Next-Generation Molecular Imaging of Thyroid Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Transporter-Targeting Probes
2.1. Sodium Iodine Symporter (NIS)-Targeting Probes
2.1.1. Radioiodine
2.1.2. [18F]Tetrafluoroborate ([18F]TFB)
2.2. Glucose Transporter-Targeting Probes
2.3. Amino Acid Transporter-Targeting Probes
2.3.1. [18F]FDOPA
2.3.2. [11C]MET
2.3.3. [18F]FGln
2.4. Nucleoside Transporter-Targeting Probes
3. Peptide-Based Probes
3.1. Somatostatin Receptor (SSTR)-Targeting Probes
3.2. αvβ3 Integrin-Targeting Probes
3.3. PSMA-Targeting Probes
3.4. Cholecystokinin-2 Receptor (CCK2R)-Targeting Probes
4. Antibody-Based Probes
4.1. Single Target Immunoglobulin G (IgG) Probes
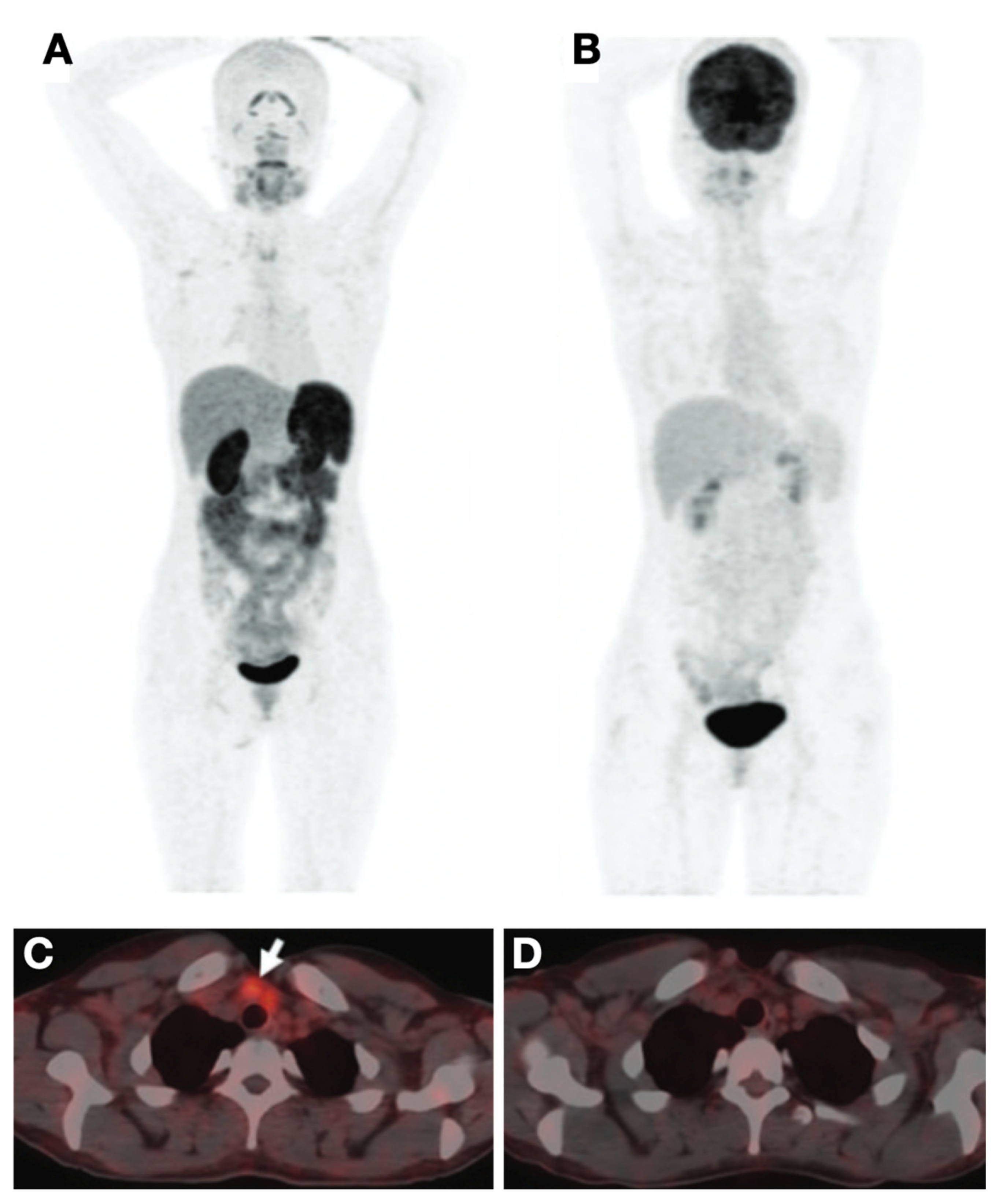
4.1.1. Epidermal Growth Factor Receptor (HER2, ERBB2)-Targeting Probes
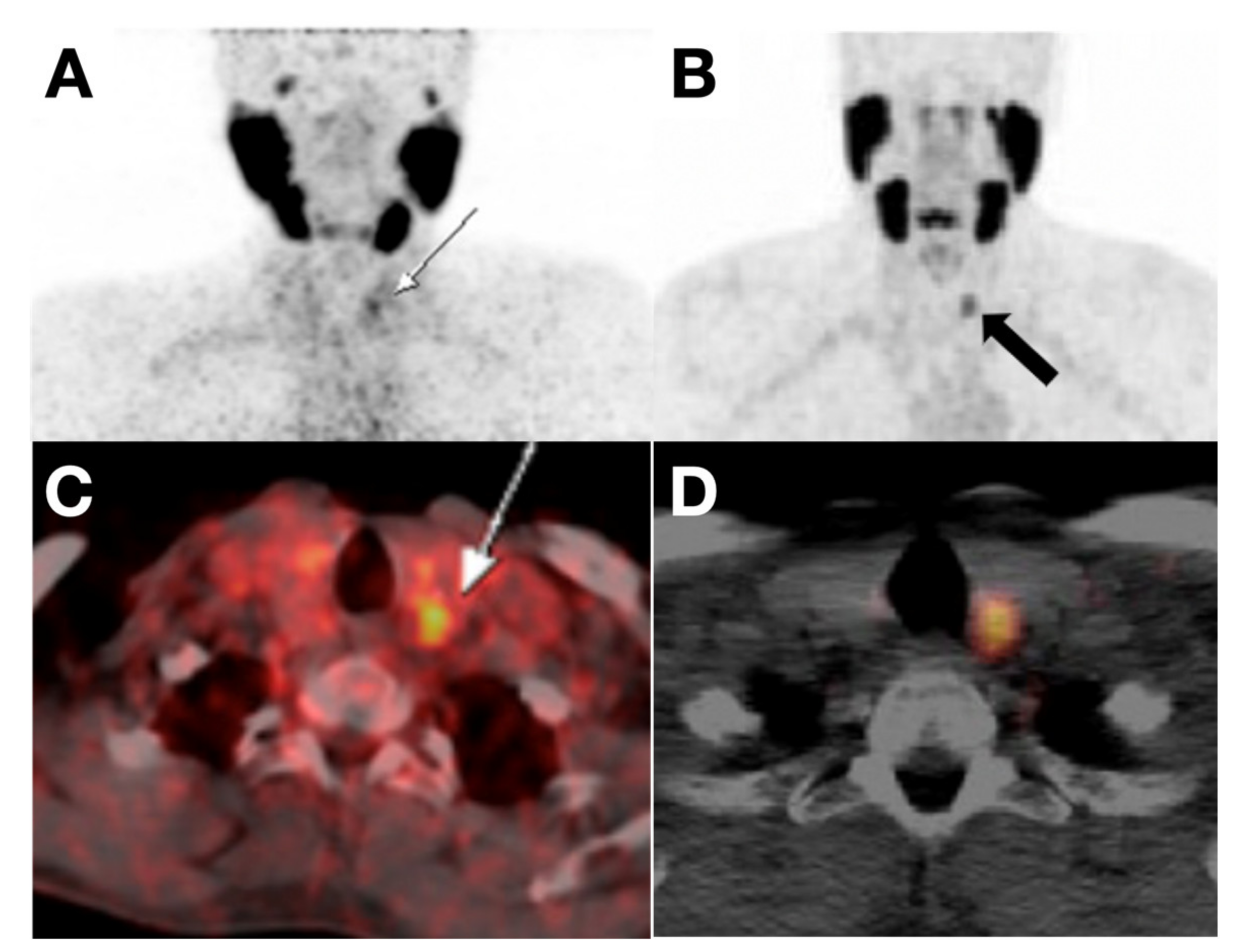
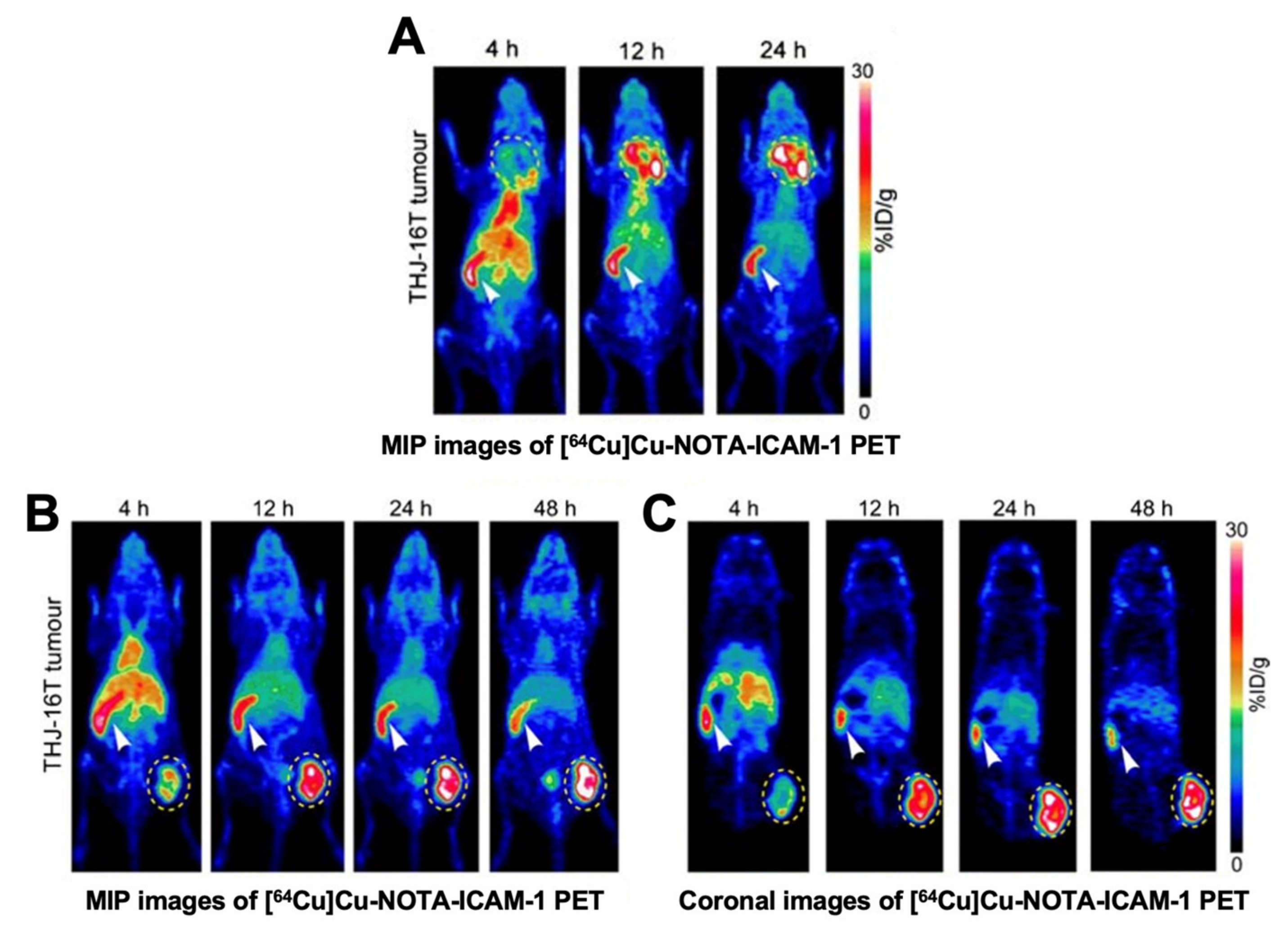
4.1.2. Intercellular Adhesion Molecule-1 (ICAM-1, CD54)-Targeting Probes
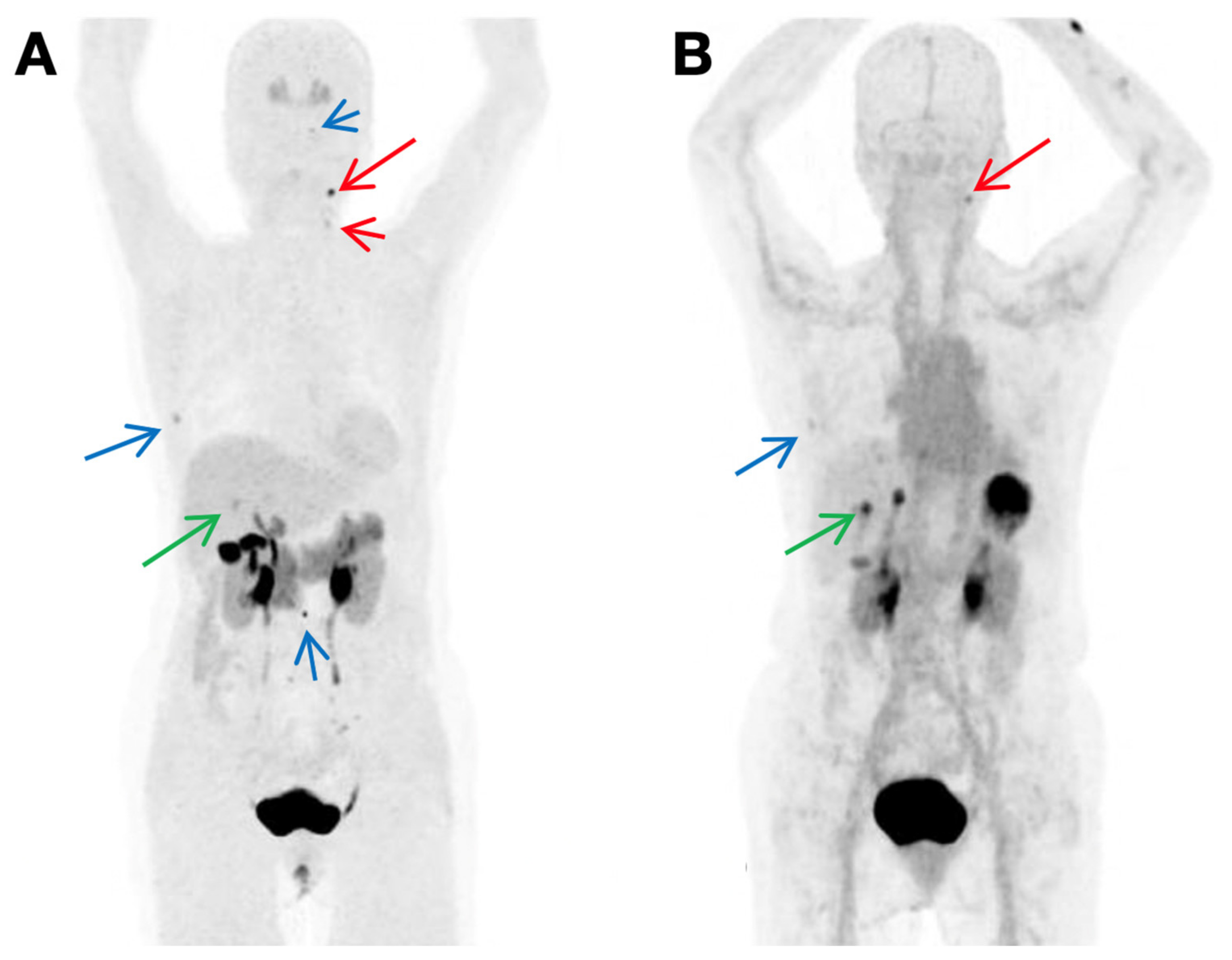
4.1.3. Lectin Galactoside-Binding Soluble 3 (LGALS3, Galectin-3, or Gal3)-Targeting Probes
4.2. Bispecific IgG Probes
4.3. Fab-Based Probes
4.4. Nanobody-Based Probes
5. Other Probes
5.1. Aptamer-Based Probes
5.1.1. Prominin 1 (PROM1, CD133)-Targeting Probes
5.1.2. PTC Tissue-Targeting Probes
5.2. Nanoparticles-Based Probes
5.2.1. EGFR-Targeting Probes
5.2.2. Protein Tyrosine Phosphatase Non-Receptor Type 11 (PTPN11, SHP2)-Targeting Probes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 2017, 317, 1338. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Vieja, A.; Riesco-Eizaguirre, G. Radio-Iodide Treatment: From Molecular Aspects to the Clinical View. Cancers 2021, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Schlumberger, M.; Leboulleux, S. Current Practice in Patients with Differentiated Thyroid Cancer. Nat. Rev. Endocrinol. 2021, 17, 176–188. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; Vathaire, F.D.; et al. Long-Term Outcome of 444 Patients with Distant Metastases from Papillary and Follicular Thyroid Carcinoma: Benefits and Limits of Radioiodine Therapy. J. Clin Endocrinol Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- ACS Thyroid Cancer Survival Rates, by Type and Stage. Available online: https://www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 5 June 2021).
- Lin, B.; Ma, H.; Ma, M.; Zhang, Z.; Sun, Z.; Hsieh, I.-Y.; Okenwa, O.; Guan, H.; Li, J.; Lv, W. The Incidence and Survival Analysis for Anaplastic Thyroid Cancer: A SEER Database Analysis. Am. J. Transl. Res. 2019, 11, 5888–5896. [Google Scholar]
- James, M.L.; Gambhir, S.S. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [Green Version]
- Wahl, R.L.; Chareonthaitawee, P.; Clarke, B.; Drzezga, A.; Lindenberg, L.; Rahmim, A.; Thackeray, J.; Ulaner, G.A.; Weber, W.; Zukotynski, K.; et al. Mars Shot for Nuclear Medicine, Molecular Imaging, and Molecularly Targeted Radiopharmaceutical Therapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 6–14. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Foppiani, L.; Treglia, G.; Ferrarazzo, G.; Massollo, M.; Bottoni, G.; Giovanella, L. PET/CT in Thyroid Nodule and Differentiated Thyroid Cancer Patients. The Evidence-Based State of the Art. Rev. Endocr. Metab. Disord. 2019, 20, 47–64. [Google Scholar] [CrossRef]
- Kendler, D.B.; Araújo, M.L., Jr.; Alencar, R.; Accioly, M.T.D.S.; Bulzico, D.A.; Pessoa, C.C.D.N.; Accioly, F.A.; De Farias, T.P.; Lopes, F.P.P.L.; Corbo, R.; et al. Somatostatin Receptor Subtype 1 Might Be a Predictor of Better Response to Therapy in Medullary Thyroid Carcinoma. Endocrine 2017, 58, 474–480. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on Molecular Imaging and Theranostics in Neuroendocrine Neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Ge, M.H.; Zhu, X.H.; Shao, Y.M.; Wang, C.; Huang, P.; Wang, Y.; Jiang, Y.; Maimaitiyiming, Y.; Chen, E.; Yang, C.; et al. Synthesis and Characterization of CD133 Targeted Aptamer–Drug Conjugates for Precision Therapy of Anaplastic Thyroid Cancer. Biomater. Sci. 2020. [Google Scholar] [CrossRef]
- Zhong, W.; Pu, Y.; Tan, W.; Liu, J.; Liao, J.; Liu, B.; Chen, K.; Yu, B.; Hu, Y.; Deng, Y.; et al. Identification and Application of an Aptamer Targeting Papillary Thyroid Carcinoma Using Tissue-SELEX. Anal. Chem. 2019, 91, 8289–8297. [Google Scholar] [CrossRef]
- Zhu, C.; Li, L.; Fang, S.; Zhao, Y.; Zhao, L.; Yang, G.; Qu, F. Selection and Characterization of an SsDNA Aptamer against Thyroglobulin. Talanta 2021, 223, 121690. [Google Scholar] [CrossRef]
- Kumarasamy, J.; Ghorui, S.K.; Gholve, C.; Jain, B.; Dhekale, Y.; Gupta, G.D.; Damle, A.; Banerjee, S.; Rajan, M.G.R.; Kulkarni, S. Production, Characterization and in-Vitro Applications of Single-Domain Antibody against Thyroglobulin Selected from Novel T7 Phage Display Library. J. Immunol. Methods 2021, 492, 112990. [Google Scholar] [CrossRef]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Achmad, A.; Bhattarai, A.; Yudistiro, R.; Heryanto, Y.D.; Higuchi, T.; Tsushima, Y. The Diagnostic Performance of 18F-FAMT PET and 18F-FDG PET for Malignancy Detection: A Meta-Analysis. BMC Med. Imaging 2017, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Enomoto, K.; Hotomi, M. Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer. Endocrinol. Metab. 2020, 35, 227–236. [Google Scholar] [CrossRef]
