The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review
Abstract
:Simple Summary
Abstract
1. Introduction
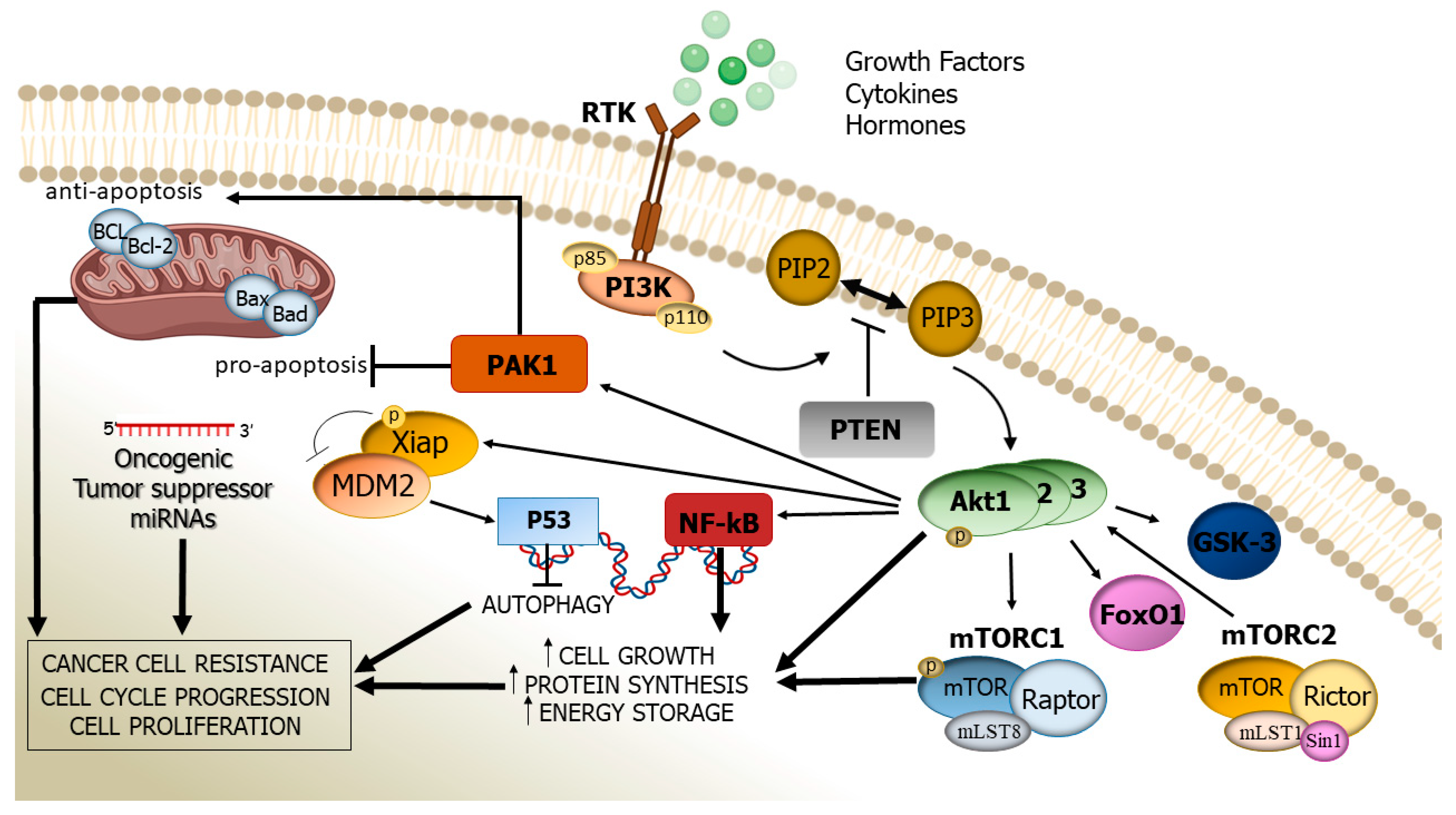
2. Structure, Activation, and Function of the PI3K/Akt Cascade
3. Altered PI3K/Akt Signalling Pathway: Its Pathogenic Role in Human Cancer
4. miRNAs’ Regulation on the PI3K/Akt Pathway
| Study | miRNA | Tumour Type | Regulation | Target | Effect |
|---|---|---|---|---|---|
| Xu X et al. [75] | miR-182-5p | RCC | Negative | AKT/FOXO3a via FLOT1 | Cancer proliferation |
| Lian JH et al. [76] | miR-122 | Positive | PI3K/AKT | Cancer proliferation | |
| Huang J et al. [69] | miR-205-5p | Negative | PI3K/Akt/mTOR and VEGFA | Tumour suppression | |
| Yang SM et al. [81] | miR-21 | Gastric | Negative | PTEN/PI3K/Akt | Drug resistance |
| Li NA et al. [83] | miR-196b | Positive | PI3K/Akt/mTOR | Tumour promoting | |
| Tsukamoto Y et al. [84] | miR-375 | Negative | PKD1/Akt and 14-3-3zeta | Tumour suppression | |
| Peng Y et al. [85] | miR-34 | Negative | PDGFR/Akt and MET | Tumour suppressor | |
| Wang T et al. [86] | miR15a, miR16 | Negative | TWIST1 | Tumour suppressor | |
| Kang W et al. [87] | YAP1 | ||||
| Wu L et al. [88] | miR137 | Negative | AKT2 | Tumour suppressor | |
| Liang M et al. [89] | miR203 | Negative | PIK3CA/Akt | Tumour suppressor | |
| Riquelme I et al. [90] | miR125b-2, miR-451a and miR101-2 | Negative | mTOR via PIK3CB and TSC1 | Tumour suppressor | |
| Deng Y et al. [91] | miR27a | Bladder | Negative | RUNX-1 | Drug resistance |
| Zhao X et al. [94] | gastric, | Negative | p21 | ||
| Li Z et al. [95] | ovarian | Negative | HIPK2 | ||
| Feng DD et al. [96] | leukaemia | Positive | MDR1 | ||
| Chen Z et al. [97] | hepatic | Positive | FZD7/β-catenin | ||
| Zhu B et al. [98] | breast | Positive | BTG2 | ||
| Geng W et al. [99] | miR-520h | Breast | Positive | OTUD3/PTEN |
5. Inhibition of the PI3K/Akt Pathway
6. The PI3K/AKT Pathway and Drug Resistance
7. Target Therapies and Future Prospective
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lei, Y.; Cong, C.; Tan, D.; Zhou, X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer (Review). Mol. Med. Rep. 2019, 19, 4529–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, F.; Evangelisti, G.; Ferro, D.L.; Domenico, S.; Ferraioli, D.; Vellone, V.G.; De Cian, F.; Ferrero, S. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin. Investig. Drugs 2019, 28, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Harari, P.M.; Wheler, D.L.; Toulany, M. Targeting AKT/PKB to improve treatment outocomes for solid tumors. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2020, 819–820, 111690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Shorning, B.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K/AKT-mTOR Pathway and prostate cancer: At the crossroads of AR, MAPK, and WNT signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/AKT signaling pathway and cancer: An update review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, S.; Xiong, S.; Xue, X.; Zhou, X. MicroRNAs and the PTEN/PI3K/Akt pathway in gastric cancer (Review). Oncol. Rep. 2019, 41, 1439–1454. [Google Scholar] [CrossRef] [Green Version]
