Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participating Centers and Total Patients
2.2. Data Collection, Division of Patients into Groups, and Comparative Analysis
2.2.1. Propensity Score Matching (PSM) Analysis
2.2.2. Inverse Probability Treatment Weighted Analysis
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Morise, Z.; Kawabe, N.; Tomishige, H.; Nagata, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 14381–14392. [Google Scholar] [CrossRef]
- Itamoto, T.; Nakahara, H.; Amano, H.; Kohashi, T.; Ohdan, H.; Tashiro, H.; Asahara, T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 2007, 141, 589–597. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Gamblin, T.C.; Geller, D.A. World review of laparoscopic liver resection-2804 patients. Ann. Surg. 2009, 250, 831–841. [Google Scholar] [CrossRef]
- Buell, J.F.; Thomas, M.T.; Rudich, S.; Marvin, M.; Nagubandi, R.; Ravindra, K.V.; McMasters, K.M. Experience with more than 500 minimally inva-sive hepatic procedures. Ann. Surg. 2008, 248, 475–486. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Wakabayashi, G. First quarter century of laparoscopic liver resection. World J. Gastroenterol. 2017, 23, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Tanabe, M.; Wakabayashi, G.; Cheung, T.T.; Lo, C.M.; et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. BJS 2020, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.; Tanabe, M.; Ito, H.; Otsuka, Y.; Nitta, H.; Abe, Y.; Hasegawa, Y.; Katagiri, T.; Takagi, C.; Itano, O.; et al. A novel difficulty scoring system for laparoscopic liver resection. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 745–753. [Google Scholar] [CrossRef]
- Xu, H.-W.; Li, H.-Y.; Liu, F.; Wei, Y.-G.; Li, B. Laparoscopic Versus Open Liver Resection for Lesions Adjacent to Major Vessels: A Propensity Score Matched Analysis. J. Laparoendosc. Adv. Surg. Technol. 2017, 27, 1002–1008. [Google Scholar] [CrossRef]
- Takahara, T.; Wakabayashi, G.; Beppu, T.; Aihara, A.; Hasegawa, K.; Gotohda, N.; Hatano, E.; Tanahashi, Y.; Mizuguchi, T.; Kamiyama, T.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Beppu, T.; Wakabayashi, G.; Hasegawa, K.; Go, W.; Mizuguchi, T.; Takahashi, Y.; Hirokawa, F.; Taniai, N.; Watanabe, M.; Katou, M.; et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: A multi-institutional Japanese study. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resec-tion? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef]