- Fu, H.; Sa, R.; Cheng, L.; Jin, Y.; Qiu, X.; Liu, M.; Chen, L. An updated review on nuclear molecular imaging of thyroid cancers. Endocr. Pract. 2020, 27, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− Symporter (NIS): Mechanism and Medical Impact. Endocr. Rev. 2013, 35, 106–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, G.; Tonacchera, M.; Grosso, M.; Orsolini, F.; Vitti, P.; Strauss, H.W. The Role of Nuclear Medicine in the Clinical Management of Benign Thyroid Disorders, Part 1: Hyperthyroidism. J. Nucl. Med. 2021, 62, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Ahn, B.-C. Molecular Mechanisms of Radioactive Iodine Refractoriness in Differentiated Thyroid Cancer: Impaired Sodium Iodide Symporter (NIS) Expression Owing to Altered Signaling Pathway Activity and Intracellular Localization of NIS. Theranostics 2021, 11, 6251–6277. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Nostrand, D.V.; Cheng, L.; Liu, M.; Chen, L. Radioiodine Refractory Differentiated Thyroid Cancer. Crit. Rev. Oncol. Hemat. 2018, 125, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nagarajah, J.; Janssen, M.; Hetkamp, P.; Jentzen, W. Iodine Symporter Targeting with 124 I/ 131 I Theranostics. J. Nucl. Med. 2017, 58, 34S–38S. [Google Scholar] [CrossRef] [Green Version]
- Wyszomirska, A. Iodine-131 for Therapy of Thyroid Diseases. Physical and Biological Basis. Nucl. Med. Rev. Cent. East. Eur. 2012, 15, 120–123. [Google Scholar]
- Gulec, S.A.; Kuker, R.A.; Goryawala, M.; Fernandez, C.; Perez, R.; Khan-Ghany, A.; Apaza, A.; Harja, E.; Harrell, M. 124 I PET/CT in Patients with Differentiated Thyroid Cancer: Clinical and Quantitative Image Analysis. Thyroid 2016, 26, 441–448. [Google Scholar] [CrossRef]
- Jentzen, W.; Verschure, F.; van Zon, A.; van de Kolk, R.; Wierts, R.; Schmitz, J.; Bockisch, A.; Binse, I. 124I PET Assessment of Response of Bone Metastases to Initial Radioiodine Treatment of Differentiated Thyroid Cancer. J. Nucl. Med. 2016, 57, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Ruhlmann, M.; Jentzen, W.; Ruhlmann, V.; Pettinato, C.; Rossi, G.; Binse, I.; Bockisch, A.; Rosenbaum-Krumme, S. High Level of Agreement Between Pretherapeutic 124I PET and Intratherapeutic 131I Imaging in Detecting Iodine-Positive Thyroid Cancer Metastases. J. Nucl. Med. 2016, 57, 1339–1342. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ruan, M.; Cheng, L.; Fu, H.; Liu, M.; Sheng, S.; Chen, L. Radioiodine Uptake and Thyroglobulin-Guided Radioiodine Remnant Ablation in Patients with Differentiated Thyroid Cancer: A Prospective, Randomized, Open-Label, Controlled Trial. Thyroid 2019, 29, 101–110. [Google Scholar] [CrossRef]
- Simon, D.; Körber, C.; Krausch, M.; Segering, J.; Groth, P.; Görges, R.; Grünwald, F.; Müller-Gärtner, H.; Schmutzler, C.; Köhrle, J.; et al. Clinical Impact of Retinoids in Redifferentiation Therapy of Advanced Thyroid Cancer: Final Results of a Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 775–782. [Google Scholar] [CrossRef]
- Plantinga, T.S.; Heinhuis, B.; Gerrits, D.; Netea, M.G.; Joosten, L.A.B.; Hermus, A.R.M.M.; Oyen, W.J.G.; Schweppe, R.E.; Haugen, B.R.; Boerman, O.C.; et al. MTOR Inhibition Promotes TTF1-Dependent Redifferentiation and Restores Iodine Uptake in Thyroid Carcinoma Cell Lines. J. Clin. Endocrinol. Metab. 2014, 99, E1368–E1375. [Google Scholar] [CrossRef] [Green Version]
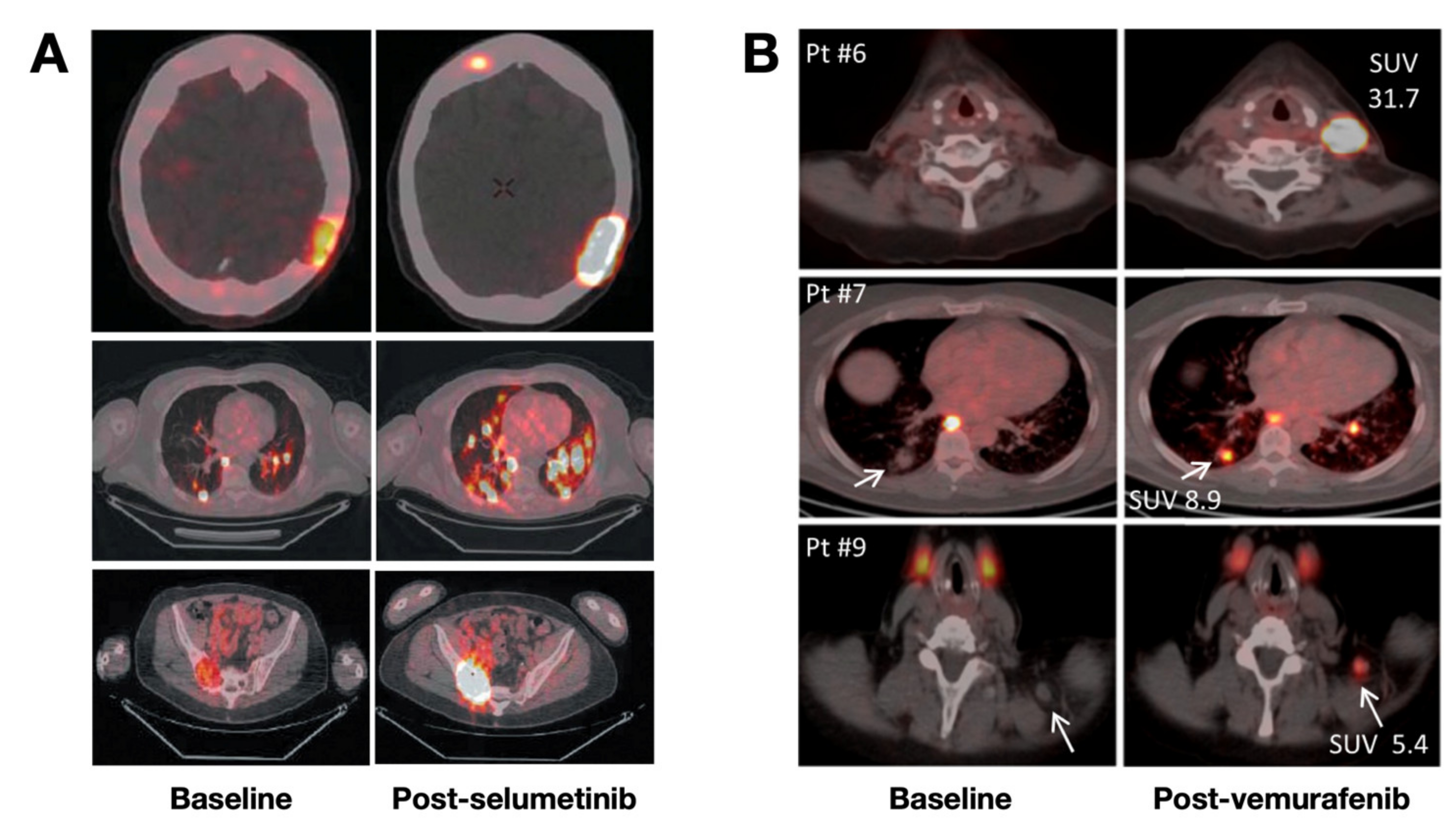
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.A.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J. Clin. Endocrinol. Metab. 2018, 104, 1417–1428. [Google Scholar] [CrossRef]
- Vaisman, F.; Carvalho, D.P.; Vaisman, M. A New Appraisal of Iodine Refractory Thyroid Cancer. Endocr. Relat. Cancer 2015, 22, R301–R310. [Google Scholar] [CrossRef]
- Misawa, A.; Inoue, S. Estrogen-Related Receptors in Breast Cancer and Prostate Cancer. Front. Endocrinol. 2015, 6, 83. [Google Scholar] [CrossRef] [Green Version]
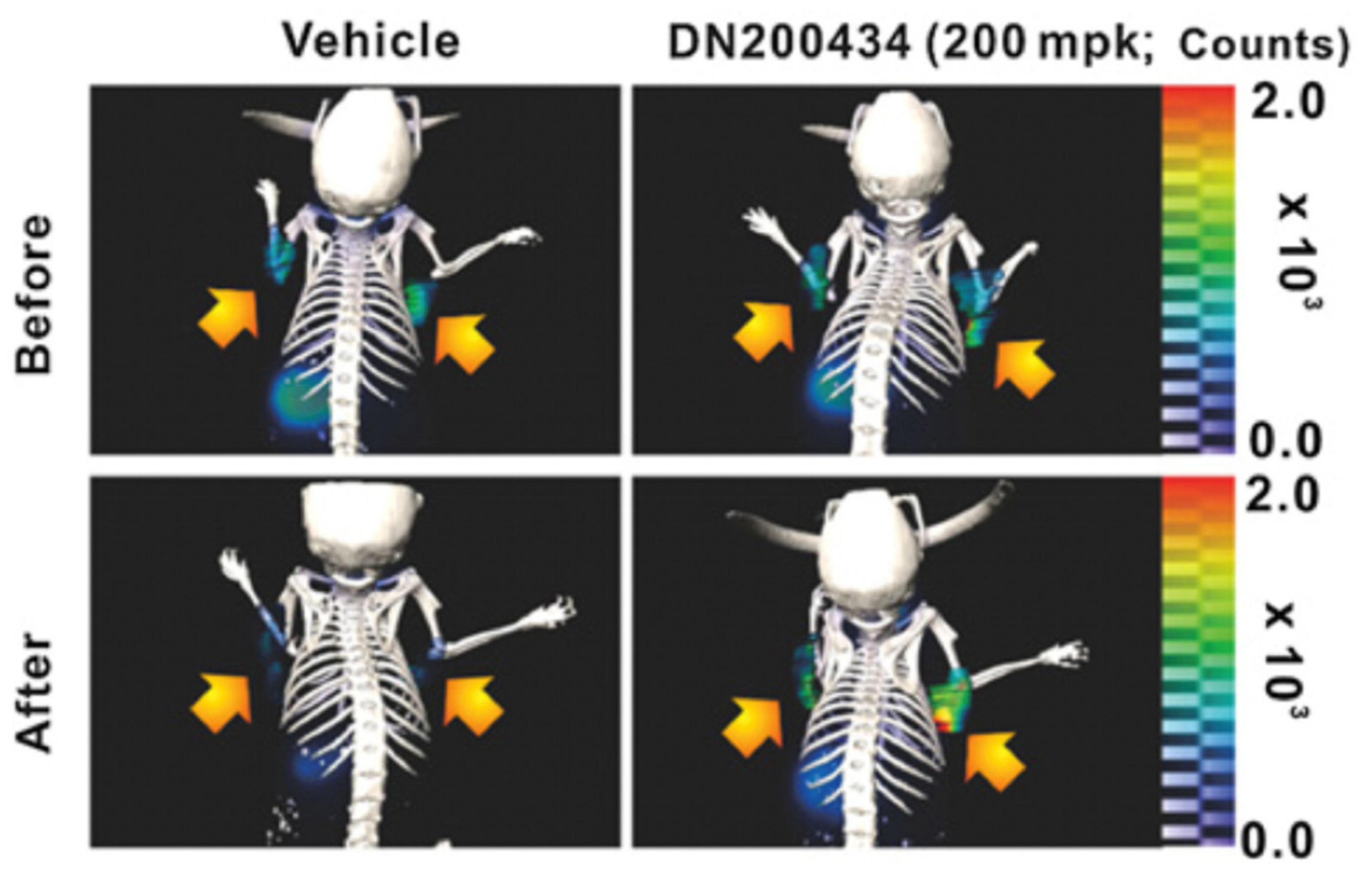
- Singh, T.D.; Jeong, S.Y.; Lee, S.-W.; Ha, J.-H.; Lee, I.-K.; Kim, S.H.; Kim, J.; Cho, S.J.; Ahn, B.-C.; Lee, J.; et al. Inverse Agonist of Estrogen-Related Receptor γ Enhances Sodium Iodide Symporter Function Through Mitogen-Activated Protein Kinase Signaling in Anaplastic Thyroid Cancer Cells. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1690–1696. [Google Scholar] [CrossRef]
- Singh, T.D.; Song, J.; Kim, J.; Chin, J.; Ji, H.D.; Lee, J.-E.; Lee, S.B.; Yoon, H.; Yu, J.H.; Kim, S.K.; et al. A Novel Orally Active Inverse Agonist of Estrogen-Related Receptor Gamma (ERRγ), DN200434, A Booster of NIS in Anaplastic Thyroid Cancer. Clin. Cancer Res. 2019, 25, 5069–5081. [Google Scholar] [CrossRef] [Green Version]
- Hershman, J.M. To Avoid Stunning, Give Treatment Doses of Radioiodine-131 within Three Days after Completing the Diagnostic Scan or Wait Seven Days. Clin. Thyroid. 2015, 27, 341–343. [Google Scholar] [CrossRef]
- Silberstein, E.B.; Alavi, A.; Balon, H.R.; Clarke, S.E.M.; Divgi, C.; Gelfand, M.J.; Goldsmith, S.J.; Jadvar, H.; Marcus, C.S.; Martin, W.H.; et al. The SNMMI Practice Guideline for Therapy of Thyroid Disease with 131I 3.0. J. Nucl. Med. 2012, 53, 1633–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silberstein, E.B. The Problem of the Patient with Thyroglobulin Elevation but Negative Iodine Scintigraphy: The TENIS Syndrome. Semin. Nucl. Med. 2011, 41, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Sun, H.J.; Song, Y.S.; Yoo, S.-K.; Kim, Y.A.; Seo, J.-S.; Park, Y.J.; Cho, S.W. CXCL16 Positively Correlated with M2-Macrophage Infiltration, Enhanced Angiogenesis, and Poor Prognosis in Thyroid Cancer. Sci. Rep. 2019, 9, 13288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Bansal, A.; Goyal, R.; Peng, K.-W.; Russell, S.J.; DeGrado, T.R. Synthesis and Evaluation of 18F-Hexafluorophosphate as a Novel PET Probe for Imaging of Sodium/Iodide Symporter in a Murine C6-Glioma Tumor Model. Bioorg. Med. Chem. 2018, 26, 225–231. [Google Scholar] [CrossRef]
- Jiang, H.; DeGrado, T.R. [18F]Tetrafluoroborate ([18F]TFB) and Its Analogs for PET Imaging of the Sodium/Iodide Symporter. Theranostics 2018, 8, 3918–3931. [Google Scholar] [CrossRef]
- Dittmann, M.; Carvalho, J.M.G.; Rahbar, K.; Schäfers, M.; Claesener, M.; Riemann, B.; Seifert, R. Incremental Diagnostic Value of [18F]Tetrafluoroborate PET-CT Compared to [131I]Iodine Scintigraphy in Recurrent Differentiated Thyroid Cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2639–2646. [Google Scholar] [CrossRef] [Green Version]
