BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
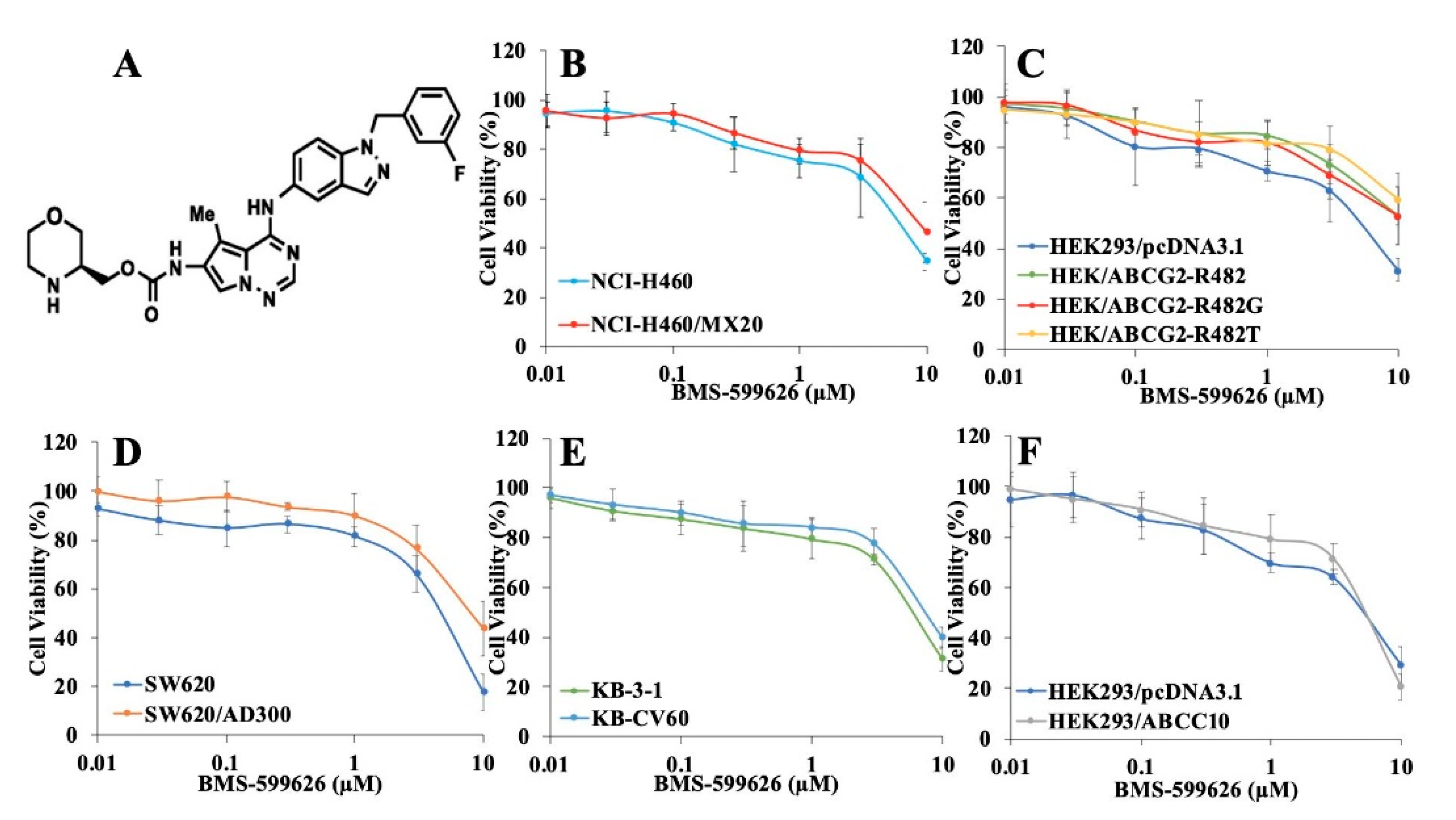
2.1. Cytotoxicity Study of BMS-599626 in Parental and ABCB1-, ABCG2-, ABCC1-, and ABCC10-Overexpressing Cells
2.2. BMS-599626 Increases the Sensitivity of ABCG2-Overexpressing Cells to the Substrates of ABCG2
2.3. BMS-599626 Increases the Sensitivity of ABCG2-Overexpressing Transfected Cells to the Substrates of ABCG2
2.4. BMS-599626 Does Not Sensitize ABCB1- or ABCC1-Overexpressing Cells to Their Respective Chemotherapeutic Agents, but Partially Sensitizes ABCC10-Overexpressing Cells
2.5. BMS-599626 Increases the Intracellular Accumulation of [3H]-Mitoxantrone in ABCG2-Overexpressing Cell Sublines
2.6. BMS-599626 Decreases the Efflux of [3H]-Mitoxantrone in ABCG2-Overexpressing Cells
2.7. BMS-599626 Does not Change the Total or Cell Membrane Expression of ABCG2
2.8. BMS-599626 Inhibits the ATPase of ABCG2
2.9. Molecular Docking Analysis of the Interaction between BMS-599626 and ABCG2: BMS-599626 Inhibits the ATPase Activity of ABCG2
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Cell Culture
4.3. Cytotoxicity Assay
4.4. [3H]-Mitoxantrone Accumulation Assay
4.5. [3H]-Mitoxantrone Efflux Assay
4.6. Immunoblotting
4.7. Immunofluorescence Assay
4.8. ATPase Assay
4.9. Molecular Docking Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amawi, H.; Sim, H.-M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. In Drug Transporters in Drug Disposition, Effects and Toxicity; Liu, X., Pan, G., Eds.; Springer: Singapore, 2019; Volume 1141, pp. 549–580. ISBN 9789811376467. [Google Scholar]
- Kathawala, R.J.; Gupta, P.; Ashby, C.R.; Chen, Z.-S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates 2015, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, M.P.; Rigalli, J.P.; Ceré, L.I.; Semeniuk, M.; Catania, V.A.; Ruiz, M.L. ABC Transporters: Regulation and Association with Multidrug Resistance in Hepatocellular Carcinoma and Colorectal Carcinoma. Curr. Med. Chem. 2019, 26, 1224–1250. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.L.; Palshof, J.; Brünner, N.; Stenvang, J.; Viuff, B.M. Implications of ABCG2 Expression on Irinotecan Treatment of Colorectal Cancer Patients: A Review. Int. J. Mol. Sci. 2017, 18, 1926. [Google Scholar] [CrossRef] [Green Version]
- Beretta, G.L.; Cassinelli, G.; Pennati, M.; Zuco, V.; Gatti, L. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur. J. Med. Chem. 2017, 142, 271–289. [Google Scholar] [CrossRef]