- Morise, Z. Status and perspective of laparoscopic repeat liver resection. World J. Hepatol. 2018, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Belli, G.; Cioffi, L.; Fantini, C.; D’Agostino, A.; Russo, G.; Limongelli, P.; Belli, A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: Feasibility, safety, and results. Surg. Endosc. 2009, 23, 1807–1811. [Google Scholar] [CrossRef]
- Shafaee, Z.; Kazaryan, A.M.; Marvin, M.R.; Cannon, R.; Buell, J.F.; Edwin, B.; Gayet, B. Is Laparoscopic Repeat Hepatectomy Feasible? A Tri-institutional Analysis. J. Am. Coll. Surg. 2011, 212, 171–179. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, G.; Xu, D.; Liu, R. Laparoscopic Repeat Resection of Recurrent Hepatocellular Carcinoma. World J. Surg. 2010, 35, 648–655. [Google Scholar] [CrossRef]
- Kanazawa, A.; Tsukamoto, T.; Shimizu, S.; Kodai, S.; Yamamoto, S.; Yamazoe, S.; Nakajima, T. Laparoscopic liver resection for treating re-current hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2013, 20, 512–517. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Felli, E.; Memeo, R.; Mascagni, P.; Abe, Y.; Kitagawa, Y.; Pessaux, P. Short-term outcomes of laparoscopic repeat liver resection after open liver resection: A systematic review. Surg. Endosc. 2019, 33, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, M.J.; Barkhatov, L.; Fuks, D.; Berardi, G.; Cipriani, F.; Aljaiuossi, A.; Lainas, P.; Dagher, I.; D’Hondt, M.; Rotellar, F.; et al. Multicentre propensity score-matched study of laparoscopic versus open repeat liver resection for colorectal liver metastases. Br. J. Surg. 2019, 106, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Sa Cunha, A.; Cherqui, D.; Ayet, B.; Goéré, D.; Bachellier, P.; Pessaux, P. French Colorectal Liver Metastases Working Group, As-sociation Française de Chirurgie. Laparoscopic compared to open repeat hepatectomy for colorectal liver metastases: A mul-ti-institutional propensity-matched analysis of short- and long-term outcomes. World J. Surg. 2017, 41, 3189–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Zhou, Z.; Xiao, W.; Hu, X.; Cao, J.; Mao, S. Systematic review and meta-analysis of laparoscopic versus open repeat hepa-tectomy for recurrent liver cancer. Surg. Oncol. 2019, 28, 19–30. [Google Scholar] [CrossRef] [PubMed]
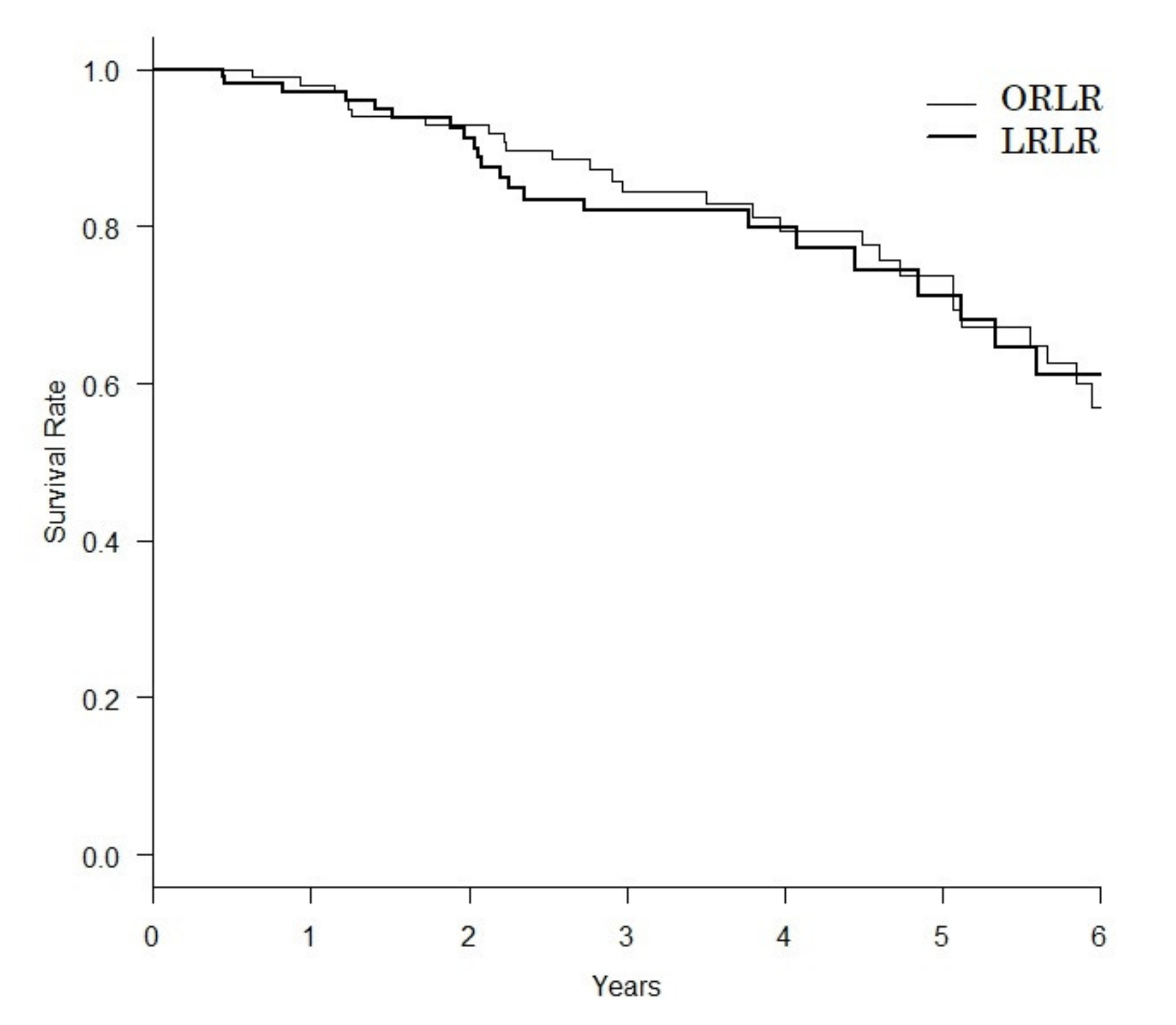
| Backgrounds | ORLR (n = 287) | LRLR (n = 328) | p Value |
|---|---|---|---|
| Age (years) | 67.3 ± 9.8 | 67.7 ± 10.8 | 0.665 |
| Sex ratio (Male:Female) | 222:61 | 252:74 | 0.734 |
| BMI (kg/m2) | 23.1 ± 3.6 | 23.9 ± 3.7 | 0.009 |
| Performance status | |||
| 0 | 249 | 256 | 0.022 |
| 1 | 36 | 71 | |
| 2 | 1 | 3 | |
| 3 | 1 | 2 | |
| Number of tumors | 1.47 ± 1.13 | 1.22 ± 0.60 | <0.001 |