- Khoshnevisan, A.; Chuamsaamarkkee, K.; Boudjemeline, M.; Jackson, A.; Smith, G.E.; Gee, A.D.; Fruhwirth, G.O.; Blower, P.J. 18F-Fluorosulfate for PET Imaging of the Sodium–Iodide Symporter: Synthesis and Biologic Evaluation In Vitro and In Vivo. J. Nucl. Med. 2017, 58, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Concilio, S.C.; Zhekova, H.R.; Noskov, S.Y.; Russell, S.J. Inter-Species Variation in Monovalent Anion Substrate Selectivity and Inhibitor Sensitivity in the Sodium Iodide Symporter (NIS). PLoS ONE 2020, 15, e0229085. [Google Scholar] [CrossRef] [Green Version]
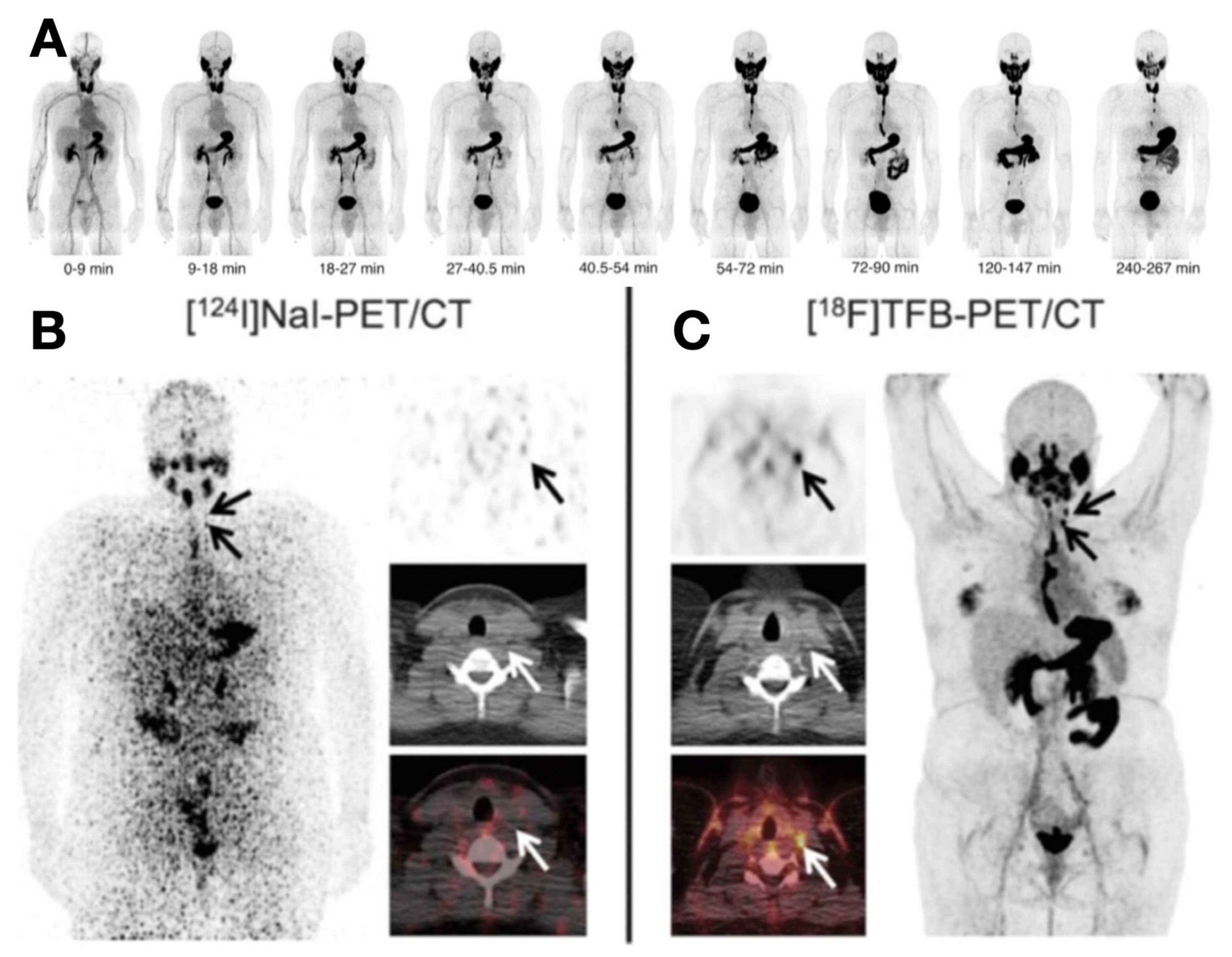
- O’Doherty, J.; Jauregui-Osoro, M.; Brothwood, T.; Szyszko, T.; Marsden, P.K.; O’Doherty, M.J.; Cook, G.J.R.; Blower, P.J.; Lewington, V. 18 F-Tetrafluoroborate, a PET Probe for Imaging Sodium/Iodide Symporter Expression: Whole-Body Biodistribution, Safety, and Radiation Dosimetry in Thyroid Cancer Patients. J. Nucl. Med. 2017, 58, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
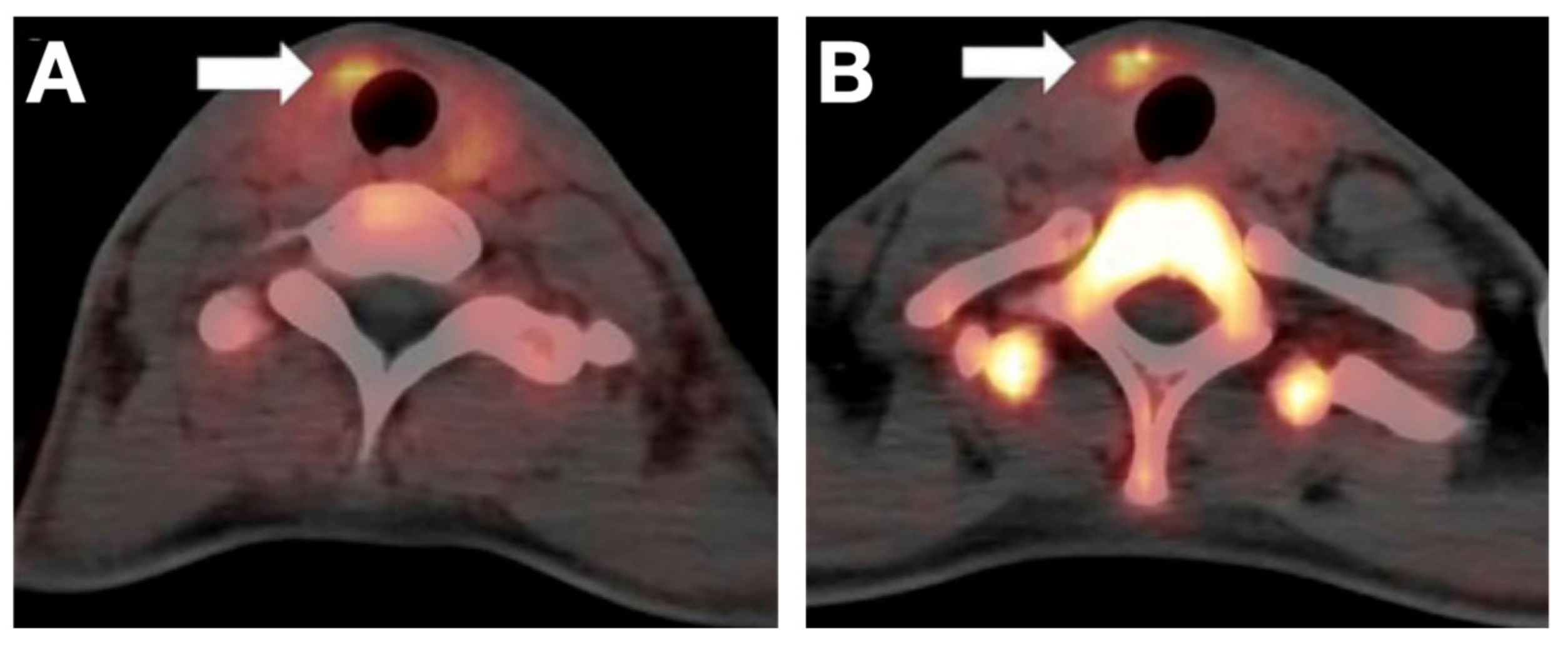
- Samnick, S.; Al-Momani, E.; Schmid, J.-S.; Mottok, A.; Buck, A.K.; Lapa, C. Initial Clinical Investigation of [18F]Tetrafluoroborate PET/CT in Comparison to [124I]Iodine PET/CT for Imaging Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 162–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J. Targeting Uptake Transporters for Cancer Imaging and Treatment. Acta Pharm. Sin. B 2019, 10, 79–90. [Google Scholar] [CrossRef]
- Nagarajah, J.; Ho, A.L.; Tuttle, R.M.; Weber, W.A.; Grewal, R.K. Correlation of BRAFV600E Mutation and Glucose Metabolism in Thyroid Cancer Patients: An 18F-FDG PET Study. J. Nucl. Med. 2015, 56, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Cheng, L.; Jin, Y.; Ruan, M.; Sheng, S.; Chen, L. Predicting 131I-Avidity of Metastases from Differentiated Thyroid Cancer Using 18F-FDG PET/CT in Postoperative Patients with Elevated Thyroglobulin. Sci. Rep. 2018, 8, 4352. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.Y.; Bang, J.-I.; Kang, K.W.; Lee, H.; Chung, J.-K. FDG PET/CT for the Early Prediction of RAI Therapy Response in Patients with Metastatic Differentiated Thyroid Carcinoma. PLoS ONE 2019, 14, e0218416. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Chen, J.; Cui, J.; Gao, T.; Gao, Y.; Zeng, W. Nuclear Imaging of Glucose Metabolism: Beyond 18F-FDG. Contrast Media Mol. Imaging 2019, 2019, 7954854. [Google Scholar] [CrossRef] [Green Version]
- Galldiks, N.; Langen, K.-J.; Albert, N.L.; Chamberlain, M.; Soffietti, R.; Kim, M.M.; Law, I.; Rhun, E.L.; Chang, S.; Schwarting, J.; et al. PET Imaging in Patients with Brain Metastasis—Report of the RANO/PET Group. Neuro-Oncology 2019, 21, 585–595. [Google Scholar] [CrossRef]
- Pagano, L.; Samà, M.T.; Morani, F.; Prodam, F.; Rudoni, M.; Boldorini, R.; Valente, G.; Marzullo, P.; Baldelli, R.; Appetecchia, M.; et al. Thyroid Incidentaloma Identified by 18F-Fluorodeoxyglucose Positron Emission Tomography with CT (FDG-PET/CT): Clinical and Pathological Relevance. Clin. Endocrinol. 2011, 75, 528–534. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; Iakovou, I.; Mihailovic, J.; Verburg, F.A.; Luster, M. EANM Practice Guideline for PET/CT Imaging in Medullary Thyroid Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 61–77. [Google Scholar] [CrossRef]
- Beheshti, M.; Pöcher, S.; Vali, R.; Waldenberger, P.; Broinger, G.; Nader, M.; Kohlfürst, S.; Pirich, C.; Dralle, H.; Langsteger, W. The Value of 18F-DOPA PET-CT in Patients with Medullary Thyroid Carcinoma: Comparison with 18F-FDG PET-CT. Eur. Radiol. 2009, 19, 1425–1434. [Google Scholar] [CrossRef]
- Liu, F.; Xu, X.; Zhu, H.; Zhang, Y.; Yang, J.; Zhang, L.; Li, N.; Zhu, L.; Kung, H.F.; Yang, Z. PET Imaging of 18 F-(2S,4R)4-Fluoroglutamine Accumulation in Breast Cancer: From Xenografts to Patients. Mol Pharm. 2018, 15, 3448–3455. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, H.; Liu, F.; Zhang, Y.; Yang, J.; Zhang, L.; Xie, Q.; Zhu, L.; Li, N.; Kung, H.F.; et al. Dynamic PET/CT Imaging of 18F-(2S, 4R)4-Fluoroglutamine in Healthy Volunteers and Oncological Patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2280–2292. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Iversen, P.; Arveschoug, A.K. Follicular Thyroid Cancer Avid on C-11 Methionine PET/CT. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Barollo, S.; Bertazza, L.; Watutantrige-Fernando, S.; Censi, S.; Cavedon, E.; Galuppini, F.; Pennelli, G.; Fassina, A.; Citton, M.; Rubin, B.; et al. Overexpression of L-Type Amino Acid Transporter 1 (LAT1) and 2 (LAT2): Novel Markers of Neuroendocrine Tumors. PLoS ONE 2016, 11, e0156044. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Camacho, C.P.; Lindsey, S.C.; Valente, F.O.F.; Andreoni, D.M.; Yamaga, L.Y.; Wagner, J.; Biscolla, R.P.M.; Maciel, R.M.B. The Combined Use of Calcitonin Doubling Time and 18F-FDG PET/CT Improves Prognostic Values in Medullary Thyroid Carcinoma: The Clinical Utility of 18F-FDG PET/CT. Endocr. Pract. 2017, 23, 942–948. [Google Scholar] [CrossRef]
- Weber, T.; Gottstein, M.; Schwenzer, S.; Beer, A.; Luster, M. Is C-11 Methionine PET/CT Able to Localise Sestamibi-Negative Parathyroid Adenomas? World J. Surg. 2017, 41, 980–985. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, J.; Kan, Y.; Yang, J.; Wang, X. The Diagnostic Value of 11C-Methionine PET in Hyperparathyroidism with Negative 99mTc-MIBI SPECT: A Meta-Analysis. Acta Radiol. 2016, 58, 558–564. [Google Scholar] [CrossRef]
