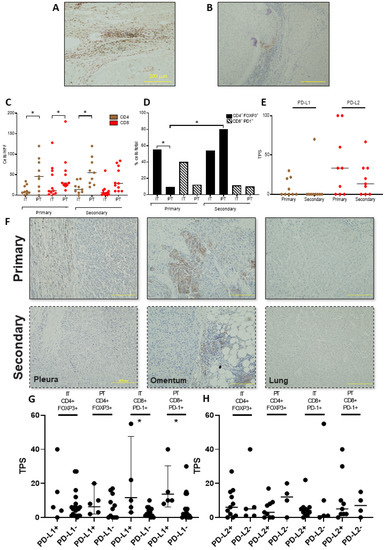
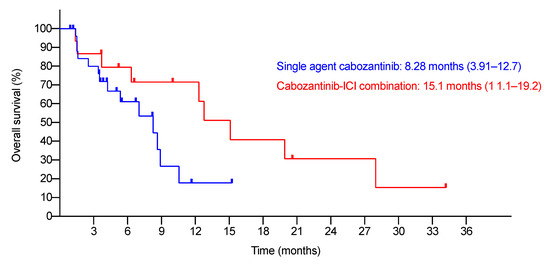
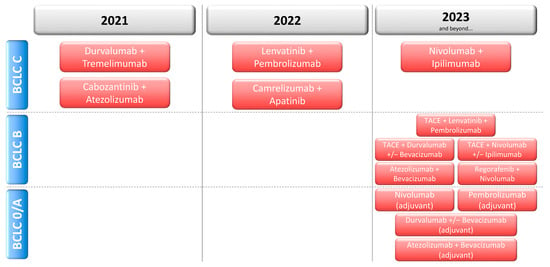
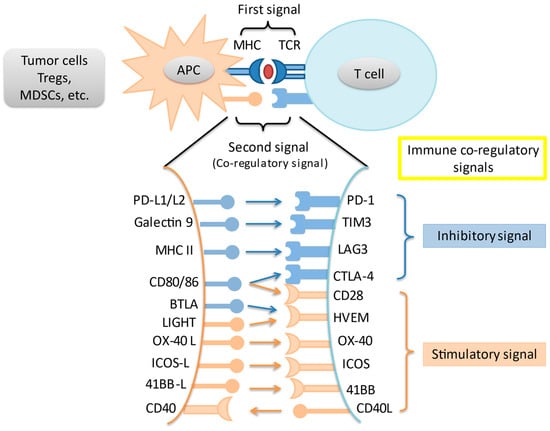
Novel Therapies for Hepatocellular Carcinoma (Closed)
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 90687Editor
2. Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (Milan), Italy
Interests: liver cancer; hepatocellular carcinoma; biliary tract cancer; cholangiocarcinoma; targeted therapies; immunotherapy; combination therapy; prognostic biomarkers; predictive biomarkers; tumor biomarkers; circulating biomarkers; clinical research; translational research
Topical Collection Information
Dear Colleagues,
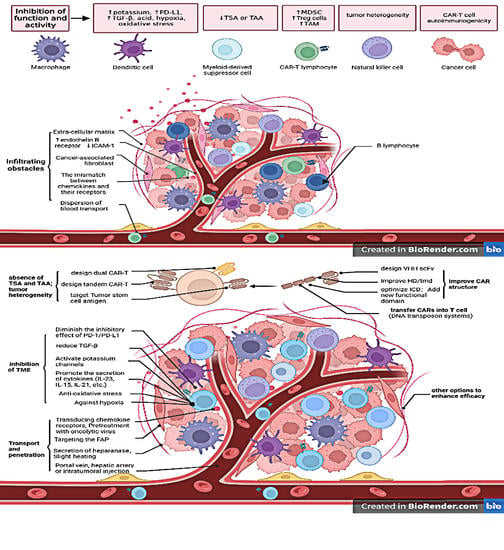
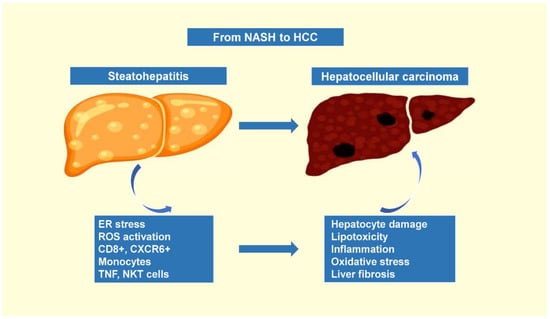
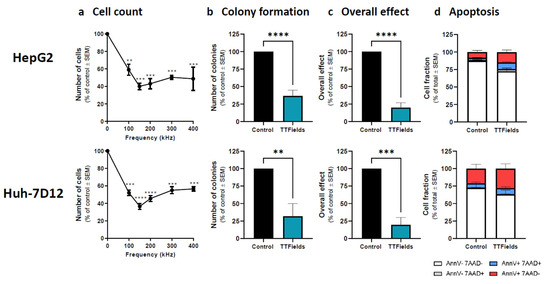
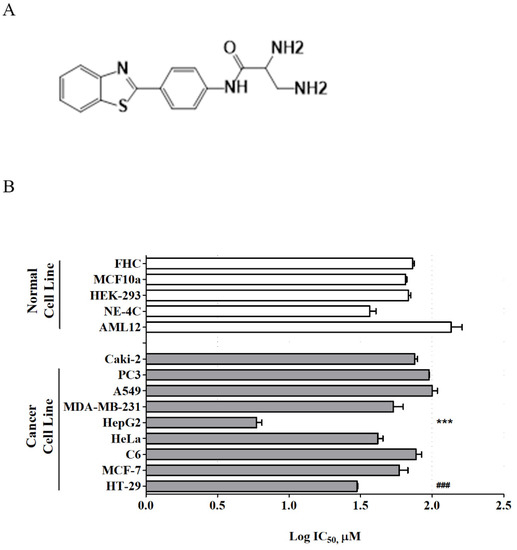
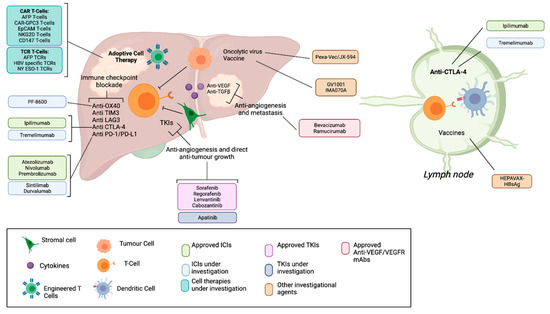
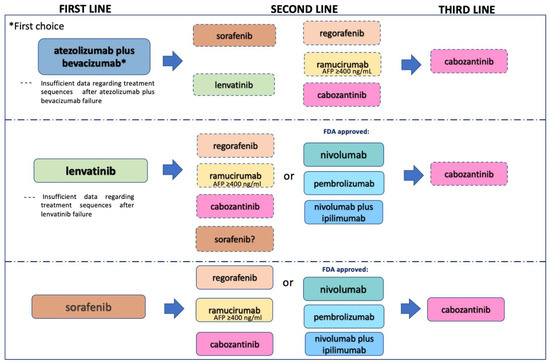
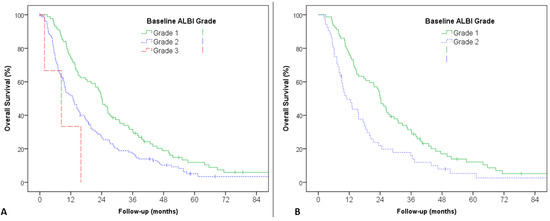
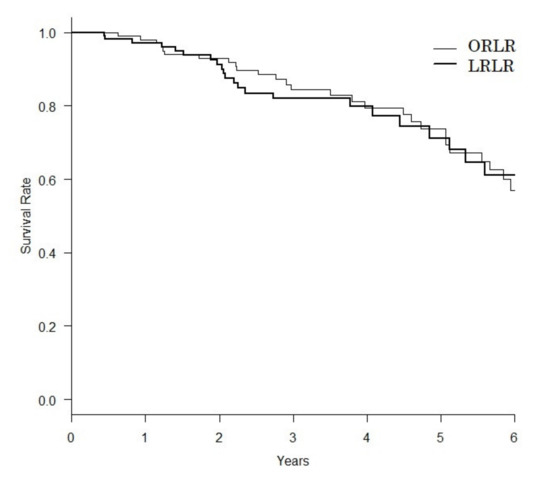
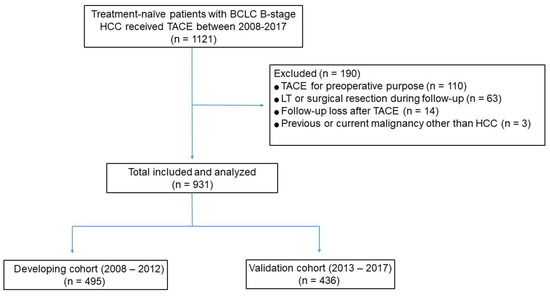
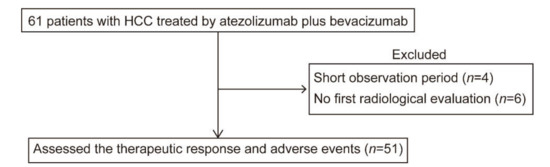
In recent years, therapeutic options and life expectancy for patients with hepatocellular carcinoma (HCC) unsuitable for locoregional therapy have improved, and new systemic therapy options have led to a real change in the approach to this disease. Several therapeutic agents are available for first-, second-, and also third-line treatment, including multikinase inhibitors and monoclonal antibodies. Furthermore, recent positive data combining immunotherapy and antiangiogenic drugs (i.e., atezolizumab and bevacizumab) in front-line settings are leading to further expansion of the therapeutic options. Finally, other combination strategies, including immune checkpoint inhibitors and multikinase inhibitors, are being tested in phase 3 trials and the results are expected to available in the near future. In addition, novel molecular targets and novel drugs, although at an earlier stage of development, may lead to further progress. However, an important yet unmet need is currently represented by the lack of clinical and/or biological factors and/or biomarkers that can guide therapeutic choices.
In this rapidly evolving scenario, it is extremely important that physicians are updated and aware of novel therapeutic options in order to make the best use of them in appropriate clinical settings.
In this Topical Collection, we are inviting both original research articles and reviews focused on the key open issues in the treatment of HCC, such as novel therapies and approaches for treating this disease in advanced stages, novel therapeutic targets, and biomarkers.
Prof. Lorenza Rimassa
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- hepatocellular carcinoma
- targeted therapies
- immunotherapy
- combination therapies
- prognostic biomarkers
- predictive biomarkers
- tumor biomarkers
- circulating biomarkers
- novel targets
- novel therapeutic agents