Circulating Tumor Cells: From the Laboratory to the Cancer Clinic
Abstract
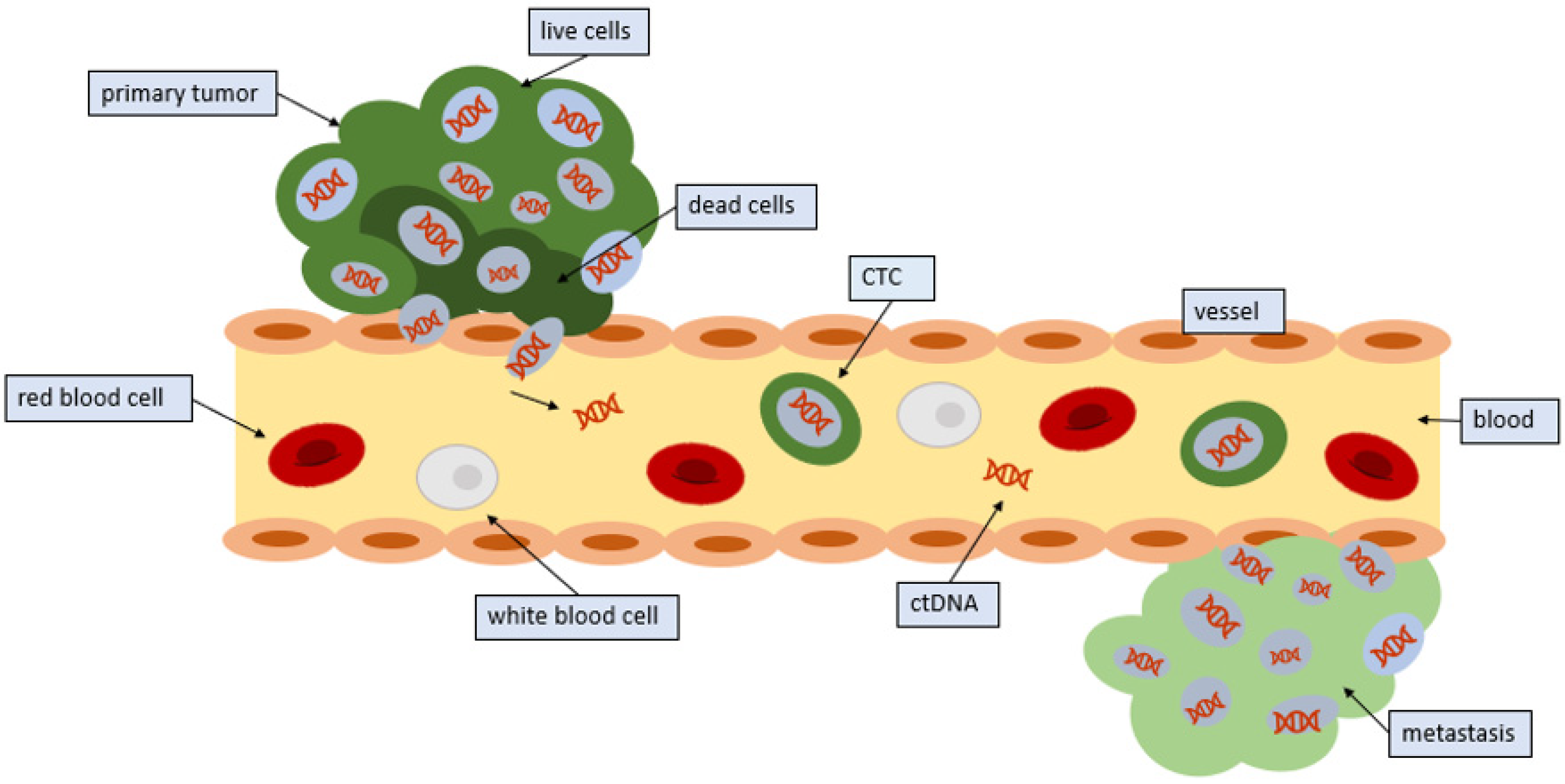
1. Introduction
2. Technological Methods to Detect CTCs
2.1. CTC Enrichment by Immunoaffinity
2.2. Positive Enrichment Strategies
2.3. Negative Enrichment Strategies
2.4. CTC Enrichment Based on Biophysical Properties
3. Mechanisms by Which Circulating Tumor Cells Enter, Leave, and Travel in the Circulation
4. Characterization of Circulating Tumor Cells by Genomic, RNA, and Protein Techniques
5. CTC-Derived Explant Models
6. Clinical Uses of Circulating Tumor Cells
7. Comparison of CTCs and Circulating Tumor DNA (ctDNA)
8. Other Circulating Vehicles: Exosomes
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kowalik, A.; Kowalewska, M.; Gozdz, S. Current approaches for avoiding the limitations of circulating tumor cells detection methods-implications for diagnosis and treatment of patients with solid tumors. Transl. Res. 2017, 185, 58–84. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Hou, J.-M.; Ward, T.H.; Blackhall, F.H.; Dive, C. Circulating tumour cells: Their utility in cancer management and predicting outcomes. Ther. Adv. Med. Oncol. 2010, 2, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, K.; Fan, Z.H. Circulating tumor cell isolation and analysis. Adv. Clin. Chem. 2016, 75, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kamps, R.; Brandao, R.D.; Bosch, B.J.; Paulussen, A.D.; Xanthoulea, S.; Blok, M.J.; Romano, A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int. J. Mol. Sci. 2017, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Millner, L.M.; Linder, M.W.; Valdes, R. Circulating tumor cells: A review of present methods and the need to identify heterogeneous phenotypes. Ann. Clin. Lab. Sci. 2013, 43, 295–304. [Google Scholar]
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J. Mol. Diagn. 2015, 17, 209–224. [Google Scholar] [CrossRef]
- Lin, E.; Cao, T.; Nagrath, S.; King, M.R. Circulating tumor cells: Diagnostic and therapeutic applications. Ann. Rev. Biomed. Eng. 2018, 20, 329–352. [Google Scholar] [CrossRef]
- Forte, V.A.; Barrak, D.K.; Elhodaky, M.; Tung, L.; Snow, A.; Lang, J.E. The potential for liquid biopsies in the precision medical treatment of breast cancer. Cancer Biol. Med. 2016, 13, 19–40. [Google Scholar] [CrossRef]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The Use of Microfluidic Technology for Cancer Applications and Liquid Biopsy. Micromachines 2018, 9, 397. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef] [PubMed]
- Osman, O.; Toru, S.; Dumas-Bouchiat, F.; Dempsey, N.M.; Haddour, N.; Zanini, L.F.; Buret, F.; Reyne, G.; Frenea-Robin, M. Microfluidic immunomagnetic cell separation using integrated permanent micromagnets. Biomicrofluidics 2013, 7, 054115. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2015, 78, 016601. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Bischoff, F.Z.; Mayer, J.A.; Wong, K.L.; Pham, T.; Bottsford-Miller, J.; Stone, R.L.; Lin, Y.G.; Jaladurgam, P.; Roh, J.W.; et al. A novel platform for detection of CK+ and CK− CTCs. Cancer Discov. 2011, 1, 580–586. [Google Scholar] [CrossRef]
- Hao, S.J.; Wan, Y.; Xia, Y.Q.; Zou, X.; Zheng, S.Y. Size-based separation methods of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 3–20. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Wang, B. Size- and deformability-based isolation of circulating tumor cells with microfluidic chips and their applications in clinical studies. AIP Adv. 2018, 8, 120701. [Google Scholar] [CrossRef]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Oliveto, S.; Mancino, M.; Manfrini, N.; Biffo, S. Role of microRNAs in translation regulation and cancer. World J. Biol. Chem. 2017, 8, 45–56. [Google Scholar] [CrossRef]
