour, W.; El-Awady, R. Interplay between Epigenetics, Expression of Estrogen Receptor- alpha, HER2/ERBB2 and Sensitivity of Triple Negative Breast Cancer Cells to Hormonal Therapy. Cancers 2018, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef]
- Zwicke, G.L.; Mansoori, G.A.; Jeffery, C.J. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nanotechnol. Rev. 2012, 3, 18496. [Google Scholar] [CrossRef] [PubMed]
- Quici, S.; Casoni, A.; Foschi, F.; Armelao, L.; Bottaro, G.; Seraglia, R.; Bolzati, C.; Salvarese, N.; Carpanese, D.; Rosato, A.; et al. Folic acid-conjugated europium complexes as luminescent probes for selective targeting of cancer cells. J. Med. Chem. 2015, 58, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Babaer, D.; Amara, S.; Ivy, M.; Zhao, Y.; Lammers, P.E.; Titze, J.M.; Tiriveedhi, V. High salt induces P-glycoprotein mediated treatment resistance in breast cancer cells through store operated calcium influx. Oncotarget 2018, 9, 25193–25205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueder, N.; Allan, G.; Telliez, M.S.; Hague, F.; Fernandez, J.M.; Sanchez-Fernandez, E.M.; Ortiz-Mellet, C.; Ahidouch, A.; Ouadid-Ahidouch, H. sp2-Iminosugar alpha-glucosidase inhibitor 1-C-octyl-2-oxa-3-oxocastanospermine specifically affected breast cancer cell migration through Stim1, beta1-integrin, and FAK signaling pathways. J. Cell. Physiol. 2017, 232, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Hammadi, M.; Chopin, V.; Matifat, F.; Dhennin-Duthille, I.; Chasseraud, M.; Sevestre, H.; Ouadid-Ahidouch, H. Human ether a-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J. Cell. Physiol. 2012, 227, 3837–3846. [Google Scholar] [CrossRef]
- Necela, B.M.; Crozier, J.A.; Andorfer, C.A.; Lewis-Tuffin, L.; Kachergus, J.M.; Geiger, X.J.; Kalari, K.R.; Serie, D.J.; Sun, Z.; Moreno-Aspitia, A.; et al. Folate receptor-alpha (FOLR1) expression and function in triple negative tumors. PLoS ONE 2015, 10, e0122209. [Google Scholar]
- Díaz-Garcia, D.; Cenariu, D.; Pérez, Y.; Cruz, P.; Del Hierro, I.; Prashar, S.; Fischer-Fodor, E.; Gómez-Ruiz, S. Modulation of the mechanism of apoptosis in cancer cell lines by treatment with silica-based nanostructured materials functionalized with different metallodrugs. Dalton Trans. 2018, 47, 12284–12299. [Google Scholar] [CrossRef]
- Ott, I.; Gust, R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm Weinh. 2007, 340, 117–126. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Markus, G.; Michael, A.J.; Bernhard, K.K. Update of the Preclinical Situation of Anticancer Platinum Complexes: Novel Design Strategies and Innovative Analytical Approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Kaluderovic, G.N.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Gómez-Ruiz, S. Synthesis and biological applications of ionic triphenyltin(IV) chloride carboxylate complexes with exceptionally high cytotoxicity. Metallomics 2010, 2, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Carrasco-García, E.; Ceballos-Torres, J.; Prashar, S.; Kaluderovic, G.N.; Ferragut, J.A.; Gómez-Ruiz, S. Study of the anticancer properties of tin(IV) carboxylate complexes on a panel of human tumor cell lines. ChemMedChem 2012, 7, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, K.S.; Lippard, S.J. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009, 28, 10651–10659. [Google Scholar] [CrossRef]
- Siccardi, M.; Martin, P.; McDonald, T.O.; Liptrott, N.J.; Giardiello, M.; Rannard, S.; Owen, A. Research Spotlight: Nanomedicines for HIV therapy. Ther. Deliv. 2013, 4, 153–156. [Google Scholar] [CrossRef]