- Lenschow, C.; Gassmann, P.; Wenning, C.; Senninger, N.; Colombo-Benkmann, M. Preoperative 11C-Methionine PET/CT Enables Focused Parathyroidectomy in MIBI-SPECT Negative Parathyroid Adenoma. World J. Surg. 2015, 39, 1750–1757. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-Methionine-PET for Differentiating Recurrent Brain Tumor from Radiation Necrosis: Radiomics Approach with Random Forest Classifier. Sci. Rep. 2019, 9, 15666. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Zhang, B.; Shi, X.; Yi, C.; Zhang, X. Diagnostic Accuracy of 13N-Ammonia PET, 11C-Methionine PET and 18F-Fluorodeoxyglucose PET: A Comparative Study in Patients with Suspected Cerebral Glioma. BMC Cancer 2019, 19, 332. [Google Scholar] [CrossRef]
- Wedman, J.; Pruim, J.; Putten, L.; Hoekstra, O.S.; Bree, R.; Dijk, B.A.C.; Laan, B.F.A.M. Is C-11 Methionine PET an Alternative to 18-F FDG-PET for Identifying Recurrent Laryngeal Cancer after Radiotherapy? Clin. Otolaryngol. 2019, 44, 124–130. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Jager, P.L.; Plukker, J.T.M.; Wolffenbuttel, B.H.R.; Dierckx, R.A.; Links, T.P. Comparison of 11C-Methionine PET and 18F-Fluorodeoxyglucose PET in Differentiated Thyroid Cancer. Nucl. Med. Commun. 2008, 29, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Kudomi, N.; Maeda, Y.; Kobata, T.; Oishi, A.; Matsumoto, K.; Monden, T.; Iwasaki, T.; Mitamura, K.; Norikane, T.; et al. Effect of Quantitative Values on Shortened Acquisition Duration in Brain Tumor 11C-Methionine PET/CT. EJNMMI Phys. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-L.; Durán, R.V. Glutamine Metabolism in Cancer Therapy. Cancer Drug Resist. 2018. [Google Scholar] [CrossRef] [Green Version]
- Sohda, M.; Miyazaki, T.; Honjyo, H.; Hara, K.; Ozawa, D.; Sakai, M.; Yokobori, T.; Higuchi, T.; Tsushima, Y.; Kuwano, H. 18F-FAMT PET Is Useful to Distinguish between Specific Uptake and Nonspecific Uptake Compared to 18F-Flourodeoxyglucose Position Emission Tomography in Esophageal Cancer Patients. Dig. Surg. 2018, 35, 383–388. [Google Scholar] [CrossRef]
- Wei, L.; Tominaga, H.; Ohgaki, R.; Wiriyasermkul, P.; Hagiwara, K.; Okuda, S.; Kaira, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y. Specific Transport of 3-fluoro-l-α-methyl-tyrosine by LAT1 Explains Its Specificity to Malignant Tumors in Imaging. Cancer Sci. 2016, 107, 347–352. [Google Scholar] [CrossRef]
- Wiriyasermkul, P.; Nagamori, S.; Tominaga, H.; Oriuchi, N.; Kaira, K.; Nakao, H.; Kitashoji, T.; Ohgaki, R.; Tanaka, H.; Endou, H.; et al. Transport of 3-Fluoro-l-α-Methyl-Tyrosine by Tumor-Upregulated L-Type Amino Acid Transporter 1: A Cause of the Tumor Uptake in PET. J. Nucl. Med. 2012, 53, 1253–1261. [Google Scholar] [CrossRef] [Green Version]
- Saidijam, M.; Afshar, S.; Ahmad, I.; Patching, S. Nucleoside Transporters in PET Imaging of Proliferating Cancer Cells Using 3ꞌ-Deoxy-3ꞌ-[18F]Fluoro-L-Thymidine. J. Diagn. Imaging 2018, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, M.; Nakajo, M.; Jinguji, M.; Tani, A.; Kajiya, Y.; Tanabe, H.; Fukukura, Y.; Nakabeppu, Y.; Koriyama, C. Diagnosis of Metastases from Postoperative Differentiated Thyroid Cancer: Comparison between FDG and FLT PET/CT Studies. Radiology 2013, 267, 891–901. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y.; Liu, T.; Li, Z.; Zhang, X.; Chen, X. Peptide-Based Imaging Agents for Cancer Detection. Adv. Drug Deliv. Rev. 2017, 110, 38–51. [Google Scholar] [CrossRef] [Green Version]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin Receptor PET Ligands-the next Generation for Clinical Practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Staderini, M.; Megia-Fernandez, A.; Dhaliwal, K.; Bradley, M. Peptides for Optical Medical Imaging and Steps towards Therapy. Bioorg. Med. Chem. 2018, 26, 2816–2826. [Google Scholar] [CrossRef]
- Treglia, G.; Tamburello, A.; Giovanella, L. Detection Rate of Somatostatin Receptor PET in Patients with Recurrent Medullary Thyroid Carcinoma: A Systematic Review and a Meta-Analysis. Horm. Athens Greece 2017, 16, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Agthoven, J.F.V.; Xiong, J.-P.; Alonso, J.L.; Rui, X.; Adair, B.D.; Goodman, S.L.; Arnaout, M.A. Structural Basis for Pure Antagonism of Integrin AVβ3 by a High-Affinity Form of Fibronectin. Nat. Struct. Mol. Biol. 2014, 21, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Parihar, A.S.; Mittal, B.R.; Kumar, R.; Shukla, J.; Bhattacharya, A. 68Ga-DOTA-RGD2 Positron Emission Tomography/Computed Tomography in Radioiodine Refractory Thyroid Cancer: Prospective Comparison of Diagnostic Accuracy with 18F-FDG Positron Emission Tomography/Computed Tomography and Evaluation Toward Potential Theranostics. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 557–567. [Google Scholar] [CrossRef]
- Parihar, A.S.; Sood, A.; Kumar, R.; Bhusari, P.; Shukla, J.; Mittal, B.R. Novel Use of 177Lu-DOTA-RGD2 in Treatment of 68Ga-DOTA-RGD2-Avid Lesions in Papillary Thyroid Cancer with TENIS. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1836–1837. [Google Scholar] [CrossRef]
- Heitkötter, B.; Steinestel, K.; Trautmann, M.; Grünewald, I.; Barth, P.; Gevensleben, H.; Bögemann, M.; Wardelmann, E.; Hartmann, W.; Rahbar, K.; et al. Neovascular PSMA Expression Is a Common Feature in Malignant Neoplasms of the Thyroid. Oncotarget 2018, 9, 9867–9874. [Google Scholar] [CrossRef] [Green Version]
- Sager, S.; Vatankulu, B.; Uslu, L.; Sönmezoglu, K. Incidental Detection of Follicular Thyroid Carcinoma in 68Ga-PSMA PET/CT Imaging. J. Nucl. Med. Technol. 2016, 44, 199–200. [Google Scholar] [CrossRef] [Green Version]
- Bychkov, A.; Vutrapongwatana, U.; Tepmongkol, S.; Keelawat, S. PSMA Expression by Microvasculature of Thyroid Tumors–Potential Implications for PSMA Theranostics. Sci. Rep. 2017, 7, 5202. [Google Scholar] [CrossRef]
- Taywade, S.K.; Damle, N.A.; Bal, C. PSMA Expression in Papillary Thyroid Carcinoma. Clin. Nucl. Med. 2016, 41, e263–e265. [Google Scholar] [CrossRef]
