Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Selection, Meta-Analysis Inclusion Criteria, and Data Extraction
2.3. Statistical Analyses
3. Results
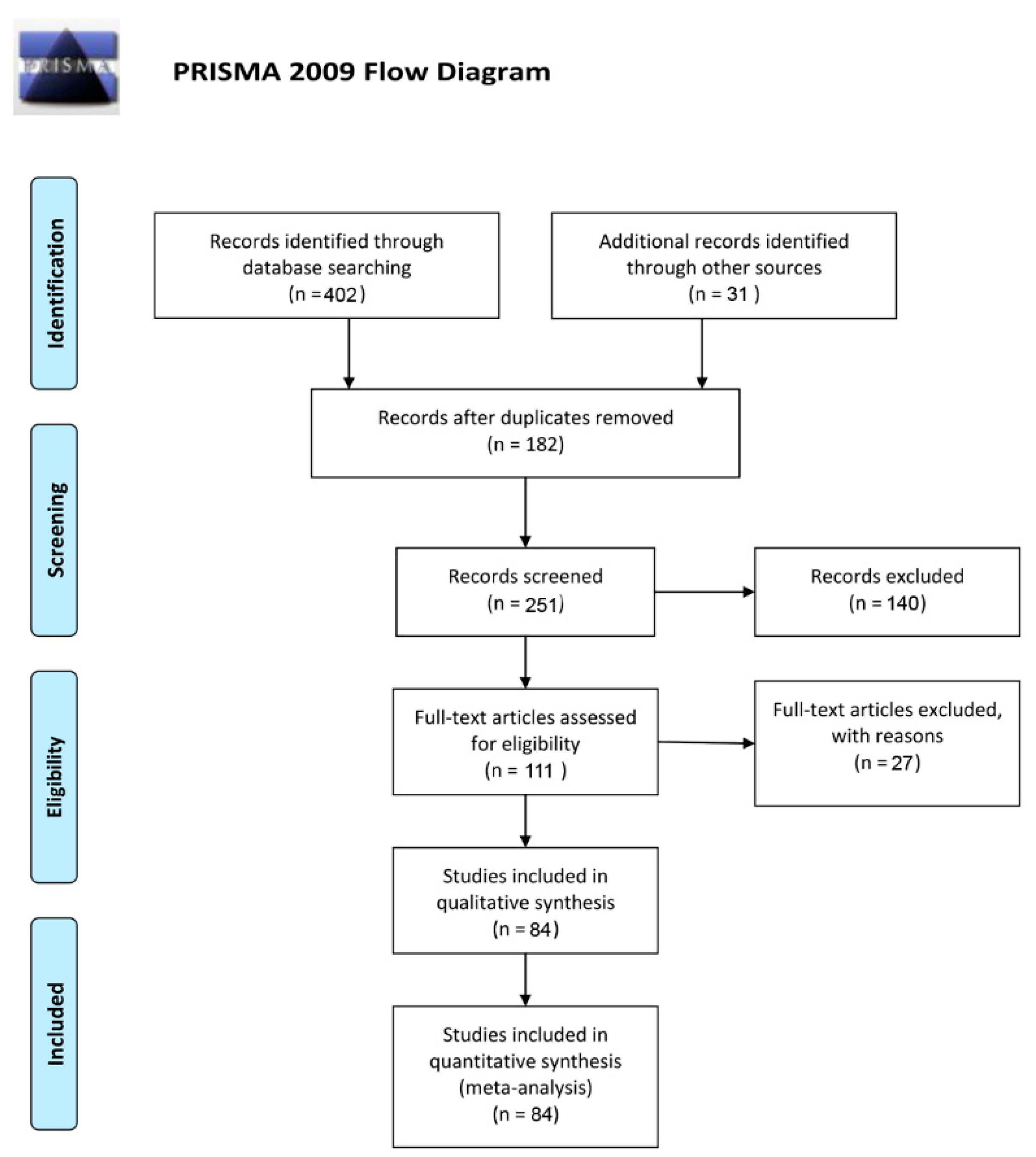
3.1. Eligible Studies and Characteristics
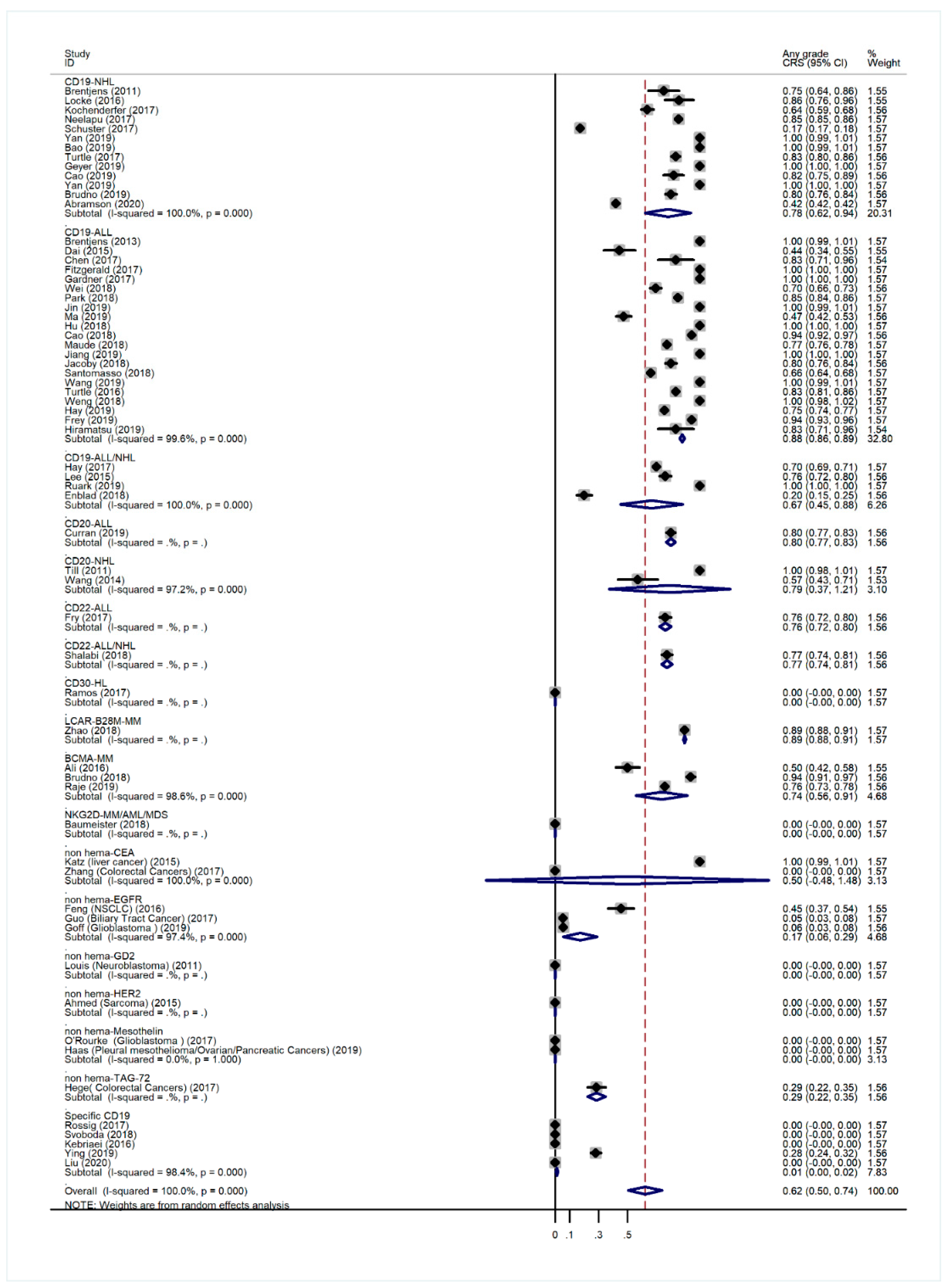
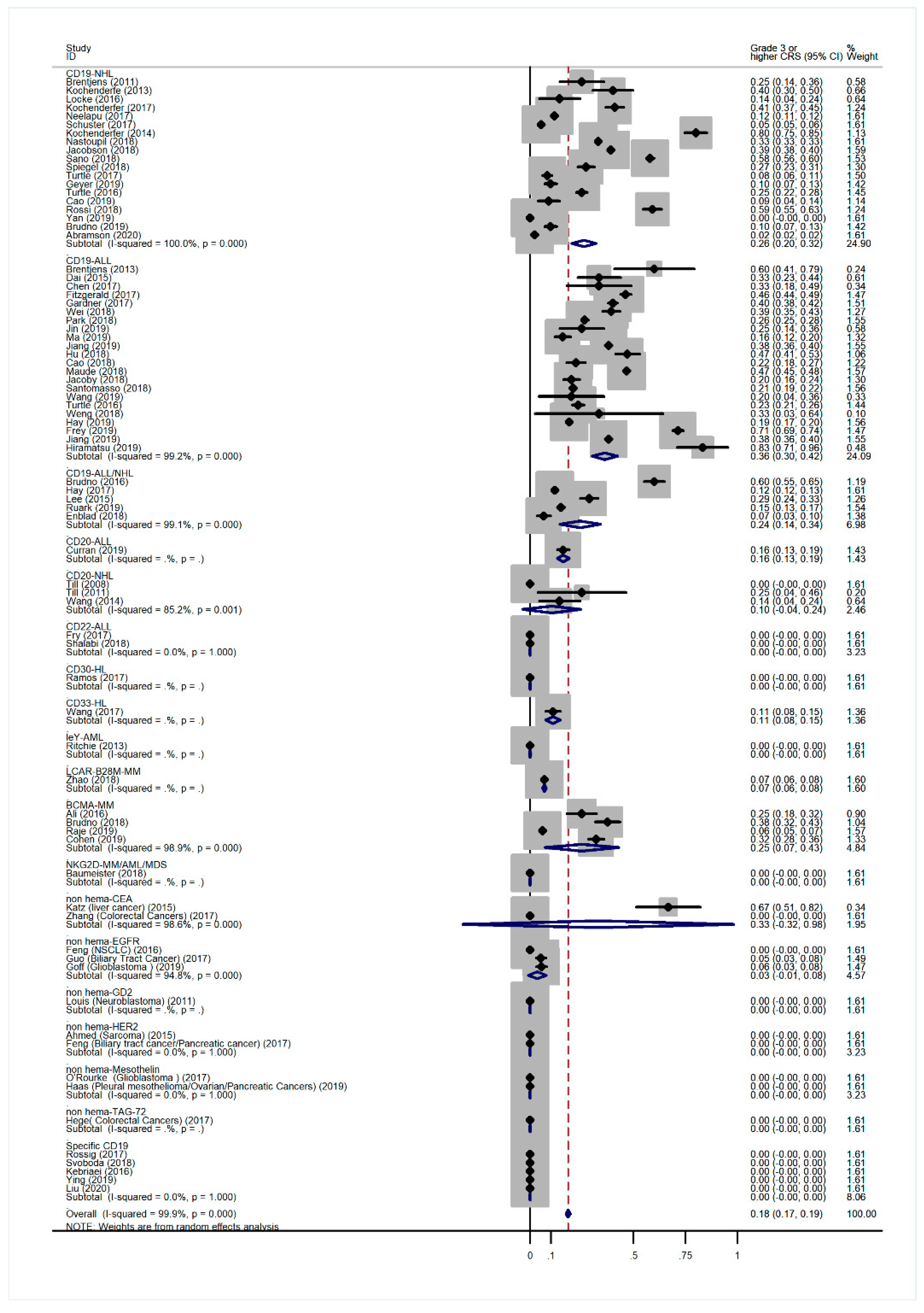
3.2. Overall Incidence of CRS and Non-Hematological AEs
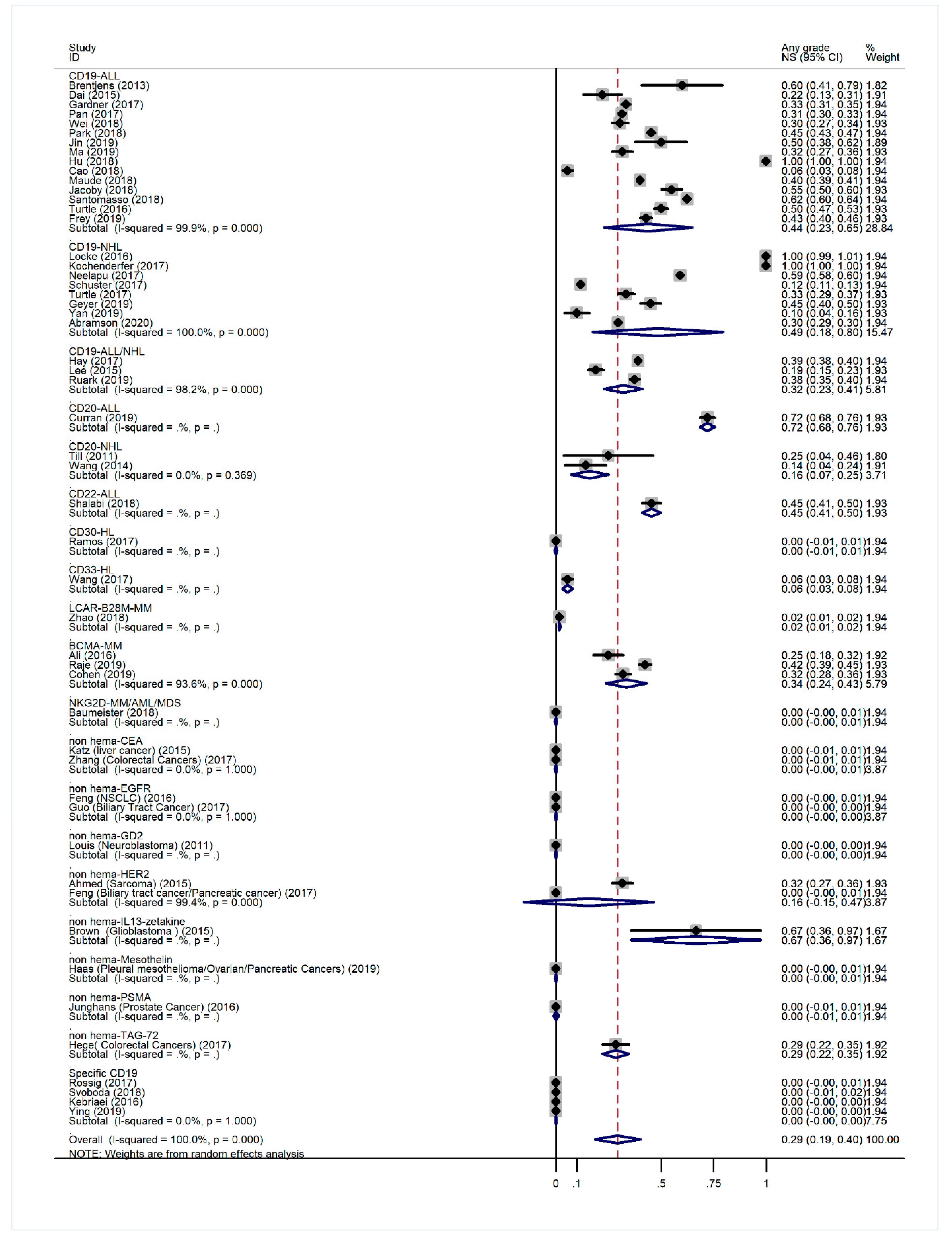
3.3. Overall Incidence of NS-Related AEs
3.4. Incidence of Treatment-Related Deaths
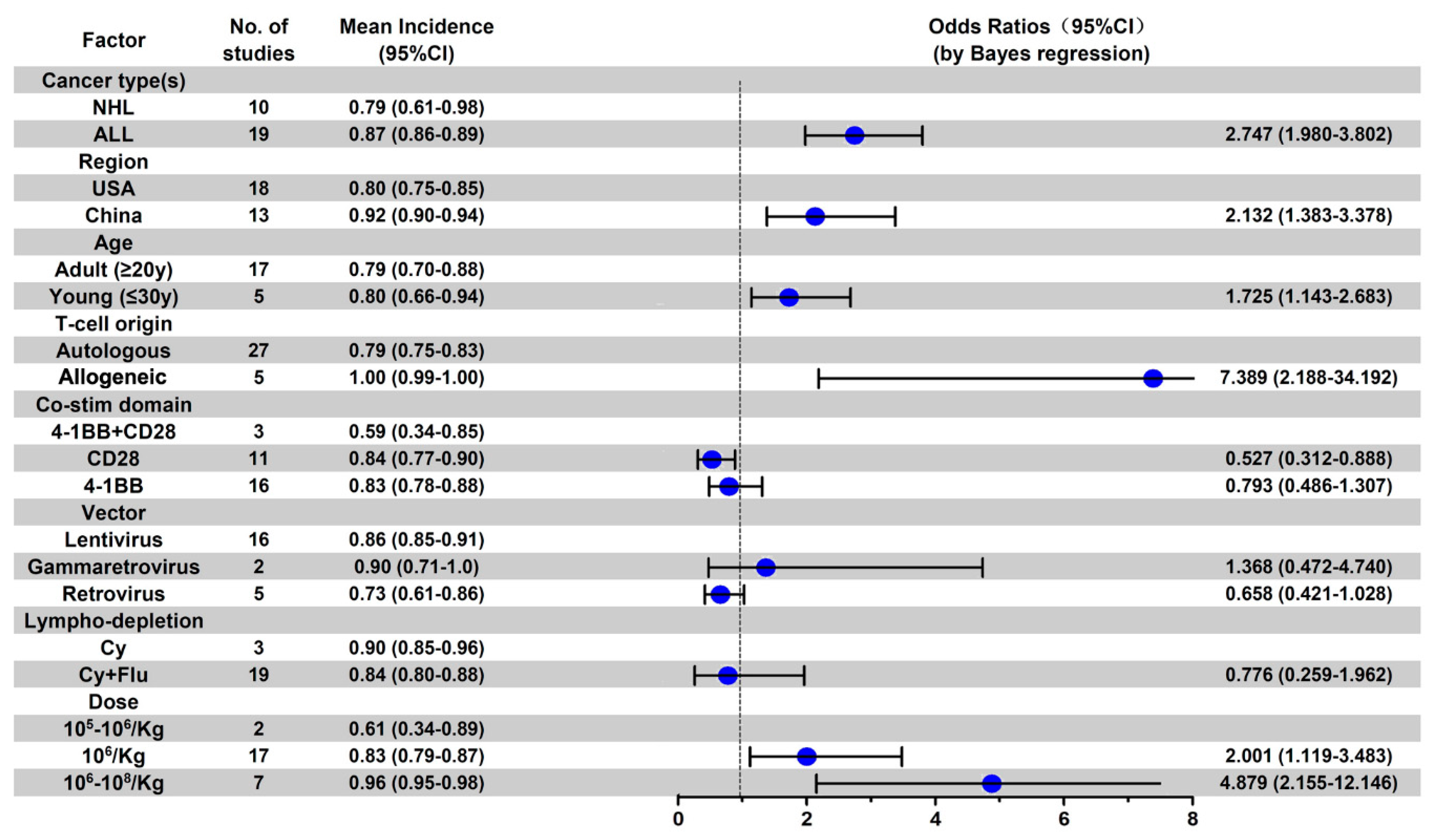
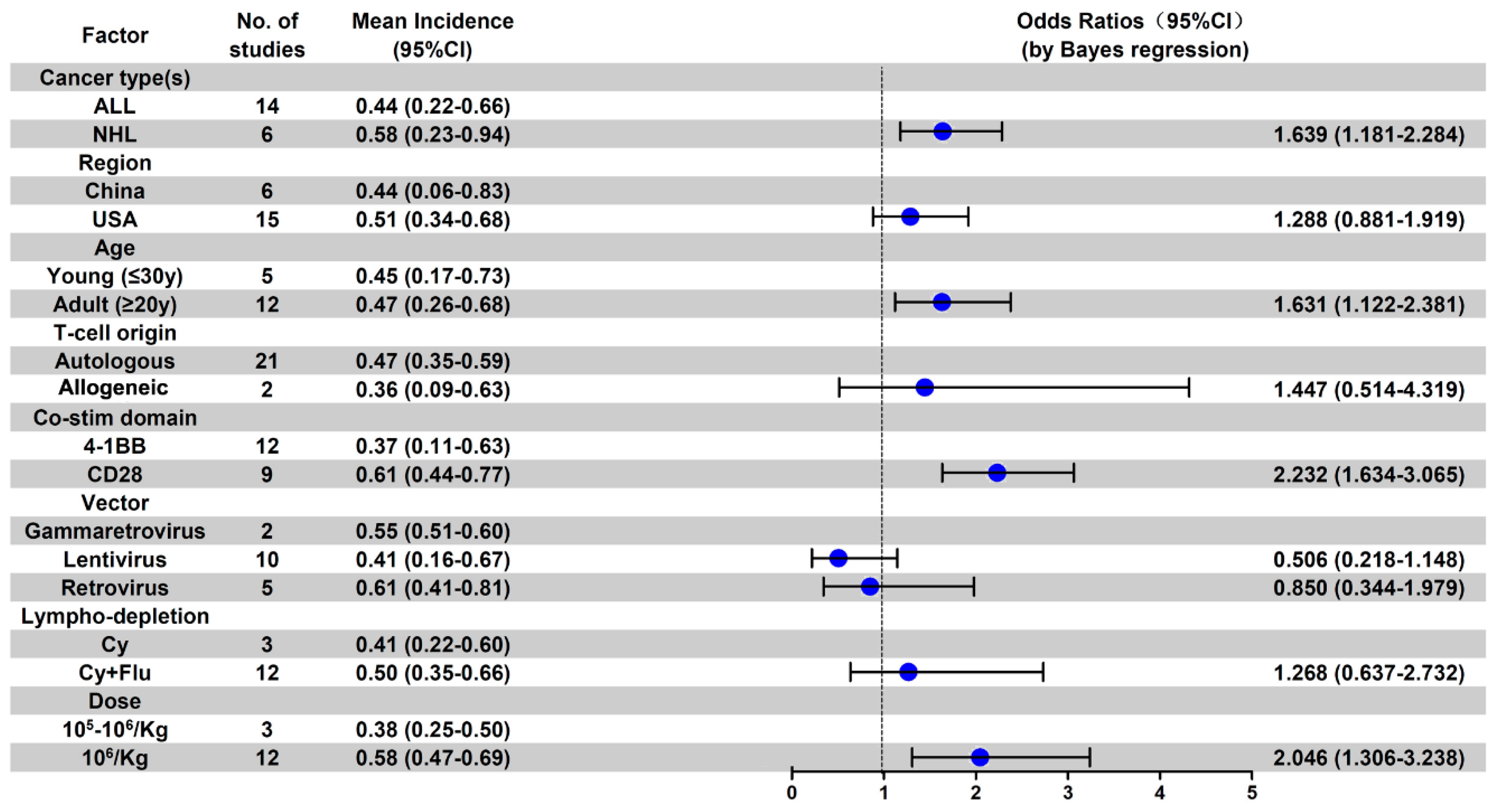
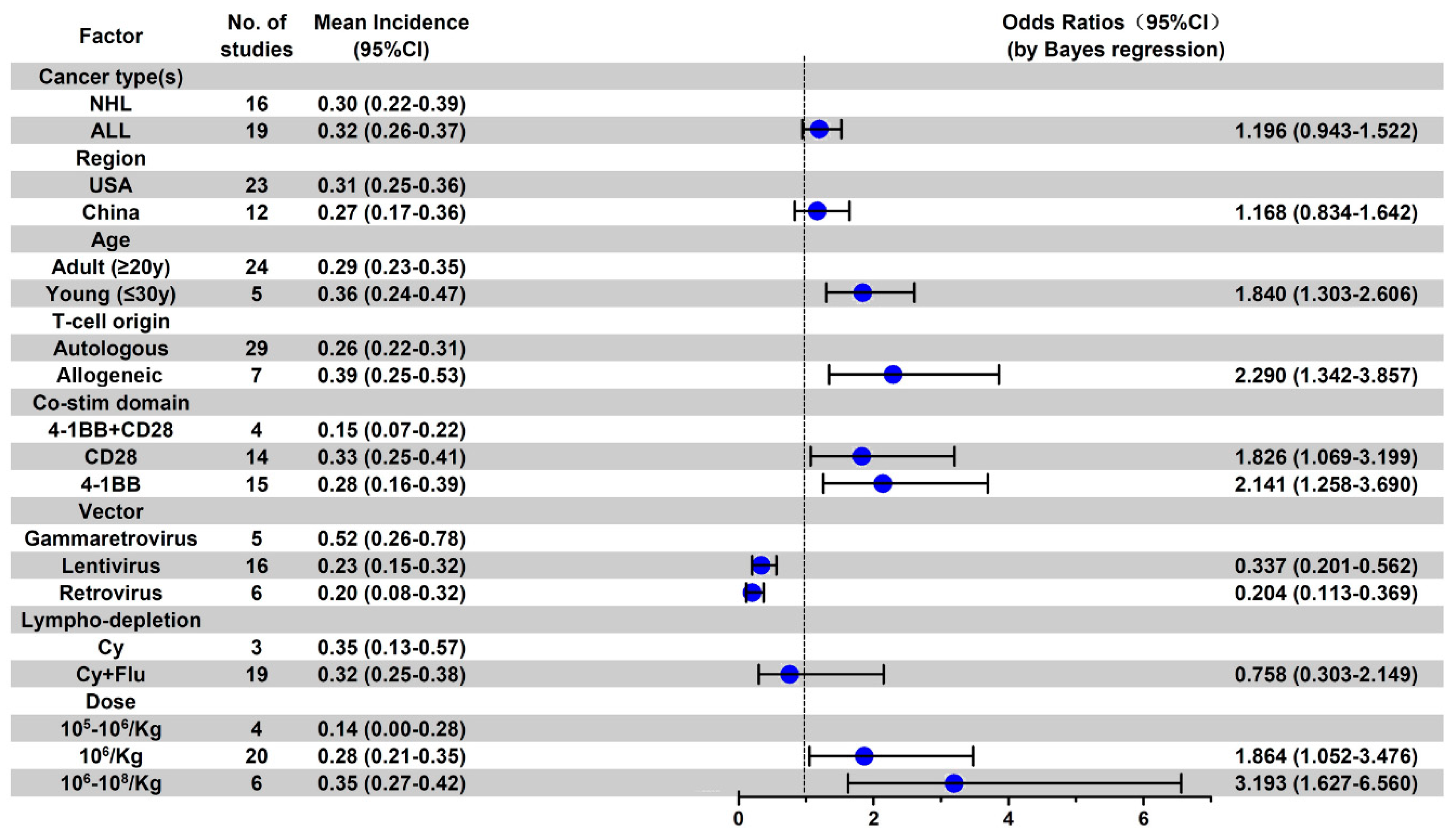
3.5. Subgroup Analysis of AE Incidence by Cancer Type
3.6. Subgroup Analysis of Anti-CD19 CAR-T-Related AE Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Cause of Death | No. (%) |
| Cytokine release syndrome | 23 (43.4) |
| Neurological symptoms | 8 (15.1) |
| Respiratory | |
| pneumonia | 3 (5.6) |
| Pulmonary embolism | 2 (3.7) |
| Cardiovascular | |
| Cardiac arrhythmia | 1 (1.9) |
| Heart failure | 2 (3.7) |
| Infectious | |
| Sepsis | 5 (9.4) |
| Coagulation dysfunction | |
| Hemorrhage | 8 (15.1) |
| aGVHD | 1 (1.9) |


References
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [Green Version]
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Hudecek, M.; Pender, B.; Robinson, E.; Hawkins, R.; Chaney, C.; Cherian, S.; Chen, X.; et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016, 8, 116r–355r. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Siddiqi, T.; Chavez, J.C.; Hosing, C.M.; Ghobadi, A.; Budde, L.E.; Bot, A.; Rossi, J.M.; et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Mol. Ther. 2017, 25, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Shalabi, H.; Wolters, P.L.; Martin, S.; Toledo-Tamula, M.A.; Roderick, M.C.; Struemph, K.; Kane, E.; Yates, B.; Delbrook, C.; Mackall, C.L.; et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J. Immunother. 2018, 41, 350–358. [Google Scholar] [CrossRef]
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Guo, Y.; Dai, H.; Wang, Y.; Li, X.; Jia, H.; Han, W. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 2016, 59, 468–479. [Google Scholar] [CrossRef] [Green Version]
- Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Yvon, E.; Myers, G.D.; Rossig, C.; Russell, H.V.; Diouf, O.; Liu, E.; et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011, 118, 6050–6056. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2018, 9, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; Lopez, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Brentjens, R.J.; Riviere, I.; Park, J.H.; Davila, M.L.; Wang, X.; Stefanski, J.; Taylor, C.; Yeh, R.; Bartido, S.; Borquez-Ojeda, O.; et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011, 118, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013, 5, 138r–177r. [Google Scholar] [CrossRef] [Green Version]