- Marciello, M.; Pellico, J.; Fernandez-Barahona, I.; Herranz, F.; Ruiz-Cabello, J.; Filice, M. Recent advances in the preparation and application of multifunctional iron oxide and liposome-based nanosystems for multimodal diagnosis and therapy. Interface Focus 2016, 6, 20160055. [Google Scholar] [CrossRef]
- Filice, M.; Palomo, J.M. Cascade Reactions Catalyzed by Bionanostructures. ACS Catal. 2014, 4, 1588–1598. [Google Scholar] [CrossRef]
- Filice, M.; Marciello, M.; Morales, M.d.P.; Palomo, J.M. Synthesis of heterogeneous enzyme–metal nanoparticle biohybrids in aqueous media and their applications in C–C bond formation and tandem catalysis. Chem. Commun. 2013, 49, 6876–6878. [Google Scholar] [CrossRef] [Green Version]
- Erami, R.S.; Ovejero, K.; Meghdadi, S.; Filice, M.; Amirnasr, M.; Rodríguez-Diéguez, A.; De La Orden, M.U.; Gómez-Ruiz, S. Applications of Nanomaterials Based on Magnetite and Mesoporous Silica on the Selective Detection of Zinc Ion in Live Cell Imaging. Nanomaterials 2018, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Zizak, Z.; Sierra, I.; Prashar, S.; Hierro, I.; Fajardo, M.; Juranic, Z.; Kaluđerović, G. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anti-cancer Applications of Titanocene-Functionalised Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, M.; Maksimovic-Ivanic, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korac, A.; Golic, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-Loaded Mesoporous Silica as a Biocompatible Strategy in Cancer Treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–8987. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Chicca, A.; Gertsch, J.; Pinar, A.B.; Gómez-Ruiz, S. Ether-Substituted Group 4 Metallocene Complexes: Cytostatic Effects and Applications in Ethylene Polymerization. Organometallics 2015, 34, 2522–2532. [Google Scholar] [CrossRef]
- Bensing, C.; Mojic, M.; Gómez-Ruiz, S.; Carralero, S.; Dojcinovic, B.; Maksimovic-Ivanic, D.; Mijatovic, S.; Kaluđerović, G. Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against A2780 ovarian carcinoma cell line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(II) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalton Trans. 2019, 48, 5940–5951. [Google Scholar] [CrossRef]
- Lee, C.-H.; Cheng, S.-H.; Wang, Y.-J.; Chen, Y.-C.; Chen, N.-T.; Souris, J.; Chen, C.-T.; Mou, C.-Y.; Yang, C.-S.; Lo, L.-W.; et al. Near-Infrared Mesoporous Silica Nanoparticles for Optical Imaging: Characterization and In Vivo Biodistribution. Adv. Funct. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Guillet-Nicolas, R.; Laprise-Pelletier, M.; Nair, M.M.; Chevallier, P.; Lagueux, J.; Gossuin, Y.; Laurent, S.; Kleitz, F.; Fortin, M.A. Manganese-impregnated mesoporous silica nanoparticles for signal enhancement in MRI cell labelling studies. Nanoscale 2013, 5, 11499–11511. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Ji, X.; Zhang, S.; Chen, H.; Zheng, Y.; Sun, Y.; Qu, H.; Wang, Z.; Li, Y.; et al. Manganese oxide-based multifunctionalized mesoporous silica nanoparticles for pH-responsive MRI, ultrasonography and circumvention of MDR in cancer cells. Biomaterials 2012, 33, 7126–7137. [Google Scholar] [CrossRef] [PubMed]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morere, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. Camb. 2009, 28, 1475–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, E.; Dehghannejad, N.; Hashemikia, S.; Ghasemnejad, M.; Tabebordbar, H. Synthesis and surface modification of mesoporous silica nanoparticles and its application as carriers for sustained drug delivery. Drug Deliv. 