- Wen, Y.; Zhao, R.; Gupta, P.; Fan, Y.; Zhang, Y.; Huang, Z.; Li, X.; Su, Y.; Liao, L.; Xie, Y.-A.; et al. The epigallocatechin gallate derivative Y6 reverses drug resistance mediated by the ABCB1 transporter both in vitro and in vivo. Acta Pharm. Sin. B 2019, 9, 316–323. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Li, J.Y.; Teng, Q.-X.; Lei, Z.-N.; Ji, N.; Cui, Q.; Zeng, L.; Pan, Y.; Yang, D.-H.; Chen, Z.-S. Venetoclax, a BCL-2 Inhibitor, Enhances the Efficacy of Chemotherapeutic Agents in Wild-Type ABCG2-Overexpression-Mediated MDR Cancer Cells. Cancers 2020, 12, 466. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Cai, C.-Y.; Gao, H.-L.; Ren, L.; Ji, N.; Gupta, P.; Yang, Y.; Shukla, S.; Ambudkar, S.V.; Yang, D.-H.; et al. Glesatinib, a c-MET/SMO Dual Inhibitor, Antagonizes P-glycoprotein Mediated Multidrug Resistance in Cancer Cells. Front. Oncol. 2019, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Gao, H.-L.; Ashar, Y.; Karadkhelkar, N.; Yoganathan, S.; Chen, Z.-S. Ciprofloxacin Enhances the Chemosensitivity of Cancer Cells to ABCB1 Substrates. Int. J. Mol. Sci. 2019, 20, 268. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Fan, Y.-F.; Cai, C.-Y.; Wang, J.-Q.; Teng, Q.-X.; Lei, Z.-N.; Zeng, L.; Gupta, P.; Chen, Z.-S. Olmutinib (BI1482694/HM61713), a Novel Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, Reverses ABCG2-Mediated Multidrug Resistance in Cancer Cells. Front. Pharmacol. 2018, 9, 1097. [Google Scholar] [CrossRef] [Green Version]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC subfamily a transporters: Multifaceted players with incipient potentialities in cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020; Volume 60, pp. 57–71. [Google Scholar] [CrossRef]
- Gavai, A.V.; Fink, B.E.; Fairfax, D.J.; Martin, G.S.; Rossiter, L.M.; Holst, C.L.; Kim, S.-H.; Leavitt, K.J.; Mastalerz, H.; Han, W.-C.; et al. Discovery and preclinical evaluation of [4-[[1-(3-fluorophenyl) methyl]-1 H-indazol-5-ylamino]-5-methylpyrrolo [2, 1-f][1,2,4] triazin-6-yl] carbamic Acid,(3 S)-3-Morpholinylmethyl Ester (BMS-599626), a selective and orally efficacious inhibitor of human epidermal growth factor receptor 1 and 2 kinases. J. Med. Chem. 2009, 52, 6527–6530. [Google Scholar] [CrossRef]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2018, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Yang, Y.; Cai, C.-Y.; Lei, Z.-N.; Wang, J.-Q.; Gupta, P.; Shukla, S.; Ambudkar, S.V.; Kong, D.; Chen, Z.-S. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1- and ABCG2-overexpressing cancer cells. Cancer Lett. 2019, 440–441, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-N.; Zhang, Y.-K.; Wang, Y.-J.; Gupta, P.; Ashby, C.R.; Alqahtani, S.; Deng, T.; Bates, S.E.; Kaddoumi, A.; Wurpel, J.N.D.; et al. Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: In vitro and in vivo. Cancer Lett. 2018, 424, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, J.; Liu, J.; Xi, Y.; Zheng, Z.; To, K.K.W.; Chen, Z.; Wang, F.; Zhang, Y.; Fu, L. CM082 Enhances the Efficacy of Chemotherapeutic Drugs by Inhibiting the Drug Efflux Function of ABCG2. Mol. Ther. Oncolytics 2020, 16, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Zhang, Y.-K.; Zhang, X.-Y.; Wang, Y.-J.; Lu, K.W.; Hall, T.; Peng, R.; Yang, D.-H.; Xie, N.; Chen, Z.-S. Voruciclib, a Potent CDK4/6 Inhibitor, Antagonizes ABCB1 and ABCG2-Mediated Multi-Drug Resistance in Cancer Cells. Cell. Physiol. Biochem. 2018, 45, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Ambjørner, S.E.B.; Wiese, M.; Köhler, S.C.; Svindt, J.; Lund, X.L.; Gajhede, M.; Saaby, L.; Brodin, B.; Rump, S.; Weigt, H.; et al. The Pyrazolo [3, 4-d] pyrimidine Derivative, SCO-201, Reverses Multidrug Resistance Mediated by ABCG2/BCRP. Cells 2020, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-K.; Wang, Y.-J.; Gupta, P.; Chen, Z.-S. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Caetano-Pinto, P.; Jansen, J.; Assaraf, Y.G.; Masereeuw, R. The importance of breast cancer resistance protein to the kidneys excretory function and chemotherapeutic resistance. Drug Resist. Updates 2017, 30, 15–27. [Google Scholar] [CrossRef]
- Hasanabady, M.H.; Kalalinia, F. ABCG2 inhibition as a therapeutic approach for overcoming multidrug resistance in cancer. J. Biosci. 2016, 41, 313–324. [Google Scholar] [CrossRef]
- Yousaf, M.; Ali, M. Modulation of ABCG2 surface expression by Rab5 and Rab21 to overcome multidrug resistance in cancer cells. Xenobiotica 2020, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Khot, M.I.; Downey, C.L.; Armstrong, G.; Svavarsdottir, H.S.; Jarral, F.; Andrew, H.; Jayne, D.G. The role of ABCG2 in modulating responses to anti-cancer photodynamic therapy. Photodiagn. Photodyn. Ther. 2020, 29, 101579. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Lee, F.Y.; Yu, C.; Luo, F.R.; Oppenheimer, S.; Zhang, H.; Smykla, R.A.; Mastalerz, H.; Fink, B.E.; Hunt, J.T.; et al. Preclinical Antitumor Activity of BMS-599626, a pan-HER Kinase Inhibitor That Inhibits HER1/HER2 Homodimer and Heterodimer Signaling. Clin. Cancer Res. 2006, 12, 6186–6193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Yang, Y.; Wang, G.; Wang, J.; Teng, Q.; Sun, L.; Lei, Z.; Lin, L.; Chen, Z.; Zou, C. Dual TTK/CLK2 inhibitor, CC-671, selectively antagonizes ABCG2-mediated multidrug resistance in lung cancer cells. Cancer Sci. 2020, 111, 2872–2882. [Google Scholar] [CrossRef]