- Paladini, L.; Fabris, L.; Bottai, G.; Raschioni, C.; Calin, G.A.; Santarpia, L. Targeting microRNA sas key modulators of tumor immune response. Exp. Clin. Cancer Res. 2016, 35, 103. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumors. Cell Biosci. 2020, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Samuels, Y.; Ericson, K. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 2006, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nature reviews. Mol. Cell Biol. 2012, 13, 283–296. [Google Scholar]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Castellano, E.; Downward, J. RAS Interaction with PI3K more than Just another Effector Pathway. Genes Cancer 2011, 2, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Long, Y.C.; Shen, H.M. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy 2015, 11, 1711–1728. [Google Scholar] [CrossRef] [Green Version]
- Ghigo, A.; Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinases in health and disease. Sub-Cell. Biochem. 2012, 58, 183–213. [Google Scholar]
- Williams, R.; Berndt, A.; Miller, S.; Hon, W.C.; Zhang, X. Form and fexibility in phosphoinositide 3-kinases. Biochem. Soc. Trans. 2009, 37, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Szymonowicz, K.; Oeck, S.; Malewicz, N.M.; Jendrossek, V. New insights into protein kinase B/Akt signaling: Role of localized Akt activation and compartment-specific target proteins for the cellular radiation response. Cancers 2018, 10, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toulany, M.; Maier, J.; Iida, M.; Rebholz, S.; Holler, M.; Grottke, A.; Jüker, M.; Wheeler, D.L.; Rothbauer, U.; Rodemann, H.P. Akt1 and Akt3 but not Akt2 through interaction with DNA-PKcs stimulate proliferation and post-irradiation cell survival of K-RAS-mutated cancer cells. Cell Death Discov. 2017, 3, 17072. [Google Scholar] [CrossRef]
- Girardi, C.; James, P.; Zanin, S.; Pinna, L.A.; Ruzzene, M. Differential phosphorylation of Akt1 and Akt2 by protein kinase CK2 may account for isoform specific functions. Biochim. Biophys. Acta 2014, 1843, 1865–1874. [Google Scholar] [CrossRef]
- Andjelković, M.; Alessi, D.R.; Meier, R.; Fernandez, A.; Lamb, N.J.; Frech, M.; Cron, P.; Cohen, P.; Lucocq, J.M.; Hemmings, B.A. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997, 272, 31515–31524. [Google Scholar] [CrossRef] [Green Version]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Hornsveld, M.; Dansen, T.B.; Derksen, P.W.; Burgering, B.M.T. Re-evaluating the role of FOXOs in cancer. Semin. Cancer Biol. 2018, 50, 90–100. [Google Scholar] [CrossRef]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 9, 92–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef]