- Kochenderfer, J.N.; Dudley, M.E.; Carpenter, R.O.; Kassim, S.H.; Rose, J.J.; Telford, W.G.; Hakim, F.T.; Halverson, D.C.; Fowler, D.H.; Hardy, N.M.; et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013, 122, 4129–4139. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, W.; Li, X.; Han, Q.; Guo, Y.; Zhang, Y.; Wang, Y.; Wang, C.; Shi, F.; Zhang, Y.; et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology 2015, 4, e1027469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudno, J.N.; Somerville, R.P.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 1112. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cheng, Y.; Suo, P.; Yan, C.; Wang, Y.; Chen, Y.; Han, W.; Xu, L.; Zhang, X.; Liu, K.; et al. Donor-derived CD19-targeted T cell infusion induces minimal residual disease-negative remission in relapsed B-cell acute lymphoblastic leukaemia with no response to donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation. Br. J. Haematol. 2017, 179, 598–605. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Weiss, S.L.; Maude, S.L.; Barrett, D.M.; Lacey, S.F.; Melenhorst, J.J.; Shaw, P.; Berg, R.A.; June, C.H.; Porter, D.L.; et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit. Care Med. 2017, 45, e124–e131. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Pan, J.; Yang, J.F.; Deng, B.P.; Zhao, X.J.; Zhang, X.; Lin, Y.H.; Wu, Y.N.; Deng, Z.L.; Zhang, Y.L.; Liu, S.H.; et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017, 31, 2587–2593. [Google Scholar] [CrossRef]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Hu, Y.; Pu, C.; Yu, J.; Luo, Y.; Shi, J.; Cui, Q.; Wu, W.; Wang, J.; Xiao, L.; et al. CD19 targeted CAR-T therapy versus chemotherapy in re-induction treatment of refractory/relapsed acute lymphoblastic leukemia: Results of a case-controlled study. Ann. Hematol. 2018, 97, 781–789. [Google Scholar] [CrossRef]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cao, Y.; Wang, L.; Sun, R.; Cheng, L.; He, X.; Xiao, X.; Jiang, Y.; Li, Q.; Zhang, H.; et al. HLA-matched and HLA-haploidentical allogeneic CD19-directed chimeric antigen receptor T-cell infusions are feasible in relapsed or refractory B-cell acute lymphoblastic leukemia before hematopoietic stem cell transplantation. Leukemia 2019, 34, 909–913. [Google Scholar] [CrossRef]
- Ruark, J.; Mullane, E.; Cleary, N.; Cordeiro, A.; Bezerra, E.D.; Wu, V.; Voutsinas, J.; Shaw, B.E.; Flynn, K.E.; Lee, S.J.; et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors after Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2020, 26, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Ho, J.Y.; Du, H.; Xuan, F.; Wu, X.; Wang, Q.; Wang, L.; Liu, Y.; Ba, M.; Wang, Y.; et al. Evidence of long-lasting anti-CD19 activity of engrafted CD19 chimeric antigen receptor-modified T cells in a phase I study targeting pediatrics with acute lymphoblastic leukemia. Hematol. Oncol. 2019, 37, 601–608. [Google Scholar] [CrossRef]
- Yan, Z.X.; Li, L.; Wang, W.; OuYang, B.S.; Cheng, S.; Wang, L.; Wu, W.; Xu, P.P.; Muftuoglu, M.; Hao, M.; et al. Clinical Efficacy and Tumor Microenvironment Influence in a Dose-Escalation Study of Anti-CD19 Chimeric Antigen Receptor T Cells in Refractory B-Cell Non-Hodgkin’s Lymphoma. Clin. Cancer Res. 2019, 25, 6995–7003. [Google Scholar] [CrossRef] [Green Version]