2014, 21, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Kesse, S.; Boakye-Yiadom, K.O.; Ochete, B.O.; Opoku-Damoah, Y.; Akhtar, F.; Filli, M.S.; Asim Farooq, M.; Aquib, M.; Maviah Mily, B.J.; Murtaza, G.; et al. Mesoporous Silica Nanomaterials: Versatile Nanocarriers for Cancer Theranostics and Drug and Gene Delivery. Pharmaceutics 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and Comparison of Mesoporous Silica Particles for Optimized Drug Delivery. Nanomater. Nanotechnol. 2014, 4, 1. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Hu, Q.; Li, J.; Qiao, S.; Hao, Z.; Tian, H.; Ma, C.; He, C. Synthesis and hydrophobic adsorption properties of microporous/mesoporous hybrid materials. J. Hazard. Mater. 2009, 164, 1205–1212. [Google Scholar] [CrossRef]
- Sanchez, A.; Ovejero Paredes, K.; Ruiz-Cabello, J.; Martinez-Ruiz, P.; Pingarron, J.M.; Villalonga, R.; Filice, M. Hybrid Decorated Core@Shell Janus Nanoparticles as a Flexible Platform for Targeted Multimodal Molecular Bioimaging of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31032–31043. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chellappan, D.R.; Bhavsar, D.V.; Ranganathan, R.; Sivanantham, B.; Subramanian, A.; Sharma, U.; Jagannathan, N.R.; Krishnan, U.M.; Sethuraman, S. Multi-functional nanoparticles as theranostic agents for the treatment & imaging of pancreatic cancer. Acta Biomater. 2017, 49, 422–433. [Google Scholar] [PubMed]
- Lazaro-Carrillo, A.; Filice, M.; Guillén, M.J.; Amaro, R.; Viñambres, M.; Tabero, A.; Paredes, K.O.; Villanueva, A.; Calvo, P.; del Puerto Morales, M.; et al. Tailor-made PEG coated iron oxide nanoparticles as contrast agents for long lasting magnetic resonance molecular imaging of solid cancers. Mater. Sci. Eng. C 2020, 107, 110262. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Aires, A.; Marciello, M. Protein-Modified Magnetic Nanoparticles for Biomedical Applications. Curr. Org. Chem. 2015, 19, 1. [Google Scholar] [CrossRef]
- Filice, M.; Ruiz-Cabello, J. Nucleic Acid Nanotheranostics: Biomedical Applications; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Zelzer, M.; Todd, S.J.; Hirst, A.R.; McDonald, T.O.; Ulijn, R.V. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2013, 1, 11–39. [Google Scholar] [CrossRef]
- Andresen, T.L.; Thompson, D.H.; Kaasgaard, T. Enzyme-triggered nanomedicine: Drug release strategies in cancer therapy. Mol. Membr. Biol. 2010, 27, 353–363. [Google Scholar] [CrossRef]
- Ruan, J.; Zheng, H.; Rong, X.; Rong, X.; Zhang, J.; Fang, W.; Zhao, P.; Luo, R. Over-expression of cathepsin B in hepatocellular carcinomas predicts poor prognosis of HCC patients. Mol. Cancer 2016, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, M.; Jane, D.; Theriault, A.; Adeli, K. Expression of cathepsin B and aryl hydrocarbon hydroxylase activities, and of apolipoprotein B in human hepatoma cells maintained long-term in a serum-free medium. Vitr. Cell. Dev. Biol. Anim. 1993, 29A, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Groborz, K.; Vizovisek, M.; Maruggi, M.; Turk, D.; Turk, B.; Powis, G.; Drag, M.; Salvesen, G.S. Fluorescent probes towards selective cathepsin B detection and visualization in cancer cells and patient samples. Chem. Sci. 2019, 10, 8461–8477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpaderska, A.; Frankfater, A. An intracellular form of cathepsin B contributes to invasiveness in cancer. Cancer Res. 2001, 61, 3493–3500. [Google Scholar] [PubMed]