- Potdar, P.D.; Lotey, N.K. Role of circulating tumor cells in future diagnosis and therapy of cancer. J. Cancer Metastasis Treat. 2015, 1, 44–56. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Trape, A.P.; Gonzalez-Angulo, A.M. Breast cancer and metastasis: On the way toward individualized therapy. Cancer Genom. Proteom. 2012, 9, 297–310. [Google Scholar]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Porras, T.B.; Kaur, P.; Ring, A.; Schechter, N.; Lang, J.E. Challenges in using liquid biopsies for gene expression profiling. Oncotarget 2018, 9, 7036–7053. [Google Scholar] [CrossRef]
- Mayer, J.A.; Pham, T.; Wong, K.L.; Scoggin, J.; Sales, E.V.; Clarin, T.; Pircher, T.J.; Mikolajczyk, S.D.; Cotter, P.D.; Bischoff, F.Z. FISH-based determination of HER2 status in circulating tumor cells isolated with the microfluidic CEE platform. Cancer Genet. 2011, 204, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.R.; Ibarra, G.H.; Vibat, C.R.T.; Singh, V.M. Detecting an ALK Rearrangement via Liquid Biopsy Enabled a Targeted Therapy-based Approach for Treating a Patient with Advanced Non-small Cell Lung Cancer. Oncol. Hematol. Rev. 2018, 14, 38–41. [Google Scholar] [CrossRef]
- Wu, Y.; Park, K.J.; Deighan, C.; Amaya, P.; Miller, B.; Pan, Q.; Zborowski, M.; Lustberg, M.; Chalmers, J. Multiparameter evaluation of the heterogeneity of circulating tumor cells using integrated RNA in situ hybridization and immunocytochemical analysis. Front. Oncol. 2016, 6, 234. [Google Scholar] [CrossRef]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef]
- Dive, C.; Frese, K.; Brady, G.; Tugwood, J.; Miller, C.; Blackhall, F. Circulating tumor cell eXplants (CDX) to advance small cell lung cancer (SCLC) research and drug development. J. Thorac. Oncol. 2016. [Google Scholar] [CrossRef][Green Version]
- Lallo, A.; Schenk, M.W.; Frese, K.K.; Blackhall, F.; Dive, C. Circulating tumor cells and CDx models as a tool for preclinical drug development. Transl. Lung Cancer Res. 2017, 6, 397–408. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Riethdorf, S.; Soave, A.; Rink, M. The current status and clinical value of circulating tumor cells and circulating cell-free tumor DNA in bladder cancer. Transl. Androl. Urol. 2017, 6, 1090. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.J.; Pantel, K. Tumor-Educated Platelets as Liquid Biopsy in Cancer Patients. Cancer Cell 2015, 28, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Babayan, A.; Hannemann, J.; Spotter, J.; Muller, V.; Pantel, K.; Joosse, S.A. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS ONE 2013, 8, e75038. [Google Scholar] [CrossRef]
- Fehm, T.; Müller, V.; Aktas, B.; Janni, W.; Schneeweiss, A.; Stickeler, E.; Lattrich, C.; Löhberg, C.R.; Solomayer, E.; Rack, B.; et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: A prospective, multicenter trial. Breast Cancer Res. Treat. 2010, 124, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Okamura, R.; Mareboina, M.; Lee, S.; Goodman, A.; Patel, S.P.; Fanta, P.T.; Schwab, R.B.; Vu, P.; Raymond, V.M.; et al. Revisiting Epidermal Growth Factor Receptor (EGFR) Amplification as a Target for Anti-EGFR Therapy: Analysis of Cell-Free Circulating Tumor DNA in Patients With Advanced Malignancies. JCO Precis. Oncol. 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Schwaederle, M.; Mohindra, M.; Fontes Jardim, D.L.; Kurzrock, R. MET alterations detected in blood-derived circulating tumor DNA correlate with bone metastases and poor prognosis. J. Hematol. Oncol. 2018, 11, 1–76. [Google Scholar] [CrossRef]