- Nastoupil, L.J.; Jain, M.D.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.A.; Lekakis, L.J.; Reagan, P.M.; Oluwole, O.O.; et al. Axicabtagene ciloleucel (Axi-cel) CD19 Chimeric Antigen Receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: Real world experience. Blood 2018, 131 (Suppl. 1), 91, (abstract). [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.; Armand, P.; Kamihara, Y.; Ritz, J.; Rodig, S.J.; Wright, K.; Lipschitz, M.; Redd, R.A.; Maus, M.V.; et al. Axicabtagene ciloleucel in the real world: Outcomes and predictors of response, resistance and toxicity. Blood 2018, 132 (Suppl. 1), 92, (abstract). [Google Scholar] [CrossRef]
- Sano, D.; Nastoupil, L.J.; Fowler, N.H.; Fayad, L.; Hagemeister, F.B.; Lee, H.J.; Samaniego, F.; Wang, M.; Rodriguez, M.A.; Iyer, S.P.; et al. Safety of Axicabtagene ciloleucel CD19 CAR T-cell therapy in elderly patients with relapsed or refractory large B-cell lymphoma. Blood 2018, 132 (Suppl. 1), 96, (abstract). [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Sahaf, B.; Hossain, N.; Frank, M.J.; Claire, G.; Abramian, M.; Latchford, T.; Villa, B.; Cancilla, J.; Oak, J.; et al. Elevated Axicabtagene ciloleucel (CAR-19) expansion by immunophenotyping is associated with toxicity in diffuse large B-cell lymphoma. Blood 2018, 132 (Suppl. 1), 576, (abstract). [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Yin, P.; Guo, T.; Liu, L.; Xia, L.; Wu, Y.; Zhou, F.; Ai, L.; Shi, W.; et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am. J. Hematol. 2019, 94, 1113–1122. [Google Scholar] [CrossRef]
- Bao, F.; Wan, W.; He, T.; Qi, F.; Liu, G.; Hu, K.; Lu, X.A.; Yang, P.; Dong, F.; Wang, J.; et al. Autologous CD19-directed chimeric antigen receptor-T cell is an effective and safe treatment to refractory or relapsed diffuse large B-cell lymphoma. Cancer Gene Ther. 2019, 26, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, J.; Wei, G.; Yu, J.; Luo, Y.; Shi, J.; Wu, W.; Zhao, K.; Xiao, L.; Zhang, Y.; et al. A retrospective comparison of allogenic and autologous chimeric antigen receptor T cell therapy targeting CD19 in patients with relapsed/refractory acute lymphoblastic leukemia. Bone Marrow Transplant. 2019, 54, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Jacoby, E.; Bielorai, B.; Avigdor, A.; Itzhaki, O.; Hutt, D.; Nussboim, V.; Meir, A.; Kubi, A.; Levy, M.; Zikich, D.; et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am. J. Hematol. 2018, 93, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Voutsinas, J.M.; Wu, Q.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; Steinmetz, R.N.; Riddell, S.R.; et al. High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 2019, 134, 636–640. [Google Scholar] [CrossRef]