- Carroll, B.; Dunlop, E.A. The lysosome: A crucial hub for AMPK and mTORC1 signalling. Biochem. J. 2017, 474, 1453–1466. [Google Scholar] [CrossRef] [Green Version]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P.; et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Jasani, B.; Griffiths, D.F.; Gumbleton, M. Phospho-4e-BP1 and eIF4E overexpression synergistically drives disease progression in clinically confined clear cell renal cell carcinoma. Am. J. Cancer Res. 2015, 5, 2838–2848. [Google Scholar] [PubMed]
- Benavente, S.; Vergés, R.; Hermosilla, E.; Fumana, V.; Casanova, N.; García, A.; Ramón y Cajal, S.; Giralt, J. Overexpression of phosphorylated 4E-BP1 predicts for tumor recurrence and reduced survival in cervical carcinoma treated with postoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Korkolopoulou, P.; Levidou, G.; El-Habr, E.A.; Piperi, C.; Adamopoulos, C.; Samaras, V.; Boviatsis, E.; Thymara, I.; Trigka, E.A.; Sakellariou, S.; et al. Phosphorylated 4E-binding protein 1 (p-4E-BP1): A novel prognostic marker in human astrocytomas. Histopathology 2012, 61, 293–305. [Google Scholar] [CrossRef]
- Karlsson, E.; Pérez-Tenorio, G.; Amin, R.; Bostner, J.; Skoog, L.; Fornander, T.; Sgroi, D.C.; Nordenskjöld, B.; Hallbeck, A.L.; Stål, O. The mTOR effectors 4EBP1 and S6K2 are frequently coexpressed, and associated with a poor prognosis and endocrine resistance in breast cancer: A retrospective study including patients from the randomised Stockholm tamoxifen trials. Breast Cancer Res. 2013, 15, R96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallone, G.; Infante, B.; Prisciandaro, C.; Grandaliano, G. mTOR and Aging: An Old Fashioned Dress. Int. J. Mol. Sci. 2019, 20, 2774. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, S.; Li, J.; Wang, D.; Li, Q. Effect of microRNA-135 on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell. Physiol. Biochem. 2017, 42, 1431–1446. [Google Scholar] [CrossRef]
- Shayesteh, L.; Lu, Y.; Kuo, W.-L.; Baldocchi, R.; Godfrey, T.; Collins, C.; Pinkel, D.; Powell, B.; Mills, G.B.; Gray, J.W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat. Genet. 1999, 21, 99–102. [Google Scholar] [CrossRef]
- Samuels, Y.; Waldman, T. Oncogenic mutations of PIK3CA in human cancers. Curr. Top. Microbiol. Immunol. 2010, 347, 21–41. [Google Scholar]
- Alqahtani, A.; Ayesh, H.S.K.; Halawani, H. PIK3CA Gene Mutations in Solid Malignancies: Association with Clinicopathological Parameters and Prognosis. Cancers 2019, 12, 93. [Google Scholar] [CrossRef] [Green Version]
- Murph, M.M.; Smith, D.L.; Hennessy, B.; Lu, Y.; Joy, C.; Coombes, K.R.; Mills, G.B. Individualized molecular medicine: Linking functional proteomics to select therapeutics targeting the PI3K pathway for specific patients. Adv. Exp. Med. Biol. 2008, 622, 183–195. [Google Scholar]