- Ahmed-Belkacem, A.; Pozza, A.; Macalou, S.; Boumendjel, A.; Di Pietro, A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2). Anti Cancer Drugs 2006, 17, 239–243. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Benndorf, R.A. 3D structure of the transporter ABCG2—What’s new? Br. J. Pharmacol. 2020, 177, 1485–1496. [Google Scholar] [CrossRef] [Green Version]
- Shafran, A.; Ifergan, I.; Bram, E.; Jansen, G.; Kathmann, I.; Peters, G.J.; Robey, R.W.; Bates, S.E.; Assaraf, Y.G. ABCG2 Harboring the Gly482 Mutation Confers High-Level Resistance to Various Hydrophilic Antifolates. Cancer Res. 2005, 65, 8414–8422. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Ripperger, A.; Krischer, A.; Robaa, D.; Sippl, W.; Benndorf, R.A. Pharmacogenetic Aspects of the Interaction of AT1 Receptor Antagonists with ATP-Binding Cassette Transporter ABCG2. Front. Pharmacol. 2018, 9, 463. [Google Scholar] [CrossRef]
- Zámbó, B.; Bartos, Z.; Mózner, O.; Szabó, E.; Várady, G.; Poór, G.; Pálinkás, M.; Andrikovics, H.; Hegedűs, T.; Homolya, L.; et al. Clinically relevant mutations in the ABCG2 transporter uncovered by genetic analysis linked to erythrocyte membrane protein expression. Sci. Rep. 2018, 8, 7487. [Google Scholar] [CrossRef]
- Choi, C.-H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodani, K.; Tiwari, A.K.; Singh, S.; Patel, A.; Xiao, Z.-J.; Chen, J.-J.; Sun, Y.-L.; Talele, T.T.; Chen, Z.-S. GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance. Biochem. Pharmacol. 2012, 83, 1613–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Ma, L.-Y.; Wang, J.-Q.; Lei, Z.-N.; Gupta, P.; Zhao, Y.-D.; Li, Z.-H.; Liu, Y.; Zhang, X.-H.; Li, Y.-N.; et al. Discovery of 5-Cyano-6-phenylpyrimidin Derivatives Containing an Acylurea Moiety as Orally Bioavailable Reversal Agents against P-Glycoprotein-Mediated Mutidrug Resistance. J. Med. Chem. 2018, 61, 5988–6001. [Google Scholar] [CrossRef] [PubMed]
- Ejendal, K.F.K.; Diop, N.K.; Schweiger, L.C.; Hrycyna, C.A. The nature of amino acid 482 of human ABCG2 affects substrate transport and ATP hydrolysis but not substrate binding. Protein Sci. 2006, 15, 1597–1607. [Google Scholar] [CrossRef] [Green Version]
- Gillet, J.-P.; Gottesman, M.M. Mechanisms of Multidrug Resistance in Cancer. In Multi-Drug Resistance in Cancer; Zhou, J., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 596, pp. 47–76. ISBN 978-1-60761-415-9. [Google Scholar]