- Geyer, M.B.; Riviere, I.; Senechal, B.; Wang, X.; Wang, Y.; Purdon, T.J.; Hsu, M.; Devlin, S.M.; Palomba, M.L.; Halton, E.; et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight 2019, 5, e122627. [Google Scholar] [CrossRef]
- Enblad, G.; Karlsson, H.; Gammelgard, G.; Wenthe, J.; Lovgren, T.; Amini, R.M.; Wikstrom, K.I.; Essand, M.; Savoldo, B.; Hallbook, H.; et al. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin. Cancer Res. 2018, 24, 6185–6194. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Gao, L.; Hu, X.; Liu, B.; Chen, J.; Zhang, W.; Wang, J.; Yu, X.; Feng, D.; Chang, A.E.; et al. Chimeric Antigen Receptor-modified Donor Lymphocyte Infusion Improves the Survival of Acute Lymphoblastic Leukemia Patients With Relapsed Diseases After Allogeneic Hematopoietic Stem Cell Transplantation. J. Immunother. 2019, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Popplewell, L.L.; Wagner, J.R.; Naranjo, A.; Blanchard, M.S.; Mott, M.R.; Norris, A.P.; Wong, C.W.; Urak, R.Z.; Chang, W.C.; et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 2016, 127, 2980–2990. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Lai, P.; Qin, L.; Lai, Y.; Jiang, Z.; Luo, C.; Huang, X.; Wu, S.; Shao, D.; Deng, C.; et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 2018, 11, 25. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, J.; Cheng, H.; Qiao, J.; Zhang, H.; Wang, Y.; Shi, M.; Lan, J.; Fei, X.; Jin, L.; et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: A single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e521–e529. [Google Scholar] [CrossRef]
- Hay, K.A.; Gauthier, J.; Hirayama, A.V.; Voutsinas, J.M.; Wu, Q.; Li, D.; Gooley, T.A.; Cherian, S.; Chen, X.; Pender, B.S.; et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019, 133, 1652–1663. [Google Scholar] [CrossRef] [Green Version]
- Garfall, A.L.; Stadtmauer, E.A.; Hwang, W.T.; Lacey, S.F.; Melenhorst, J.J.; Krevvata, M.; Carroll, M.P.; Matsui, W.H.; Wang, Q.; Dhodapkar, M.V.; et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight 2019, 4, e127684. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T.; et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, W.; Sun, R.; Jin, X.; Cheng, L.; He, X.; Wang, L.; Yuan, T.; Lyu, C.; Zhao, M. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B-Cell Non-hodgkin Lymphoma. Front. Oncol. 2019, 9, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, J.; Paczkowski, P.; Shen, Y.W.; Morse, K.; Flynn, B.; Kaiser, A.; Ng, C.; Gallatin, K.; Cain, T.; Fan, R.; et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood 2018, 132, 804–814. [Google Scholar] [CrossRef] [Green Version]
- Rossig, C.; Pule, M.; Altvater, B.; Saiagh, S.; Wright, G.; Ghorashian, S.; Clifton-Hadley, L.; Champion, K.; Sattar, Z.; Popova, B.; et al. Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia 2017, 31, 1087–1095. [Google Scholar] [CrossRef]
- Svoboda, J.; Rheingold, S.R.; Gill, S.I.; Grupp, S.A.; Lacey, S.F.; Kulikovskaya, I.; Suhoski, M.M.; Melenhorst, J.J.; Loudon, B.; Mato, A.R.; et al. Nonviral RNA chimeric antigen receptor-modified T cells in patients with Hodgkin lymphoma. Blood 2018, 132, 1022–1026. [Google Scholar] [CrossRef]