- Derlin, T.; Kreipe, H.-H.; Schumacher, U.; Soudah, B. PSMA Expression in Tumor Neovasculature Endothelial Cells of Follicular Thyroid Adenoma as Identified by Molecular Imaging Using 68Ga-PSMA Ligand PET/CT. Clin. Nucl. Med. 2017, 42, e173–e174. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholom?, M.; Ezziddin, S. 225 Ac-PSMA-617/ 177 Lu-PSMA-617 Tandem Therapy of Metastatic Castration-Resistant Prostate Cancer: Pilot Experience. Nuklearmed 2020, 59, 93. [Google Scholar] [CrossRef]
- Klingler, M.; Rangger, C.; Summer, D.; Kaeopookum, P.; Decristoforo, C.; Guggenberg, E. von Cholecystokinin-2 Receptor Targeting with Novel C-Terminally Stabilized HYNIC-Minigastrin Analogs Radiolabeled with Technetium-99m. Pharm 2019, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Klingler, M.; Hörmann, A.A.; Guggenberg, E.V. Cholecystokinin-2 Receptor Targeting with Radiolabeled Peptides: Current Status and Future Directions. Curr. Med. Chem. 2020, 27, 7112–7132. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; Guggenberg, E. von DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use. J. Nucl. Med. 2018, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprimny, C.; von Guggenberg, E.; Svirydenka, A.; Mikołajczak, R.; Hubalewska-Dydejczyk, A.; Virgolini, I.J. Comparison of PET/CT Imaging with [18F]FDOPA and Cholecystokinin-2 Receptor Targeting [68Ga]Ga-DOTA-MGS5 in a Patient with Advanced Medullary Thyroid Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–2. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharm. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Warram, J.M.; de Boer, E.; Sorace, A.G.; Chung, T.K.; Kim, H.; Pleijhuis, R.G.; van Dam, G.M.; Rosenthal, E.L. Antibody-Based Imaging Strategies for Cancer. Cancer Metastasis Rev. 2014, 33, 809–822. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Jiang, D.; Lee, H.J.; Li, M.; Kutyreff, C.J.; Engle, J.W.; Liu, J.; Cai, W. Development and Characterization of CD54-Targeted ImmunoPET Imaging in Solid Tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2765–2775. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Jiang, D.; Im, H.-J.; Sun, H.; Valdovinos, H.F.; England, C.G.; Ehlerding, E.B.; Nickles, R.J.; Lee, D.S.; Cho, S.Y.; Huang, P.; et al. Radiolabeled Pertuzumab for Imaging of Human Epidermal Growth Factor Receptor 2 Expression in Ovarian Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1296–1305. [Google Scholar] [CrossRef]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER Kinase Inhibition in Patients with HER2- and HER3-Mutant Cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrè, G.; Giovanella, L.; Siracusa, M.; Simone, A.; Branca, G.; Scarfì, R.; Trimarchi, F.; Ieni, A.; et al. HER2 Analysis in Sporadic Thyroid Cancer of Follicular Cell Origin. Int. J. Mol. Sci. 2016, 17, 2040. [Google Scholar] [CrossRef] [Green Version]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the Mutational Landscape of Anaplastic Thyroid Cancer via Whole-Exome Sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Sami, A.; Xiang, J. HER2-Directed Therapy: Current Treatment Options for HER2-Positive Breast Cancer. Breast Cancer 2015, 22, 101–116. [Google Scholar] [CrossRef]
- Pondé, N.F.; Zardavas, D.; Piccart, M. Progress in Adjuvant Systemic Therapy for Breast Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44. [Google Scholar] [CrossRef]
- Bardhan, P.; Bui, M.M.; Minton, S.; Loftus, L.; Carter, W.B.; Laronga, C.; Ismail-Khan, R. HER2-Positive Male Breast Cancer with Thyroid Cancer: An Institutional Report and Review of Literature. Ann. Clin. Lab. Sci. 2012, 42, 135–139. [Google Scholar]
- Handkiewicz-Junak, D.; Swierniak, M.; Rusinek, D.; Oczko-Wojciechowska, M.; Dom, G.; Maenhaut, C.; Unger, K.; Detours, V.; Bogdanova, T.; Thomas, G.; et al. Gene Signature of the Post-Chernobyl Papillary Thyroid Cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1267–1277. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 Expression Status in Diverse Cancers: Review of Results from 37,992 Patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, Y.; Mehta, A.; Boufraqech, M.; Davis, S.; Wang, J.; Tian, Z.; Yu, Z.; Boxer, M.B.; Kiefer, J.A.; et al. Dual Inhibition of HDAC and EGFR Signaling with CUDC-101 Induces Potent Suppression of Tumor Growth and Metastasis in Anaplastic Thyroid Cancer. Oncotarget 2015, 6, 9073–9085. [Google Scholar] [CrossRef] [Green Version]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of Feedback Inhibition of HER3 Transcription by RAF and MEK Inhibitors Attenuates Their Antitumor Effects in BRAF-Mutant Thyroid Carcinomas. Cancer Discov. 2013, 3, 520–533. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Jiang, D.; Rosenkrans, Z.T.; Barnhart, T.E.; Engle, J.W.; Luo, Q.; Cai, W. HER2-Targeted Multimodal Imaging of Anaplastic Thyroid Cancer. Am. J. Cancer Res. 2019, 9, 2413–2427. [Google Scholar]
- Reina, M.; Espel, E. Role of LFA-1 and ICAM-1 in Cancer. Cancers 2017, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Goodrich, C.; Fu, C.; Dong, C. Melanoma Upregulates ICAM-1 Expression on Endothelial Cells through Engagement of Tumor CD44 with Endothelial E-selectin and Activation of a PKCα–P38-SP-1 Pathway. FASEB J. 2014, 28, 4591–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buitrago, D.; Keutgen, X.M.; Crowley, M.; Filicori, F.; Aldailami, H.; Hoda, R.; Liu, Y.-F.; Hoda, R.S.; Scognamiglio, T.; Jin, M.; et al. Intercellular Adhesion Molecule-1 (ICAM-1) Is Upregulated in Aggressive Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 2012, 19, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Galore-Haskel, G.; Baruch, E.N.; Berg, A.L.; Barshack, I.; Zilinsky, I.; Avivi, C.; Besser, M.J.; Schachter, J.; Markel, G. Histopathological Expression Analysis of Intercellular Adhesion Molecule 1 (ICAM-1) along Development and Progression of Human Melanoma. Oncotarget 2014, 5, 99580–99586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, I.M.; Shevlin, E.; Vedvyas, Y.; Zaman, M.; Wyrwas, B.; Scognamiglio, T.; Moore, M.D.; Wang, W.; Park, S.; Park, S.; et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 7569–7583. [Google Scholar] [CrossRef] [Green Version]