- Mardinian, K.; Okamura, R.; Kato, S.; Kurzrock, R. Temporal and spatial effects and survival outcomes associated with concordance between tissue and blood KRAS alterations in the pan-cancer setting. Int. J. Cancer 2020, 146, 566–576. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Alison, S.; Jeri, M.; Craig, M.M.; James, M.R.; Gerald, V.D.; Jeffrey, W.A.; Leon, W.M.M.T.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Pachmann, K.; Camara, O.; Andreas, K.; Sabine, K.; Nele, M.; Mieczyslaw, G.; Torsten, K.; Cornelia, J.; Ulrike, H.; Annelore, A.-H.; et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J. Clin. Oncol. 2008, 26, 1208–1215. [Google Scholar] [CrossRef]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nolé, F.; Gisbert-Criado, R.; Mavroudis, D.; Grisanti, S.; Generali, D.; Garcia-Saenz, J.A.; Stebbing, J.; et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.; Hughes, B.G.M.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of Circulating Tumor Cells and Circulating Tumor DNA in Non-Small Cell Lung Cancer: Association with Clinical Endpoints in a Phase II Clinical Trial of Pertuzumab and Erlotinib. Clin. Cancer Res. 2012, 18, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Long-Mira, E.; Butori, C.; Hofman, V.; Coelle, C.; Mauro, V.; Zahaf, K.; Marquette, C.H.; Mouroux, J.; Paterlini-Brechot, P.; et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann. Oncol. 2012, 23, 2907–2913. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cao, L.; Chen, L.; Li, J.; Zhang, X.-F.; Qian, H.; Kang, X.-Y.; Zhang, Y.; Liao, J.; Shi, L.; et al. Isolation of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Using a Novel Cell Separation Strategy. Clin. Cancer Res. 2011, 17, 3783–3793. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef]
- Hoshimoto, S.; Faries, M.B.; Morton, D.L.; Shingai, T.; Kuo, C.; Wang, H.-J.; Elashoff, R.; Mozzillo, N.; Kelley, M.C.; Thompson, J.F.; et al. Assessment of Prognostic Circulating Tumor Cells in a Phase III Trial of Adjuvant Immunotherapy After Complete Resection of Stage IV Melanoma. Ann. Surg. 2012, 255, 357–362. [Google Scholar] [CrossRef]
- Steinestel, J.; Luedeke, M.; Arndt, A.; Schnoeller, T.J.; Lennerz, J.K.; Wurm, C. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 2019, 10, 4213. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Neumann, M.H.D.; Bender, S.; Krahn, T.; Schlange, T. ctDNA and CTCs in Liquid Biopsy—Current Status and where we Need to Progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef]
- Qin, Z.; Ljubimov, V.A.; Zhou, C.; Tong, Y.; Liang, J. Cell-free circulating tumor DNA in cancer. Chin. J. Cancer 2016, 35, 36. [Google Scholar] [CrossRef]
- Choi, I.S.; Kato, S.; Fanta, P.T.; Leichman, L.; Okamura, R.; Raymond, V.M.; Lanman, R.B.; Lippman, S.M.; Kurzrock, R. Genomic Profiling of Blood-Derived Circulating Tumor DNA from Patients with Colorectal Cancer: Implications for Response and Resistance to Targeted Therapeutics. Mol. Cancer Ther. 2019, 18, 1852–1862. [Google Scholar] [CrossRef]