- Kebriaei, P.; Singh, H.; Huls, M.H.; Figliola, M.J.; Bassett, R.; Olivares, S.; Jena, B.; Dawson, M.J.; Kumaresan, P.R.; Su, S.; et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Investig. 2016, 126, 3363–3376. [Google Scholar] [CrossRef]
- Frey, N.V.; Shaw, P.A.; Hexner, E.O.; Pequignot, E.; Gill, S.; Luger, S.M.; Mangan, J.K.; Loren, A.W.; Perl, A.E.; Maude, S.L.; et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2020, 38, 415–422. [Google Scholar] [CrossRef]
- Hiramatsu, H.; Adachi, S.; Umeda, K.; Kato, I.; Eldjerou, L.; Agostinho, A.C.; Natsume, K.; Tokushige, K.; Watanabe, Y.; Grupp, S.A. Efficacy and safety of tisagenlecleucel in Japanese pediatric and young adult patients with relapsed/refractory B cell acute lymphoblastic leukemia. Int. J. Hematol. 2020, 111, 303–310. [Google Scholar] [CrossRef]
- Brudno, J.N.; Lam, N.; Vanasse, D.; Shen, Y.W.; Rose, J.J.; Rossi, J.; Xue, A.; Bot, A.; Scholler, N.; Mikkilineni, L.; et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 2020, 26, 270–280. [Google Scholar] [CrossRef]
- Wang, N.; Hu, X.; Cao, W.; Li, C.; Xiao, Y.; Cao, Y.; Gu, C.; Zhang, S.; Chen, L.; Cheng, J.; et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 2020, 135, 17–27. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Till, B.G.; Jensen, M.C.; Wang, J.; Chen, E.Y.; Wood, B.L.; Greisman, H.A.; Qian, X.; James, S.E.; Raubitschek, A.; Forman, S.J.; et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008, 112, 2261–2271. [Google Scholar] [CrossRef] [Green Version]
- Till, B.G.; Jensen, M.C.; Wang, J.; Qian, X.; Gopal, A.K.; Maloney, D.G.; Lindgren, C.G.; Lin, Y.; Pagel, J.M.; Budde, L.E.; et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: Pilot clinical trial results. Blood 2012, 119, 3940–3950. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.Y.; Han, Q.W.; Liu, Y.; Dai, H.R.; Guo, Y.L.; Bo, J.; Fan, H.; Zhang, Y.; Zhang, Y.J.; et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin. Immunol. 2014, 155, 160–175. [Google Scholar] [CrossRef]
- Curran, K.J.; Margossian, S.P.; Kernan, N.A.; Silverman, L.B.; Williams, D.A.; Shukla, N.; Kobos, R.; Forlenza, C.J.; Steinherz, P.; Prockop, S.; et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 2019, 134, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef]
- Wang, C.M.; Wu, Z.Q.; Wang, Y.; Guo, Y.L.; Dai, H.R.; Wang, X.H.; Li, X.; Zhang, Y.J.; Zhang, W.Y.; Chen, M.X.; et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2017, 23, 1156–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, D.S.; Neeson, P.J.; Khot, A.; Peinert, S.; Tai, T.; Tainton, K.; Chen, K.; Shin, M.; Wall, D.M.; Honemann, D.; et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 2013, 21, 2122–2129. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Zhang, R.; Xiong, Z.; Wei, Z.; Shen, J.