- Vedvyas, Y.; McCloskey, J.E.; Yang, Y.; Min, I.M.; Fahey, T.J.; Zarnegar, R.; Hsu, Y.-M.S.; Hsu, J.-M.; Besien, K.V.; Gaudet, I.; et al. Manufacturing and Preclinical Validation of CAR T Cells Targeting ICAM-1 for Advanced Thyroid Cancer Therapy. Sci. Rep. 2019, 9, 10634. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Vasilyeva, E.; Wiseman, S.M. Beyond Immunohistochemistry and Immunocytochemistry: A Current Perspective on Galectin-3 and Thyroid Cancer. Expert Rev. Anticancer 2019, 19, 1017–1027. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Application of an Immunodiagnostic Method for Improving Preoperative Diagnosis of Nodular Thyroid Lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Galectin-3-Expression Analysis in the Surgical Selection of Follicular Thyroid Nodules with Indeterminate Fine-Needle Aspiration Cytology: A Prospective Multicentre Study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- D’Alessandria, C.; Braesch-Andersen, S.; Bejo, K.; Reder, S.; Blechert, B.; Schwaiger, M.; Bartolazzi, A. Noninvasive In Vivo Imaging and Biologic Characterization of Thyroid Tumors by ImmunoPET Targeting of Galectin-3. Cancer Res. 2016, 76, 3583–3592. [Google Scholar] [CrossRef] [Green Version]
- Rose, F.D.; Braeuer, M.; Braesch-Andersen, S.; Otto, A.M.; Steiger, K.; Reder, S.; Mall, S.; Nekolla, S.; Schwaiger, M.; Weber, W.A.; et al. Galectin-3 Targeting in Thyroid Orthotopic Tumors Opens New Ways to Characterize Thyroid Cancer. J. Nucl. Med. 2019, 60, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Nair, L.M.; Anila, K.R.; Sreekumar, A.; Pradeep, V.M. Renal Metastasis from Papillary Carcinoma Thyroid Detected by Whole Body Iodine Scan: A Case Report and Review of the Literature. Indian J. Nucl. Med. IJNM Off. J. Soc. Nucl. Med. India 2016, 31, 232–234. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Wang, Z.; Hao, M.; Li, J. Bispecific Antibodies and Their Applications. J. Hematol. Oncol. 2015, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, A.; Jimeno, A. Bispecific Antibodies for Cancer Therapy: A Review. Pharm. Ther. 2017, 185, 122–134. [Google Scholar] [CrossRef]
- Oudoux, A.; Salaun, P.-Y.; Bournaud, C.; Campion, L.; Ansquer, C.; Rousseau, C.; Bardet, S.; Borson-Chazot, F.; Vuillez, J.-P.; Murat, A.; et al. Sensitivity and Prognostic Value of Positron Emission Tomography with F-18-Fluorodeoxyglucose and Sensitivity of Immunoscintigraphy in Patients with Medullary Thyroid Carcinoma Treated with Anticarcinoembryonic Antigen-Targeted Radioimmunotherapy. J. Clin. Endocrinol. Metab. 2007, 92, 4590–4597. [Google Scholar] [CrossRef] [Green Version]
- Peltier, P.; Curtet, C.; Chatal, J.F.; Doussal, J.M.L.; Daniel, G.; Aillet, G.; Gruaz-Guyon, A.; Barbet, J.; Delaage, M. Radioimmunodetection of Medullary Thyroid Cancer Using a Bispecific Anti-CEA/Anti-Indium-DTPA Antibody and an Indium-111-Labeled DTPA Dimer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1993, 34, 1267–1273. [Google Scholar]
- Barbet, J.; Peltier, P.; Bardet, S.; Vuillez, J.P.; Bachelot, I.; Denet, S.; Olivier, P.; Leccia, F.; Corcuff, B.; Huglo, D.; et al. Radiotheranostics: A Roadmap for Future Development. Lancet Oncol. 2020, 21, e146–e156. [Google Scholar] [CrossRef]
- Kraeber-Bodéré, F.; Salaun, P.-Y.; Ansquer, C.; Drui, D.; Mirallié, E.; Faivre-Chauvet, A.; Barbet, J.; Goldenberg, D.M.; Chatal, J.-F. Pretargeted Radioimmunotherapy (PRAIT) in Medullary Thyroid Cancer (MTC). Tumor Biol. 2012, 33, 601–606. [Google Scholar] [CrossRef]
- Schoffelen, R.; Boerman, O.C.; Goldenberg, D.M.; Sharkey, R.M.; van Herpen, C.M.L.; Franssen, G.M.; McBride, W.J.; Chang, C.-H.; Rossi, E.A.; van der Graaf, W.T.A.; et al. Development of an Imaging-Guided CEA-Pretargeted Radionuclide Treatment of Advanced Colorectal Cancer: First Clinical Results. Brit. J. Cancer 2013, 109, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Bodet-Milin, C.; Faivre-Chauvet, A.; Carlier, T.; Rauscher, A.; Bourgeois, M.; Cerato, E.; Rohmer, V.; Couturier, O.; Drui, D.; Goldenberg, D.M.; et al. Immuno-PET Using Anticarcinoembryonic Antigen Bispecific Antibody and 68Ga-Labeled Peptide in Metastatic Medullary Thyroid Carcinoma: Clinical Optimization of the Pretargeting Parameters in a First-in-Human Trial. J. Nucl. Med. 2016, 57, 1505–1511. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.; Power, C.A. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies 2019, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Kulmala, A.; Huovinen, T.; Lamminmäki, U. Improvement of Fab Expression by Screening Combinatorial Synonymous Signal Sequence Libraries. Microb. Cell Factories 2019, 18, 157. [Google Scholar] [CrossRef] [Green Version]
- Xenaki, K.T.; Oliveira, S.; van Bergen En Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Duan, H.; Huang, H.; Jing, G. An Antibody Fab Fragment-Based Chimeric Antigen Receptor Could Efficiently Eliminate Human Thyroid Cancer Cells. J. Cancer 2019, 10, 1890–1895. [Google Scholar] [CrossRef] [Green Version]
- Peplau, E.; Rose, F.D.; Reder, S.; Mittelhäuser, M.; Scafetta, G.; Schwaiger, M.; Weber, W.A.; Bartolazzi, A.; Skerra, A.; D’Alessandria, C. Development of a Chimeric Antigen-Binding Fragment Directed Against Human Galectin-3 and Validation as an Immuno-Positron Emission Tomography Tracer for the Sensitive In Vivo Imaging of Thyroid Cancer. Thyroid 2020, 30, 1314–1326. [Google Scholar] [CrossRef]
- Peplau, E.; Rose, F.D.; Eichinger, A.; Reder, S.; Mittelhäuser, M.; Scafetta, G.; Schwaiger, M.; Weber, W.A.; Bartolazzi, A.; D’Alessandria, C.; et al. Effective Rational Humanization of a PASylated Anti-Galectin-3 Fab for the Sensitive PET Imaging of Thyroid Cancer in Vivo. Sci. Rep. 2021, 11, 7358. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, S.; Ying, T. Single-Domain Antibodies as Therapeutics against Human Viral Diseases. Front. Immunol. 2017, 8, 1802. [Google Scholar] [CrossRef] [PubMed]