- Cheung, L.W.T.; Hennessy, B.T.; Li, J.; Yu, S.; Myers, A.P.; Djordjevic, B.; Lu, Y.; Stemke-Hale, K.; Dyer, M.D.; Zhang, F.; et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011, 1, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncology 2012, 14, v1–v49. [Google Scholar] [CrossRef]
- Zhao, H.F.; Wang, J.; Shao, W.; Wu, C.P.; Chen, Z.P.; To, S.T.; Li, W.P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Weber, G.L.; Parat, M.O.; Binder, Z.A.; Gallia, G.L.; Riggins, G.J. Abrogation of PIK3CA or PIK3R1 reduces proliferation, migration, and invasion in glioblastoma multiforme cells. Oncotarget 2011, 2, 833–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keniry, M.; Parsons, R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 16, 5–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.Q.; Ruggeri, B.; Klein, W.M.; Sonoda, G.; Altomare, D.A.; Watson, D.K.; Testa, J.R. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc. Natl. Acad. Sci. USA. 1996, 93, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M. The AKT genes and their roles in various disorders. Am. J. Med. Genet. A 2013, 161, 2931–2937. [Google Scholar] [CrossRef]
- Siess, K.M.; Leonard, T.A. Lipid-dependent Akt-ivity: Where, when, and how. Biochem. Soc. Trans. 2019, 47, 897–908. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Z.; Wu, S.C.; Chang, C.M.; Lin, C.L.; Kwan, A.L. Arctigenin, a potent ingredient of Arctium lappa L., induces endothelial nitric oxide synthase and attenuates subarachnoid hemorrhage-induced vasospasm through PI3K/Akt pathway in a rat model. BioMed Res. Int. 2015, 2015, 490209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, Y.R.; Toker, A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol. Cell. 2010, 38, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Ghadami, E.; Dadkhah, T.; Akhavan-Niaki, H. PI3k/AKT signaling pathway: Erythropoiesis and beyond. J. Cell. Physiol. 2019, 234, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Mazloumi Gavgani, F.; Smith Arnesen, V.; Jacobsen, R.G.; Krakstad, C.; Hoivik, E.A.; Lewis, A.E. Class I phosphoinositide 3-Kinase PIK3CA /p110α and PIK3CB/p110β isoforms in endometrial cancer. Int. J. Mol. Sci. 2018, 19, 3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, J.; Gallardo, A.; Cuatrecasas, M.; Catasús, L. Endometrial carcinoma: Pathology and genetics. Pathology 2007, 39, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Martinez-Outschoorn, U.E.; Schilder, R.J.; Kim, C.H.; Richard, S.D.; Rosenblum, N.G.; Johnson, J.M. Metformin as a therapeutic target in endometrial cancers. Front. Oncol. 2018, 8, 341. [Google Scholar] [CrossRef]
- Hua, L.; Zhang, L.; Zhang, X.; Cui, Z. PI3K/AKT/mTOR pathway promotes progestin resistance in endometrial cancer cells by inhibition of autophagy. OncoTargets Ther. 2017, 10, 2865–2871. [Google Scholar]
- Mabuchi, S.; Kuroda, H.; Takahashi, R.; Sasano, T. The PI3K/AKT/mTOR pathway as a therapeutic target in ovarian cancer. Gynecol. Oncol. 2015, 137, 173–179. [Google Scholar] [CrossRef]