| Tumor size (mm) | 17.5 ± 11.5 | 16.3 ± 12.0 | 0.222 |
| Tumor location | |||
| Anterolateral | 178 | 247 | 0.001 |
| Posterosuperior | 109 | 85 | |
| Background liver pathology | |||
| NL | 30 | 40 | <0.001 |
| CH | 52 | 21 | |
| LF | 88 | 103 | |
| LC | 100 | 166 | |
| Total Billirubin (mg/dL) | 0.79 ± 0.44 | 0.82 ± 0.53 | 0.404 |
| Prothronbin time (C-P score) | |||
| 1:>70% (<1.7) | 269 | 313 | 0.983 |
| 2: 40–70% (1.7–2.3) | 7 | 8 | |
| 3:<40 (>2.3) | 3 | 3 | |
| Creatinine(mg/dL) | 0.88 ± 0.68 | 0.90 ± 0.60 | 0.606 |
| Albumin(g/L) | 4.05 ± 0.44 | 4.01 ± 0.47 | 0.255 |
| Platelet count (×103/) μL | 165.5 ± 180.9 | 216.1 ± 350.7 | 0.028 |
| ICG R15 | 14.6 ± 8.4 | 15.1 ± 10.1 | 0.537 |
| Ascites (C-P score) | |||
| 1 | 282 | 314 | 0.011 |
| 2 | 5 | 19 | |
| 3 | 0 | 0 | |
| Encephalopathy (C-P score) | |||
| 1 | 286 | 331 | 0.652 |
| 2 | 1 | 2 | |
| 3 | 0 | 0 | |
| Varices (C-P score) | |||
| 1 | 256 | 288 | 0.033 |
| 2 | 11 | 29 | |
| 3 | 6 | 12 | |
| Extent of resection | |||
| Partial resection/segmentectomy | 252 | 309 | 0.008 |
| Sectionectomy | 20 | 20 | |
| ≥2 sections | 14 | 3 | |
| Previous surgery | |||
| Open Liver resection | 241 | 120 | <0.001 |
| Laparoscopic liver resection | 23 | 149 | |
| Outcomes | ORLR (n = 286) | LRLR (n = 328) | p Value |
|---|---|---|---|
| Intraoperative Blood Loss (mL) | 629.0 ± 882.3 | 246.6 ± 570.5 | <0.001 |
| Blood transfusion | |||
| No | 243 | 306 | 0.008 |
| Yes | 40 | 25 | |
| Operation time (min) | 261.1 ± 159.6 | 235.2 ± 144.7 | 0.036 |
| 90-day morbidity ≥ CD II | |||
| No | 243 | 296 | 0.059 |
| Yes | 42 | 32 | |
| 90-day morbidity ≥ CD IIIa | |||
| No | 260 | 316 | 0.008 |
| Yes | 25 | 12 | |
| 90-day mortality | |||
| No | 282 | 326 | 0.253 |
| Yes | 3 | 1 | |
| Postoperative hospital stay (days) | 13.5 ± 11.6 | 9.6 ± 8.7 | <0.001 |
| Backgrounds | ORLR (n = 115) | LRLR (n = 115) | p Value |
|---|---|---|---|
| Age (years old) | 67.5 ± 9.5 | 68.2 ± 10.3 | 0.565 |
| Sex ratio (Male: Female) | 94:21 | 91:24 | 0.618 |
| BMI (kg/m2) | 23.6 ± 3.5 | 23.6 ± 3.6 | 0.933 |
| Performance status | |||
| 0 | 103 | 99 | 0.367 |
| 1 | 11 | 16 | |
| 2 | 1 | 0 | |
| 3 | 0 | 0 | |
| Number of tumors | 1.45 ± 1.05 | 1.28 ± 0.82 | 0.164 |
| Tumor size (mm) | 14.5 ± 9.0 | 14.9 ± 12.7 | 0.792 |
| Tumor location | |||
| Anterolateral | 72 | 83 | 0.122 |
| Posterosuperior | 43 | 32 | |
| Liver fibrosis | |||
| 1 NL | 11 | 16 | <0.001 |
| 2 CH | 28 | 4 | |
| 3 LF | 37 | 51 | |
| 4 LC | 39 | 44 | |
| Total Billirubin (mg/dL) | 0.76 ± 0.36 | 0.82 ± 0.37 | 0.258 |
| Prothronbin time (C-P score) | |||
| 1:>70% (<1.7) | 109 | 109 | 0.842 |