- Pleiner, T.; Bates, M.; Trakhanov, S.; Lee, C.-T.; Schliep, J.E.; Chug, H.; Böhning, M.; Stark, H.; Urlaub, H.; Görlich, D. Nanobodies: Site-Specific Labeling for Super-Resolution Imaging, Rapid Epitope-Mapping and Native Protein Complex Isolation. Elife 2015, 4, e11349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive Imaging of Tumor Progression, Metastasis, and Fibrosis Using a Nanobody Targeting the Extracellular Matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhou, C.; Dong, B.; Zhong, H.; Chen, S.; Li, Q.; Wang, Z. Single Domain Antibody-Based Bispecific Antibody Induces Potent Specific Anti-Tumor Activity. Cancer Biol. 2016, 17, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Zhang, C.; Ma, T.; Liu, X.; Chen, Z.; Li, S.; Deng, Y. Advances in Aptamer Screening and Aptasensors’ Detection of Heavy Metal Ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as Targeted Therapeutics: Current Potential and Challenges. Nat. Rev. Drug Discov. 2016, 16, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Behrooz, A.B.; Syahir, A.; Ahmad, S. CD133: Beyond a Cancer Stem Cell Biomarker. J. Drug Target. 2018, 27, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.-L.; Zheng, Q.; Yan, J.; Pan, Y.; Wang, Z.-G. Upregulated CD133 Expression in Tumorigenesis of Colon Cancer Cells. World J. Gastroenterol. 2011, 17, 932–937. [Google Scholar] [CrossRef]
- Dhaybi, R.A.; Sartelet, H.; Powell, J.; Kokta, V. Expression of CD133+ Cancer Stem Cells in Childhood Malignant Melanoma and Its Correlation with Metastasis. Mod. Pathol. 2010, 23, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Brown, R.E. Immunohistochemical Detection of Epithelialmesenchymal Transition Associated with Stemness Phenotype in Anaplastic Thyroid Carcinoma. Int. J. Clin. Exp. Pathol. 2010, 3, 755–762. [Google Scholar]
- Glumac, P.M.; LeBeau, A.M. The Role of CD133 in Cancer: A Concise Review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Friedman, S.; Lu, M.; Schultz, A.; Thomas, D.; Lin, R.-Y. CD133+ Anaplastic Thyroid Cancer Cells Initiate Tumors in Immunodeficient Mice and Are Regulated by Thyrotropin. PLoS ONE 2009, 4, e5395. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, X.; Li, W.; Sun, M.; Peng, M.; Yang, H.; Chen, L.; Zhang, C.; Cai, L.; Li, Y. Association of Cancer Stem Cell Markers with Aggressive Tumor Features in Papillary Thyroid Carcinoma. Cancer Control. 2015, 22, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sui, G.; Teng, D.; Wang, Q.; Qu, J.; Zhu, L.; Ran, H.; Wang, Z.; Jin, C.; Wang, H. Low Intensity Focused Ultrasound (LIFU) Triggered Drug Release from Cetuximab-Conjugated Phase-Changeable Nanoparticles for Precision Theranostics against Anaplastic Thyroid Carcinoma. Biomater. Sci. 2018, 7, 196–210. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, J.; Li, T.; Guo, J. Thyroid Cancer MR Molecular Imaging via SHP2-Targeted Nanoparticles. Int. J. Nanomed. 2019, 14, 7365–7373. [Google Scholar] [CrossRef] [Green Version]
- Ayati, A.; Moghimi, S.; Toolabi, M.; Foroumadi, A. Pyrimidine-Based EGFR TK Inhibitors in Targeted Cancer Therapy. Eur. J. Med. Chem. 2021, 221, 113523. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Ma, R.; Zhang, C.-M.; Li, J.; Li, L.; Hu, Z.-T.; Gao, Q.; Li, W.-M. Expression and Clinical Significance of Tyrosine Phosphatase SHP2 in Thyroid Carcinoma. Oncol. Lett. 2015, 10, 1507–1512. [Google Scholar] [CrossRef]
- Wei, W.-J.; Hardin, H.; Luo, Q.-Y. Targeting Autophagy in Thyroid Cancers. Endocr. Relat. Cancer 2019, 1, R181–R194. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Liu, M.; Sa, R.; Fu, H.; Cheng, L.; Chen, L. Mouse Models of Thyroid Cancer: Bridging Pathogenesis and Novel Therapeutics. Cancer Lett 2020, 469, 35–53. [Google Scholar] [CrossRef]
- Nakajo, M.; Nakajo, M.; Kajiya, Y.; Jinguji, M.; Mori, S.; Aridome, K.; Suenaga, T.; Tanaka, S. High FDG and Low FLT Uptake in a Thyroid Papillary Carcinoma Incidentally Discovered by FDG PET/CT. Clin. Nucl. Med. 2012, 37, 607. [Google Scholar] [CrossRef]
- Souteiro, P.; Gouveia, P.; Ferreira, G.; Belo, S.; Costa, C.; Carvalho, D.; Duarte, H.; Sampaio, I.L. 68Ga-DOTANOC and 18F-FDG PET/CT in Metastatic Medullary Thyroid Carcinoma: Novel Correlations with Tumoral Biomarkers. Endocrine 2019, 64, 322–329. [Google Scholar] [CrossRef]
- Budiawan, H.; Salavati, A.; Kulkarni, H.R.; Baum, R.P. Peptide Receptor Radionuclide Therapy of Treatment-Refractory Metastatic Thyroid Cancer Using (90)Yttrium and (177)Lutetium Labeled Somatostatin Analogs: Toxicity, Response and Survival Analysis. Am. J. Nucl. Med. Mol. Imaging 2013, 4, 39–52. [Google Scholar] [CrossRef]
- Salavati, A.; Puranik, A.; Kulkarni, H.R.; Budiawan, H.; Baum, R.P. Peptide Receptor Radionuclide Therapy (PRRT) of Medullary and Nonmedullary Thyroid Cancer Using Radiolabeled Somatostatin Analogues. Semin. Nucl. Med. 2016, 46, 215–224. [Google Scholar] [CrossRef]
- Assadi, M.; Ahmadzadehfar, H. 177 Lu-DOTATATE and 177 Lu-Prostate-Specific Membrane Antigen Therapy in a Patient with Advanced Metastatic Radioiodine-Refractory Differentiated Thyroid Cancer after Failure of Tyrosine Kinase Inhibitors Treatment. World J. Nucl. Med. 2019, 18, 406. [Google Scholar] [CrossRef]
- De Vries, L.H.; Lodewijk, L.; Braat, A.J.A.T.; Krijger, G.C.; Valk, G.D.; Lam, M.G.E.H.; Rinkes, I.H.M.B.; Vriens, M.R.; Keizer, B. de 68Ga-PSMA PET/CT in Radioactive Iodine-Refractory Differentiated Thyroid Cancer and First Treatment Results with 177Lu-PSMA-617. EJNMMI Res. 2020, 10, 18. [Google Scholar] [CrossRef]