- Ruan, H.; Hao, S.; Young, P.; Zhang, H. Targeting Cathepsin B for Cancer Therapies. Horiz. Cancer Res. 2015, 56, 23–40. [Google Scholar] [PubMed]
- Barwal, I.; Kumar, R.; Kateriya, S.; Dinda, A.K.; Yadav, S.C. Targeted delivery system for cancer cells consist of multiple ligands conjugated genetically modified CCMV capsid on doxorubicin GNPs complex. Sci. Rep. 2016, 6, 37096. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Xu, Y.; Sun, K.; Wang, H.; Zhang, F.; Zhou, Z.; Zhang, J.; Zhang, F.; Caliskan, B.; Qiu, Z.; et al. Identification of a peptide for folate receptor alpha by phage display and its tumor targeting activity in ovary cancer xenograft. Sci. Rep. 2018, 8, 8426. [Google Scholar] [CrossRef] [Green Version]
- Lecomte, N.; Njardarson, J.T.; Nagorny, P.; Yang, G.; Downey, R.; Ouerfelli, O.; Moore, M.A.S.; Danishefsky, S.J. Emergence of potent inhibitors of metastasis in lung cancer via syntheses based on migrastatin. Proc. Natl. Acad. Sci. USA 2011, 108, 15074–15078. [Google Scholar] [CrossRef] [Green Version]
- Hai, J.; Zhu, C.Q.; Bandarchi, B.; Wang, Y.H.; Navab, R.; Shepherd, F.A.; Jurisica, I.; Tsao, M.S. L1 cell adhesion molecule promotes tumorigenicity and metastatic potential in non-small cell lung cancer. Clin. Cancer Res. 2012, 18, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Rubin, B.; Suzuki, A.; Patel, P.S.; Gahl, W.A.; Joshi, B.H.; Puri, R.K. Cysteamine suppresses invasion, metastasis and prolongs survival by inhibiting matrix metalloproteinases in a mouse model of human pancreatic cancer. PLoS ONE 2012, 7, e34437. [Google Scholar] [CrossRef]
- Scarpa, E.; Mayor, R. Collective cell migration in development. J. Cell Biol. 2016, 212, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Reinhart-King, C.A. Chapter 3 Endothelial Cell Adhesion and Migration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2008; Volume 443, pp. 45–64. [Google Scholar]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollu, V.S.; Barui, A.K.; Mondal, S.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodriguez-Dieguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin-loaded silica-based mesoporous materials: Synthesis, characterization and cytotoxic properties against cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Yang, Y.; Hao, G.; Yi, X.; Zhang, S.; Pan, Y.; Xing, B.; Gao, M. Multifunctional Magnetic Mesoporous Silica Nanoagents for in vivo Enzyme-Responsive Drug Delivery and MR Imaging. Nanotheranostics 2018, 2, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenholm, J.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomed. Lond. Engl. 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. https://doi.org/10.3390/cancers12010187
Ovejero Paredes K, Díaz-García D, García-Almodóvar V, Lozano Chamizo L, Marciello M, Díaz-Sánchez M, Prashar S, Gómez-Ruiz S, Filice M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers. 2020; 12(1):187. https://doi.org/10.3390/cancers12010187
Chicago/Turabian StyleOvejero Paredes, Karina, Diana Díaz-García, Victoria García-Almodóvar, Laura Lozano Chamizo, Marzia Marciello, Miguel Díaz-Sánchez, Sanjiv Prashar, Santiago Gómez-Ruiz, and Marco Filice. 2020. "Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer" Cancers 12, no. 1: 187. https://doi.org/10.3390/cancers12010187
APA StyleOvejero Paredes, K., Díaz-García, D., García-Almodóvar, V., Lozano Chamizo, L., Marciello, M., Díaz-Sánchez, M., Prashar, S., Gómez-Ruiz, S., & Filice, M. (2020). Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers, 12(1), 187. https://doi.org/10.3390/cancers12010187