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA (+) Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Haas, A.R.; Tanyi, J.L.; O’Hara, M.H.; Gladney, W.L.; Lacey, S.F.; Torigian, D.A.; Soulen, M.C.; Tian, L.; McGarvey, M.; Nelson, A.M.; et al. Phase I Study of Lentiviral-Transduced Chimeric Antigen Receptor-Modified T Cells Recognizing Mesothelin in Advanced Solid Cancers. Mol. Ther. 2019, 27, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Junghans, R.P.; Ma, Q.; Rathore, R.; Gomes, E.M.; Bais, A.J.; Lo, A.S.; Abedi, M.; Davies, R.A.; Cabral, H.J.; Al-Homsi, A.S.; et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016, 76, 1257–1270. [Google Scholar] [CrossRef]
- Hege, K.M.; Bergsland, E.K.; Fisher, G.A.; Nemunaitis, J.J.; Warren, R.S.; McArthur, J.G.; Lin, A.A.; Schlom, J.; June, C.H.; Sherwin, S.A. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer 2017, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.L.; Hua, Z.C. Chimeric Antigen Receptor T-cell (CAR T) Therapy for Hematologic and Solid Malignancies: Efficacy and Safety-A Systematic Review with Meta-Analysis. Cancers 2019, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Grigor, E.J.M.; Fergusson, D.; Kekre, N.; Montroy, J.; Atkins, H.; Seftel, M.D.; Daugaard, M.; Presseau, J.; Thavorn, K.; Hutton, B.; et al. Risks and Benefits of Chimeric Antigen Receptor T-Cell (CAR-T) Therapy in Cancer: A Systematic Review and Meta-Analysis. Transfus. Med. Rev. 2019, 33, 98–110. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.; Yin, P.; Guo, T.; Liu, L.; Xia, L.; Wu, Y.; Zhou, F.; Ai, L.; Shi, W.; et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann. Hematol. 2019, 98, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B.; Danish, H.H.; Ali, A.B.; Li, K.; LaRose, S.; Monk, A.D.; Cote, D.J.; Spendley, L.; Kim, A.H.; Robertson, M.S.; et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain 2019, 142, 1334–1348. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.J.; Maziarz, R.T.; Rusch, E.S.; Li, J.; Signorovitch, J.E.; Romanov, V.V.; Locke, F.L.; Maloney, D.G. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020, 4, 1432–1439. [Google Scholar] [CrossRef]




| First Author | Year | Cancer Type(s) | Patients Evaluated n (% Male) | Age, Years Mean (Range) | Country | CAR-T Type |
|---|---|---|---|---|---|---|
| Hematologic Malignancies | ||||||
| Brentjens [21] | 2011 | CLL/ALL | 8 (80) | 63.9 (51–73) | USA | CD19 |
| Brentjens [22] | 2013 | ALL | 5 (80) | 52.4 (23–66) | USA | CD19 |
| Kochenderfer [23] | 2013 | CLL/DLBCL/MCL | 10 (80) | 52.4 (44–66) | USA | CD19 |
| Dai [24] | 2015 | ALL | 9 (44) | 38.9 (15–65) | China | CD19 |
| Brudno [25] | 2016 | CLL/DLBCL/MCL/ALL | 20 (55) | 48 (20–68) | USA | CD19 |
| Locke [5] | 2016 | NHL | 7 (NR) | NR | USA | CD19 |
| Chen [26] | 2017 | ALL | 6 (17) | 26.5 (8–44) | China | CD19 |
| Fitzgerald [27] * | 2017 | ALL | 39 (51) | 11 (5–22) | USA | CD19 |
| Gardner [28] | 2017 | ALL | 45 (51) | 12.2 (1.3–25.3) | USA | CD19 |
| Hay [18] | 2017 | ALL/CLL/NHL | 133 (70) | 54 (20–73) | USA | CD19 |
| Kochenderfer [29] ** | 2017 | DLBCL/MCL/FL | 22 (NR) | 47.4 (38–64) | USA | CD19 |