- Shatsky, R.; Parker, B.A.; Bui, N.Q.; Helsten, T.; Schwab, R.B.; Boles, S.G.; Kurzrock, R. Next-Generation Sequencing of Tissue and Circulating Tumor DNA: The UC San Diego Moores Center for Personalized Cancer Therapy Experience with Breast Malignancies. Mol. Cancer Ther. 2019, 18, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Okamura, R.; Baumgartner, J.M.; Patel, H.; Leichman, L.; Kelly, K.; Sicklick, J.K.; Fanta, P.T.; Lippman, S.M.; Kurzrock, R. Analysis of Circulating Tumor DNA and Clinical Correlates in Patients with Esophageal, Gastroesophageal Junction, and Gastric Adenocarcinoma. Clin. Cancer Res. 2018, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.M.; Raymond, V.M.; Lanman, R.B.; Tran, L.; Kelly, K.J.; Lowy, A.M.; Kurzrock, R. Preoperative Circulating Tumor DNA in Patients with Peritoneal Carcinomatosis is an Independent Predictor of Progression-Free Survival. Ann. Surg. Oncol. 2018, 25, 2400–2408. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Farinas, S.; Jantus-Lewintre, E.; Herreros-Pomares, A.; Camps, C. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl. Lung Cancer Res. 2016, 5, 466–482. [Google Scholar] [CrossRef]
- Tan, C.R.; Zhou, L.; El-Deiry, W.S. Circulating tumor cells versus circulating tumor DNA in colorectal cancer: Pros and cons. Curr. Colorectal Cancer Rep. 2016, 12, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, S.; Daryani, S.; Eslami, S.Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Keivan, M.-A.; Rezvan, E. Exosomes in cancer liquid biopsy: A focus on breast cancer. Mol. Ther. Nucleic Acids 2018, 10131–10141. [Google Scholar] [CrossRef]
- dos Anjos Pultz, B.; da Luz, F.A.C.; Faria, S.S.; de Souza, L.P.F.; Tavares, P.C.B.; Goulart, V.A.; Fontes, W.; Goulart, L.R.; Silva, M.J.B. The multifaceted role of extracellular vesicles in metastasis: Priming the soil for seeding. Int. J. Cancer 2017, 140, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Hench, I.B.; Hench, J.; Tolnay, M. Liquid biopsy in clinical management of breast, lung, and colorectal cancer. Front Med. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Su, D.W.; Nieva, J. Biophysical technologies for understanding circulating tumor cell biology and metastasis. Transl. Lung Cancer Res. 2017, 6, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Koopman, C.D.; Werb, Z. Cancer. Circulating tumor cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
| Technique | Advantages | Limitations |
|---|---|---|
| CellSearch | Ferrofluid nanoparticles with EpCAM antibodies | Dependent on EpCAM |
| AdnaTest | Immunomagnetic detection of EpCAM, utilizes RT-qPCR for protein expression | Dependent on EpCAM |
| ISET | Size of CTC, immunodetection on EpCAM | Dependent on EpCAM, size variability |
| EPISPOT | Enrichment through antigen expression, enzymatic activity | Variability of enzymatic activity |
| FISH | Detection of chromosomal DNA sequence | Requires cell permeabilization fluorescence probe, time-consuming/labor intensive |
| FAST | Detection through antigen expression | Half of CTCs are lost in the process, highly sensitive to EpCAM marker down-regulation |
| Density Gradient | Enrichment based on cell size, efficient, inexpensive, not dependent on cell surface marker expression | Low specificity, high contamination, CTC loss |
| Microfiltration (3D) | Efficient, captures live CTCs | Costly, dilution of blood required |
| Microflow | Efficient CTC enrichment, undiluted blood | Prototype, false positives and negative results, based on presence of antigens on CTC surface |
| Cancer Type | Setting | Results |
|---|---|---|
| Breast cancer | In a prospective multicenter study, 177 patients with measurable metastatic breast cancer were tested for CTCs before starting new treatment and at first follow up. | The number of circulating CTCs before treatment was an independent predictor of PFS and OS in patients with metastatic breast cancer [38] |
| Breast cancer | Nonmetastatic 91 primary (non-metastatic) breast cancer patients given adjuvant treatment | CTCs are influenced by adjuvant chemotherapy and an increase (even after initial response to therapy) of ≥10-fold at the end of therapy is a strong predictor of relapse and a surrogate marker for the [39] |