- Aoki, S.; Chen, Z.S.; Higasiyama, K.; Setiawan, A.; Akiyama, S.; Kobayashi, M. Reversing effect of agosterol A, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Jpn. J. Cancer Res. 2001, 92, 886–895. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Wei, L.; Anreddy, N.; Chen, K.; Patel, A.; Alqahtani, S.; Zhang, Y.-K.; Wang, Y.-J.; Sodani, K.; Kaddoumi, A.; et al. The small molecule tyrosine kinase inhibitor NVP-BHG712 antagonizes ABCC10-mediated paclitaxel resistance: A preclinical and pharmacokinetic study. Oncotarget 2015, 6, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Ambudkar, S.V. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1998; Volume 292, pp. 504–514. [Google Scholar] [CrossRef]








| Treatment | IC50 ± SD a (μM) (FR b) | |
| SW620 | SW620/AD300 | |
| Doxorubicin | 0.09 ± 0.006 (1) | 20.58 ± 2.11 (228.67) |
| +100 nM BMS-599626 | 0.09 ± 0.009 (1) | 19.85 ± 1.08 (220.55) |
| +300 nM BMS-599626 | 0.09 ± 0.008 (1) | 17.67 ± 2.01 (196.33) |
| +300 nM Verapamil | 0.09 ± 0.009 (1) | 5.95 ± 0.06 (66.11) * |
| Treatment | IC50 ± SD a (μM) (FR b) | |
| KB-3-1 | KB-CV60 | |
| Vincristine | 0.09 ± 0.009 (1) | 41.99 ± 4.23 (446.55) |
| +100 nM BMS-599626 | 0.09 ± 0.004 (1) | 31.57 ± 3.27 (350.77) |
| +300 nM BMS-599626 | 0.08 ± 0.006 (0.89) | 29.82 ± 3.01 (331.33) |
| +25 μM MK571 | 0.09 ± 0.008 (1) | 3.09 ± 0.04 (34.33) * |
| Treatment | IC50 ± SD a (μM) (FR b) | |
| HEK293/pcDNA3.1 | HEK293/ABCC10 | |
| Vincristine | 0.08 ± 0.009 (1) | 1.09 ± 0.02 (13.62) |
| +100 nM BMS-599626 | 0.09 ± 0.10 (1.1) | 0.61 ± 0.005 (7.62) |
| +300 nM BMS-599626 | 0.07 ± 0.008 (0.87) | 0.50 ± 0.007 (6.25) |
| +300 nM Cepharathine | 0.07 ± 0.006 (0.87) | 0.23 ± 0.003 (2.87) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashar, Y.V.; Zhou, J.; Gupta, P.; Teng, Q.-X.; Lei, Z.-N.; Reznik, S.E.; Lusvarghi, S.; Wurpel, J.; Ambudkar, S.V.; Chen, Z.-S. BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance. Cancers 2020, 12, 2502. https://doi.org/10.3390/cancers12092502
Ashar YV, Zhou J, Gupta P, Teng Q-X, Lei Z-N, Reznik SE, Lusvarghi S, Wurpel J, Ambudkar SV, Chen Z-S. BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance. Cancers. 2020; 12(9):2502. https://doi.org/10.3390/cancers12092502
Chicago/Turabian StyleAshar, Yunali V., Jingchun Zhou, Pranav Gupta, Qiu-Xu Teng, Zi-Ning Lei, Sandra E. Reznik, Sabrina Lusvarghi, John Wurpel, Suresh V. Ambudkar, and Zhe-Sheng Chen. 2020. "BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance" Cancers 12, no. 9: 2502. https://doi.org/10.3390/cancers12092502
APA StyleAshar, Y. V., Zhou, J., Gupta, P., Teng, Q.-X., Lei, Z.-N., Reznik, S. E., Lusvarghi, S., Wurpel, J., Ambudkar, S. V., & Chen, Z.-S. (2020). BMS-599626, a Highly Selective Pan-HER Kinase Inhibitor, Antagonizes ABCG2-Mediated Drug Resistance. Cancers, 12(9), 2502. https://doi.org/10.3390/cancers12092502