- Bodet-Milin, C.; Bailly, C.; Touchefeu, Y.; Frampas, E.; Bourgeois, M.; Rauscher, A.; Lacoeuille, F.; Drui, D.; Arlicot, N.; Goldenberg, D.M.; et al. Clinical Results in Medullary Thyroid Carcinoma Suggest High Potential of Pretargeted Immuno-PET for Tumor Imaging and Theranostic Approaches. Front. Med. 2019, 6, 124. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Lin, F. The Potential Diagnostic Utility of TROP-2 in Thyroid Neoplasms. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Roelli, M.A.; Forster, S.; Wasmer, M.-H.; Brühl, F.; Maire, R.S.; Pancrazio, S.D.; Ruepp, M.-D.; Giger, R.; Perren, A.; et al. Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 2019, 29, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Elmageed, Z.Y.A.; Moroz, K.; Kandil, E. Clinical Significance of CD146 and Latexin during Different Stages of Thyroid Cancer. Mol. Cell Biochem. 2013, 381, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-Targeted Molecular Imaging of Cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Ma, M.T. Site-Specific Chelator-Antibody Conjugation for PET and SPECT Imaging with Radiometals. Drug Discov. Today Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Crow, D.; Turatti, F.; Bading, J.R.; Anderson, A.-L.; Poku, E.; Yazaki, P.J.; Carmichael, J.; Leong, D.; Wheatcroft, D.; et al. Site-Specific Conjugation of Monodispersed DOTA-PEGn to a Thiolated Diabody Reveals the Effect of Increasing PEG Size on Kidney Clearance and Tumor Uptake with Improved 64-Copper PET Imaging. Bioconjugate Chem. 2011, 22, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Acchione, M.; Kwon, H.; Jochheim, C.M.; Atkins, W.M. Impact of Linker and Conjugation Chemistry on Antigen Binding, Fc Receptor Binding and Thermal Stability of Model Antibody-Drug Conjugates. mAbs 2012, 4, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Adumeau, P.; Sharma, S.K.; Brent, C.; Zeglis, B.M. Site-Specifically Labeled Immunoconjugates for Molecular Imaging—Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol. 2016, 18, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-Y.; Wang, Y.-X.; Lin, Y.; Zhang, J.-S.; Yang, F.; Zhou, Q.-L.; Liao, Y.-Y. Advance of Molecular Imaging Technology and Targeted Imaging Agent in Imaging and Therapy. BioMed Res. Int. 2014, 2014, 819324. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum-Krumme, S.J.; Görges, R.; Bockisch, A.; Binse, I. 18F-FDG PET/CT Changes Therapy Management in High-Risk DTC after First Radioiodine Therapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1373–1380. [Google Scholar] [CrossRef]
- Müller, C.; van der Meulen, N.P.; Benešová, M.; Schibli, R. Therapeutic Radiometals Beyond 177 Lu and 90 Y: Production and Application of Promising α-Particle, β−-Particle, and Auger Electron Emitters. J. Nucl. Med. 2017, 58, 91S–96S. [Google Scholar] [CrossRef] [Green Version]

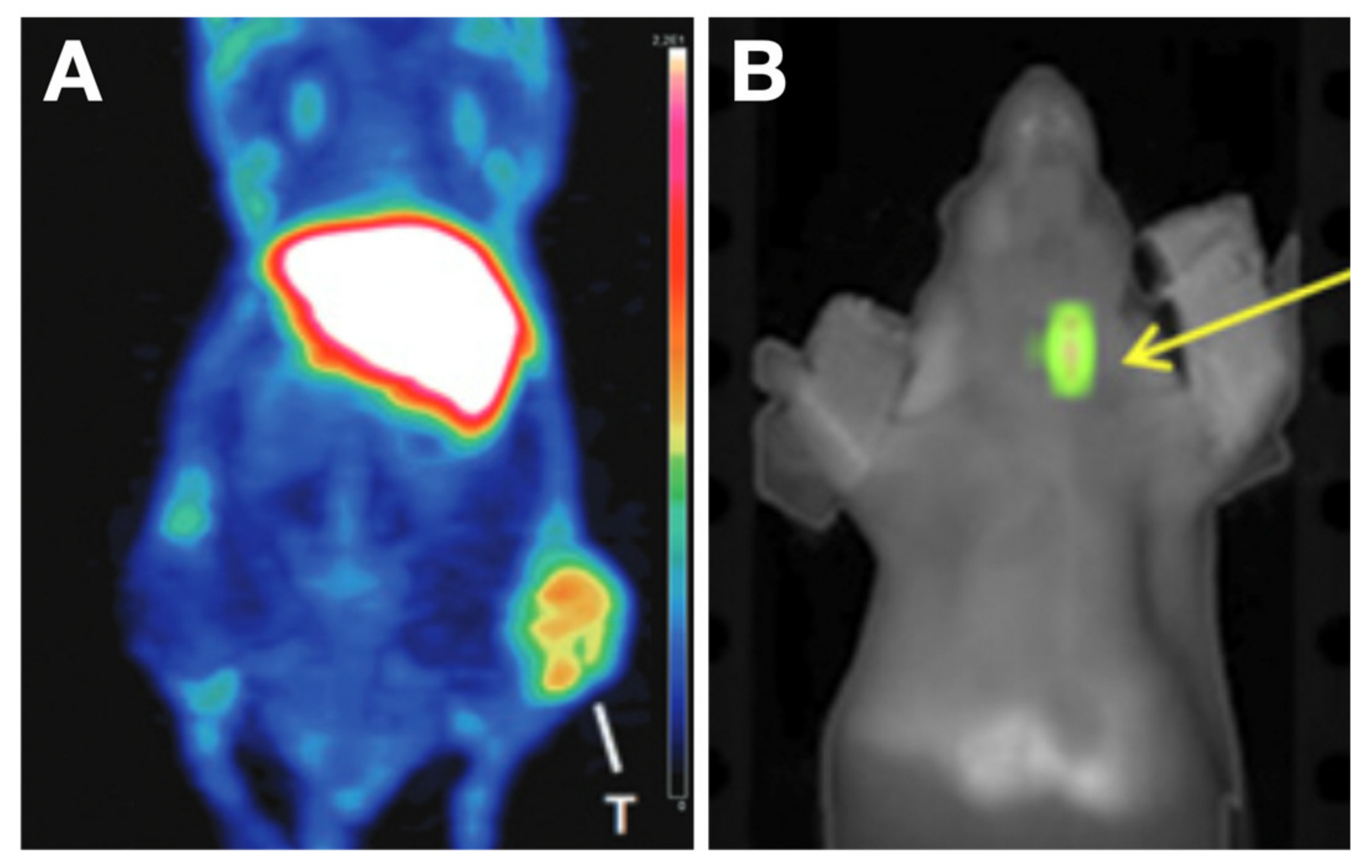
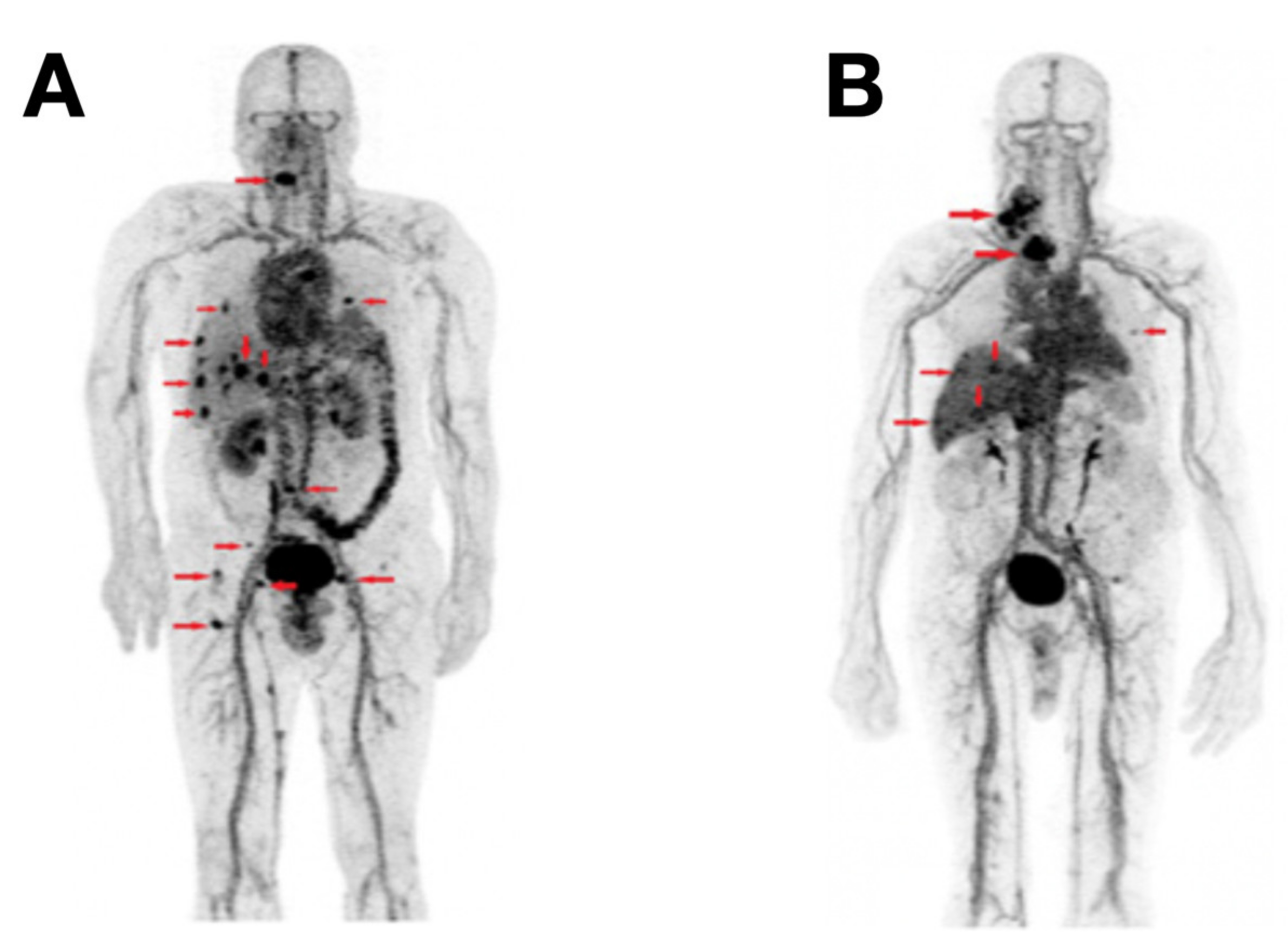

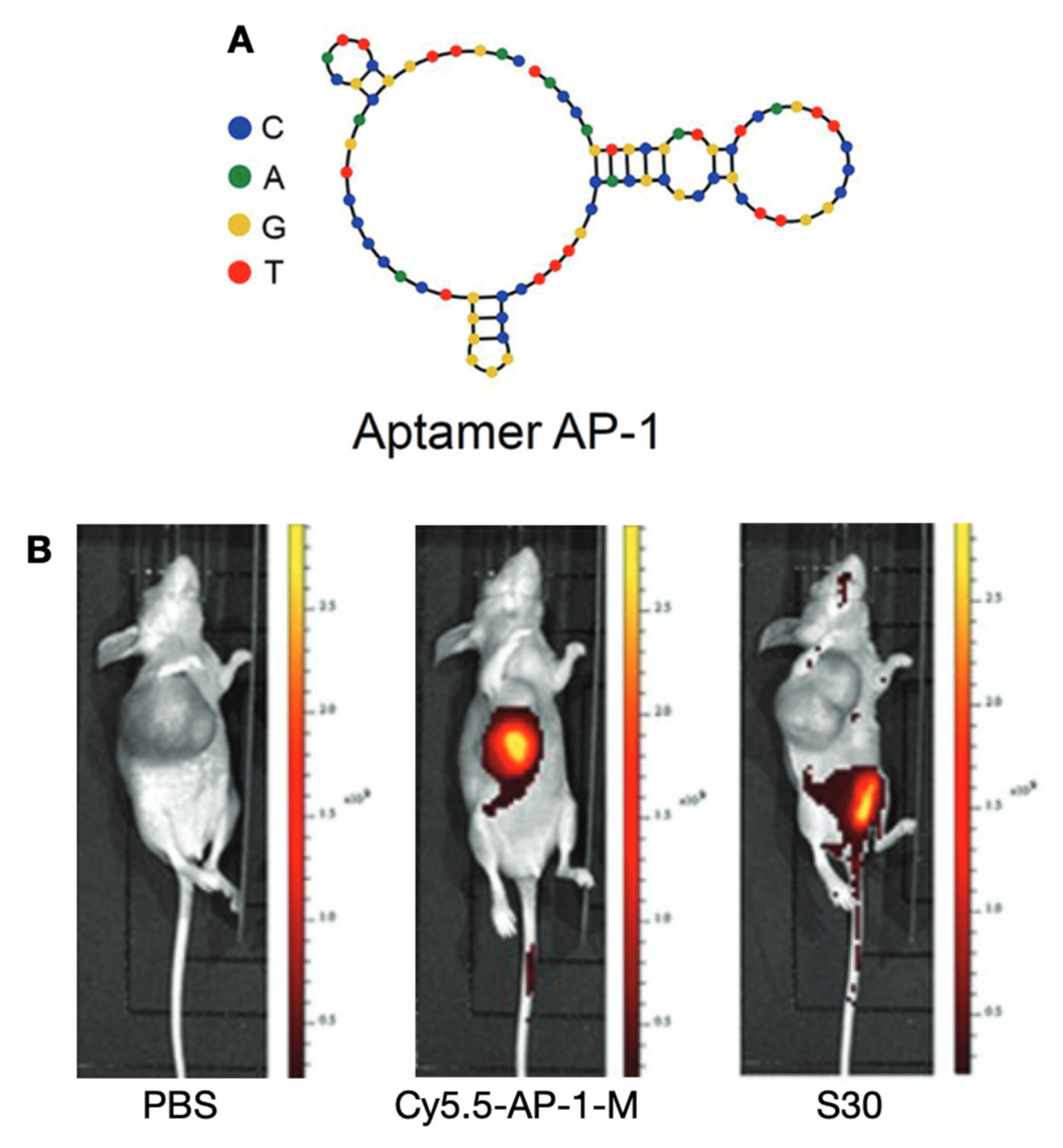
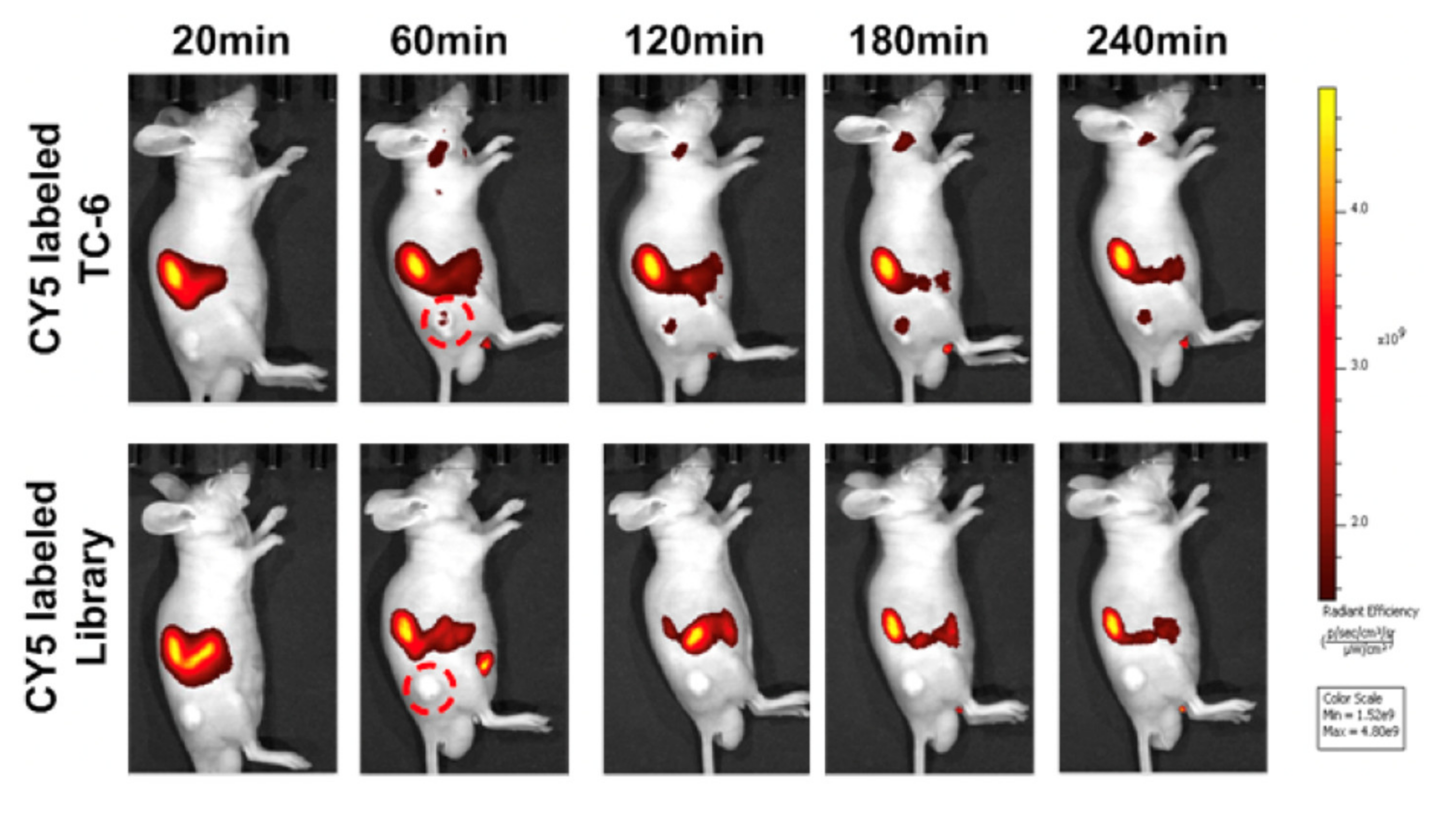


| Tracer Types | Target | TC Type | Typical Roles | LoE [Ref.] |
|---|---|---|---|---|
| Transporter-targeting probes | ||||
| [124I]NaI, [131I]NaI | NIS | DTC | LL, TS, PE, TT, RD | Clinical [35,36] |
| [18F]TFB, [18F]FS, [18F]HFP | NIS | DTC | LL, TS, PE, IUE | Clinical [50,51] |
| [18F]FDG | GLUT1 | TC | LL, TS, PE, PIU | Clinical [5,54,55] |
| [18F]FDOPA | SLC7A5, SLC7A8 | MTC | LL, TS, PE | Clinical [59,65] |
| [11C]MET | SLC7A5 | FTC | LL, TS, PE | Clinical [63] |
| [18F]FGln | SLC7A5, SLC1A5, SLC38A1 | PTC | LL, TS, PE | Clinical [62] |
| [18F]FLT | ENT1 | DTC | LL, TS, PE | Clinical [79,160] |
| Peptide-based probes | ||||
| 68Ga/177Lu/90Y/111In labelled somatostatin analogue | SSTRs | TC | LL, TS, PE, PRRT | Clinical [161,162,163] |
| 68Ga/177Lu labelled RGD2 | αvβ3 | TC | LL, TS, PE, PRRT | Clinical [85,86] |
| 68Ga/177Lu labelled PSMA-ligand | PSMA | DTC | LL, TS, PE, PRRT | Clinical [88,91,164,165] |
| 68Ga/177Lu labelled MGS5 | CCK2R | MTC | LL, TS, PE, PRRT | Clinical [96] and preclinical [95] |
| Antibody-based probes | ||||
| Single target IgG-based probes | ||||
| 89Zr or RDye 800CW labeled pertuzumab | HER2 | ATC | LL, TS | Preclinical [114] |
| 64Cu or RDye 800CW labeled ICAM-1 Ab | ICAM-1 | ATC | LL, TS | Preclinical [101] |
| 89Zr labeled Gal3 Ab | Gal3 | DTC | LL, TS | Preclinical [124] |
| Bispecific IgG-based probes | ||||
| 68Ga/177Lu labelled IMP288 plus TF2 BsAb | CEA × HSG | MTC | LL, TS, PE, PRRT | Clinical [134,166] |
| Fab-based probes | ||||
| 89Zr or Cy5.5 labeled αGal3-Fab | Gal3 | DTC | LL, TS | Preclinical [125,139,140] |
| Nanobody-based probes | ||||
| Targeting TROP-2 probes | TROP-2 | TC (SP [167]) | LL, TS, PRRT | Preclinical (OS) |
| Targeting CD47 probes | CD47 | TC (SP [168]) | LL, TS, PRRT | Preclinical (OS) |
| Targeting CD146 probes | CD146 | TC (SP [169]) | LL, TS, PRRT | Preclinical (OS) |
| Other probes | ||||
| Aptamer-based probes | ||||
| Cy5.5-AP-1-M | CD133 | ATC | LL, TS | Preclinical [15] |
| Cy5-TC-6 | PTC tissue | PTC | LL, TS | Preclinical [16] |
| Nanoparticles-based probes | ||||
| C-HPNs | EGFR | ATC | LL, TS, TT | Preclinical [154] |
| NPs-SHP2 | SHP2 | TC | LL, TS | Preclinical [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Liu, B.; Younis, M.H.; Huang, G.; Liu, J.; Cai, W.; Wei, W. Next-Generation Molecular Imaging of Thyroid Cancer. Cancers 2021, 13, 3188. https://doi.org/10.3390/cancers13133188
Jin Y, Liu B, Younis MH, Huang G, Liu J, Cai W, Wei W. Next-Generation Molecular Imaging of Thyroid Cancer. Cancers. 2021; 13(13):3188. https://doi.org/10.3390/cancers13133188
Chicago/Turabian StyleJin, Yuchen, Beibei Liu, Muhsin H. Younis, Gang Huang, Jianjun Liu, Weibo Cai, and Weijun Wei. 2021. "Next-Generation Molecular Imaging of Thyroid Cancer" Cancers 13, no. 13: 3188. https://doi.org/10.3390/cancers13133188
APA StyleJin, Y., Liu, B., Younis, M. H., Huang, G., Liu, J., Cai, W., & Wei, W. (2021). Next-Generation Molecular Imaging of Thyroid Cancer. Cancers, 13(13), 3188. https://doi.org/10.3390/cancers13133188