| Neelapu [30] ** | 2017 | NHL | 110 (64) | 58 (23–76) | USA | CD19 |
| Pan [31] | 2017 | ALL | 51 (63) | NR (2–68) | China | CD19 |
| Schuster [32] * | 2017 | DLBCL/FL | 28 (61) | NR (25–77) | USA | CD19 |
| Lee [3] | 2015 | ALL/NHL | 21 (66) | 14.7 (5–27) | USA | CD19 |
| Wei [33] | 2018 | ALL | 23 (43) | 35.8 (8–57) | China | CD19 |
| Kochenderfer [4] | 2015 | NHL/CLL | 15 (53) | 56 (30–68) | USA | CD19 |
| Park [34] | 2018 | ALL | 53 (NR) | 44 (23–74) | USA | CD19 |
| Jin [35] | 2019 | ALL | 8 (NR) | NR (30–81) | China | CD19 |
| Ruark [36] | 2019 | ALL/NHL/CLL | 40 (63) | 54 (22–74) | USA | CD19 |
| Ma [37] | 2019 | ALL | 19 (40) | NR (3–13) | China | CD19 |
| Yan [38] | 2019 | NHL | 10 (80) | 47 (32–59) | China | CD19 |
| Nastoupil [39] ** | 2018 | NHL | 274 (NR) | 60 (21–82) | NR | CD19 |
| Jacobson [40] ** | 2018 | NHL | 104 (NR) | 64 (21–84) | NR | CD19 |
| Sano [41] ** | 2018 | NHL | 52 (NR) | 42 (23–64) | NR | CD19 |
| Spiegel [42] ** | 2018 | DLBCL | 22 (NR) | NR | NR | CD19 |
| Jiang [43] | 2019 | ALL | 58 (53) | NR | China | CD19 |
| Bao [44] | 2019 | DLBCL | 5 (NR) | NR (31–67) | China | CD19 |
| Hu [45] | 2018 | ALL | 31 (64) | 31.6 (8–57) | China | CD19 |
| Turtle [46] | 2017 | CLL | 24 (NR) | 61 (40–73) | USA | CD19 |
| Maude [47] * | 2018 | ALL | 75 (NR) | 11 (3–23) | USA | CD19 |
| Jacoby [48] | 2018 | ALL | 20 (60) | 11 (5–48) | Israel | CD19 |
| Santomasso [19] | 2018 | ALL | 53 (75) | NR | USA | CD19 |
| Hirayama [49] | 2019 | FL | 21 (67) | 56 (51–62) | USA | CD19 |
| Geyer [50] | 2019 | CLL/NHL | 20 (70) | 63 (43–75) | USA | CD19 |
| Enblad [51] | 2018 | NHL/ALL | 15 (46) | 61 (24–71) | Sweden | CD19 |
| Wang [52] | 2019 | ALL | 5 (40) | 31 (14–54) | China | CD19 |
| Wang [53] | 2016 | DLBCL/MCL | 16 (56) | 60 (23–75) | USA | CD19 |
| Turtle [1] | 2016 | ALL | 30 (NR) | 40 (20–73) | USA | CD19 |
| Turtle [2] | 2016 | NHL | 32 (84) | 57 (22–70) | USA | CD19 |
| Weng [54] | 2018 | ALL | 3 (66) | 20 (15–34) | China | CD19 |
| Yan [55] | 2019 | MM | 21 (48) | 58 (49.5–61) | China | CD19 + BCMA |
| Hay [56] | 2019 | ALL | 53 (57) | 39 (20–76) | USA | CD19 |
| Garfall [57] | 2018 | MM | 12 (33) | 61 (48–68) | USA | CD19 |
| Ying [58] | 2019 | FL/DLBCL | 25 (52) | NR (24–76) | China | CD19-BBz (86) |
| Cao [59] | 2019 | NHL | 11 (NR) | 65 (26–75) | China | CD19 |
| Rossi [60] | 2018 | HL/NHL | 22 (77) | NR (28–67) | USA | CD19 |
| Rossig [61] | 2017 | ALL | 11 (83) | 9 (2–12) | Germany | CD19 |
| Svoboda [62] | 2018 | HL | 4 (NR) | NR (21–42) | USA | CD19 |
| Kebriaei [63] | 2016 | NHL/ALL | 26 (NR) | 40 (21–61) | USA | CD19 |
| Frey [64] | 2019 | ALL | 35 (69) | 34 (21–70) | USA | CD19 |
| Hiramatsu [65] * | 2019 | ALL | 6 (66) | (5–24) | Japan | CD19 |
| Brudno [66] | 2019 | NHL | 20 (NR) | NR | USA | CD19 (Hu19-CD828Z) |
| Wang [67] | 2020 | ALL/NHL | 89 | 36 (9–71) | China | CD19/22 |
| Abramson [68] *** | 2020 | DLBCL | 269 (65) | 63 (54–70) | USA | CD19 |
| Liu [69] | 2020 | CLL/NHL | 11 (63) | 60 (47–70) | USA | CD19 CAR–NK |