| Breast cancer | 17 centers provided data for 1944 eligible patients with metastatic breast cancer from 20 studies. | Aggressiveness of the tumor cells. Independent prognostic effect of CTC count on progression-free survival and overall survival [40] |
| Non-small-cell lung carcinoma | 41 patients on a phase II clinical trial of erlotinib and pertuzumab. | Correlation between decreases in CTCs and radiographic response in patients with advanced lung cancer [41] |
| Non-small-cell lung carcinoma | 87 patients with lung cancer showing CTCs were screened ALK status in both tumor tissue and in CTCs | ALK status can be determined in CTCs isolated from patients with lung cancer by immunocytochemistry and FISH analyses [42] |
| Hepatocellular carcinoma (HCC) | CTCs were detected by a novel method. Prevalence of CTCs was examined in samples from HCC patients, healthy volunteers, and patients with benign liver diseases or non-HCC cancers. | No healthy, benign liver disease or non-HCC subjects had CTCs detected. CTCs were identified in 69 of 85 (81%) HCC patients, with an average of 19 ± 24 CTCs per 5 mL Both the positivity rate and the number of CTCs were significantly associated with tumor size, portal vein tumor thrombus, differentiation status, and the disease extent as classified by TNM (tumor-node-metastasis). ERBB2 gene amplification and TP53 gene deletion were detected in CTCs [43] |
| Prostate carcinoma | Prospectively enrolled patients with metastatic, castration-resistant prostate cancer initiating taxane chemotherapy | Detection of AR-V7 in CTCs from men with metastatic castration resistant prostate cancer was not associated with primary resistance to taxane chemotherapy. In AR-V7-positive men, taxanes were more efficacious than androgen blockers enzalutamide or abiraterone therapy, whereas in AR-V7-negative men, taxanes and enzalutamide or abiraterone had comparable efficacy [44] |
| Melanoma | Phase III trial of adjuvant immunotherapy after complete resection of stage Iv melanoma | Pretreatment CTCs (>0 vs. O) status was associated with shorter disease free and overall survival. Serial CTCs was also associated with disease free survival [45] |
| CTCs | ctDNA |
|---|---|
| More steps for isolation than ctDNA | Easier to isolate than CTCs |
| Can be cultured and used for functional assays (both in vitro and in vivo) | Cannot be cultured |
| Can analyze DNA, RNA and protein | DNA is more stable than RNA |
| Sampling bias of captured cells (only cells of high affinity, size based) | Small quantities of ctDNA in circulation |
| Levels of CTCs can be used to predict response and resistance to therapy | Levels of ctDNA can be used to predict response and resistance to therapy |
| Heterogeneity can confound analysis | Cell death under therapy could modify ctDNA levels |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agashe, R.; Kurzrock, R. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers 2020, 12, 2361. https://doi.org/10.3390/cancers12092361
Agashe R, Kurzrock R. Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers. 2020; 12(9):2361. https://doi.org/10.3390/cancers12092361
Chicago/Turabian StyleAgashe, Ruchi, and Razelle Kurzrock. 2020. "Circulating Tumor Cells: From the Laboratory to the Cancer Clinic" Cancers 12, no. 9: 2361. https://doi.org/10.3390/cancers12092361
APA StyleAgashe, R., & Kurzrock, R. (2020). Circulating Tumor Cells: From the Laboratory to the Cancer Clinic. Cancers, 12(9), 2361. https://doi.org/10.3390/cancers12092361