| 2: 40–70% (1.7–2.3) | 4 | 3 | |
| 3:<40 (>2.3) | 2 | 3 | |
| Creatinine(mg/dL) | 0.85 ± 0.62 | 0.90 ± 0.82 | 0.667 |
| Albumin(g/L) | 4.09 ± 0.40 | 4.04 ± 0.46 | 0.382 |
| Platelet count (×103/) μL | 145.0 ± 77.0 | 150.9 ± 157.1 | 0.715 |
| ICG R15 | 15.6 ± 9.2 | 14.5 ± 10.3 | 0.378 |
| Ascites (C-P score) | |||
| 1 | 115 | 113 | 0.155 |
| 2 | 0 | 2 | |
| 3 | 0 | 0 | |
| Encephalopathy (C-P score) | |||
| 1 | 115 | 115 | - |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| Varices (C-P score) | |||
| 1 | 109 | 109 | 1.000 |
| 2 | 5 | 5 | |
| 3 | 1 | 1 | |
| Extent of resection | |||
| Partial resection/segmentectomy | 103 | 108 | 0.332 |
| Sectionectomy | 8 | 6 | |
| ≥2 sections | 4 | 1 | |
| Previous surgery | |||
| Open Liver resection | 101 | 90 | 0.053 |
| Laparoscopic liver resection | 14 | 25 | |
| Outcomes | ORLR (n = 115) | LRLR (n = 115) | p Value |
|---|---|---|---|
| Intraoperative Blood Loss (mL) | 603.5 ± 664.9 | 283.3 ± 823.0 | 0.001 |
| Blood transfusion | |||
| No | 99 | 103 | 0.420 |
| Yes | 16 | 12 | |
| Operation time (min) | 270.0 ± 129.6 | 260.6 ± 158.3 | 0.623 |
| 90-day morbidity ≥ CD II | |||
| No | 94 | 105 | 0.034 |
| Yes | 21 | 10 | |
| 90-day morbidity ≥ CD IIIa | |||
| No | 101 | 110 | 0.031 |
| Yes | 14 | 5 | |
| 90-day mortality | |||
| No | 115 | 115 | - |
| Yes | 0 | 0 | |
| Postoperative hospital stay (days) | 13.2 ± 12.1 | 10.2 ± 11.3 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyama, A.; Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Cheung, T.-T.; Lo, C.-M.; Tanaka, S.; Kubo, S.; Okamura, Y.; et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers 2021, 13, 3187. https://doi.org/10.3390/cancers13133187
Miyama A, Morise Z, Aldrighetti L, Belli G, Ratti F, Cheung T-T, Lo C-M, Tanaka S, Kubo S, Okamura Y, et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers. 2021; 13(13):3187. https://doi.org/10.3390/cancers13133187
Chicago/Turabian StyleMiyama, Arimasa, Zenichi Morise, Luca Aldrighetti, Giulio Belli, Francesca Ratti, Tan-To Cheung, Chung-Mau Lo, Shogo Tanaka, Shoji Kubo, Yukiyasu Okamura, and et al. 2021. "Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels" Cancers 13, no. 13: 3187. https://doi.org/10.3390/cancers13133187
APA StyleMiyama, A., Morise, Z., Aldrighetti, L., Belli, G., Ratti, F., Cheung, T.-T., Lo, C.-M., Tanaka, S., Kubo, S., Okamura, Y., Uesaka, K., Monden, K., Sadamori, H., Hashida, K., Kawamoto, K., Gotohda, N., Chen, K., Kanazawa, A., Takeda, Y., ... Wakabayashi, G. (2021). Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers, 13(13), 3187. https://doi.org/10.3390/cancers13133187