| Till [70] | 2008 | idon–NHL | 9 (89) | 60.2 (43–77) | USA | CD20 |
| Till [71] | 2011 | idon-NHL | 4 (100) | 59.2 (28–80) | USA | CD20 |
| Wang [72] | 2014 | DLBCL | 7 (85) | 62.4 (37–85) | China | CD20 |
| Curran [73] | 2019 | ALL | 25 (NR) | (1–22.5) | USA | CD20 |
| Fry [6] | 2017 | ALL | 21 (62) | 19 (7–30) | USA | CD22 |
| Shalabi [7] | 2018 | ALL/DLBCL | 22 (63) | 17.9 (7.3–30.5) | USA | CD22 |
| Ramos [74] | 2017 | HL | 9 (67) | 34.6 (20–65) | USA | CD30 |
| Wang [75] | 2017 | HL | 18 (72) | 33 (13–77) | China | CD33 |
| Ritchie [76] | 2013 | AML | 5 (33) | 70.6 (64–78) | Australia | LeY |
| Zhao [8] | 2018 | MM | 57 (60) | 54 (27–72) | China | LCAR-B38M |
| Ali [9] | 2016 | MM | 12 (NR) | NR | USA | BCMA |
| Brudno [10] | 2018 | MM | 16 (NR) | NR | USA | BCMA |
| Raje [11] | 2019 | MM | 33 (64) | 60 (37–75) | USA | BCMA |
| Cohen [12] | 2019 | MM | 25 (68) | 58 (44–75) | USA | BCMA |
| Baumeister [77] | 2018 | AML/MDS/MM | 12 (75) | 70 (44–79) | USA | NKG2D |
| Solid Malignancies | ||||||
| Katz [78] | 2015 | Liver Cancer | 6 (67) | 57 (51–66) | USA | CEA |
| Zhang [79] | 2017 | Colorectal Cancer | 10 (70) | 58 (48.8–67) | China | CEA |
| Feng [15] | 2016 | Non Small Cell Lung Cancer | 11 (45) | 58 (40–66) | China | EGFR |
| Guo [80] | 2017 | Biliary Tract Cancer | 19 (53) | 57 (39–70) | China | EGFR |
| Goff [81] | 2019 | Glioblastoma | 18 (83) | NR (43–64) | USA | EGFRvIII |
| Louis [16] | 2011 | Neuroblastoma | 19 (47) | 7 (3–20) | USA | GD2 |
| Ahmed [82] | 2015 | Sarcoma | 19 (47) | 17 (7.7–29.6) | USA | HER2 |
| Feng [17] | 2017 | Biliary Tract Cancer /Pancreatic Cancer | 11 (82) | 60.5 (50–75) | China | HER2 |
| Brown [13] | 2015 | Glioblastoma | 3 (NR) | NR | USA | IL13-zetakine |
| O’Rourke [14] | 2017 | Glioblastoma | 10 (50) | 59.5 (45–76) | USA | Mesothelin |
| Haas [83] | 2019 | Pleural Mesothelioma /Ovarian Carcinoma /Pancreatic Cancer | 15 (67) | 69 (48–75) | USA | Mesothelin |
| Junghans [84] | 2016 | Prostate Cancer | 5 (100) | 61 (51–75) | USA | PSMA |
| Hege [85] | 2017 | Colorectal Cancer | 14 (NR) | NR | USA | TAG-72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Xie, M.; Jiang, Q.; Xu, N.; Li, P.; Liang, A.; Young, K.H.; Qian, W. Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 3912. https://doi.org/10.3390/cancers13153912
Lei W, Xie M, Jiang Q, Xu N, Li P, Liang A, Young KH, Qian W. Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(15):3912. https://doi.org/10.3390/cancers13153912
Chicago/Turabian StyleLei, Wen, Mixue Xie, Qi Jiang, Nengwen Xu, Ping Li, Aibin Liang, Ken H. Young, and Wenbin Qian. 2021. "Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis" Cancers 13, no. 15: 3912. https://doi.org/10.3390/cancers13153912
APA StyleLei, W., Xie, M., Jiang, Q., Xu, N., Li, P., Liang, A., Young, K. H., & Qian, W. (2021). Treatment-Related Adverse Events of Chimeric Antigen Receptor T-Cell (CAR T) in Clinical Trials: A Systematic Review and Meta-Analysis. Cancers, 13(15), 3912. https://doi.org/10.3390/cancers13153912