- Levine, D.A.; Bogomolniy, F.; Yee, C.J.; Lash, A.; Barakat, R.R.; Borgen, P.I.; Boyd, J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 2005, 11, 2875–2878. [Google Scholar] [CrossRef] [Green Version]
- Andorfer, P.; Heuwieser, A.; Heinzel, A.; Lukas, A.; Mayer, B.; Perco, P. Vascular endothelial growth factor A as predictive marker for mTOR inhibition in relapsing high-grade serous ovarian cancer. BMC Syst. Biol. 2016, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Stemke-Hale, K.; Gonzalez-Angulo, A.M.; Lluch, A.; Neve, R.M.; Kuo, W.L.; Davies, M.; Carey, M.; Hu, Z.; Guan, Y.; Sahin, A.; et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008, 68, 6084–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Cheung, L.W.; Li, J.; Ju, Z.; Yu, S.; Stemke-Hale, K.; Dogruluk, T.; Lu, Y.; Liu, X.; Gu, C.; et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012, 22, 2120–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netti, G.S.; Lucarelli, G.; Spadaccino, F.; Castellano, G.; Gigante, M.; Divella, C.; Rocchetti, M.T.; Rascio, F.; Mancini, V.; Stallone, G.; et al. PTX3 modulates the immunoflogosis in tumor microenvironment and is a prognostic factor for patients with clear cell renal cell carcinoma. Aging (Albany NY) 2020, 12, 7585–7602. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Ditonno, P.; Bettocchi, C.; Vavallo, A.; Rutigliano, M.; Galleggiante, V.; Larocca, A.M.; Castellano, G.; Gesualdo, L.; Grandaliano, G.; et al. Diagnostic and prognostic role of preoperative circulating CA 15-3, CA 125, and beta-2 microglobulin in renal cell carcinoma. Dis. Markers 2014, 2014, 689795. [Google Scholar] [CrossRef]
- Dawood, M.; Mills, G.B.; Ding, Z. Shrewd AKT regulation to survive. Oncoscience 2014, 1, 113–114. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Liu, Q.; Wu, F.; Liu, N.; Qu, H.; Yuan, Y.; Li, Y.; Gao, H.; Ge, J.; Xu, Y.; et al. Prognostic significance of PI3K/AKT/mTOR signaling pathway members in clear cell renal cell carcinoma. PeerJ 2020, 8, e9261. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Wen, G.; Ren, Y. miRNA-205-5p functions as a tumor suppressor by negatively regulating VEGFA and PI3K/Akt/mTOR signaling in renal carcinoma cells. Oncol. Rep. 2019, 42, 1677–1688. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Taheri, M.; Shirvani-Farsani, Z.; Ghafouri-Fard, S. Expression profile of microRNAs in bladder cancer and their application as biomarkers. Biomed. Pharmacother. 2020, 131, 110703. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaccino, F.; Gigante, M.; Netti, G.S.; Rocchetti, M.T.; Franzin, R.; Gesualdo, L.; Castellano, G.; Stallone, G.; Ranieri, E. The ambivalent role of miRNAs in carcinogenesis: Involvement in renal cell carcinoma and their clinical applications. Pharmaceuticals 2021, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mo, Y.Y. The Akt-associated microRNAs. Cell. Mol. Life Sci. 2012, 69, 3601–3612. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wu, J.; Li, S.; Hu, Z.; Xu, X.; Zhu, Y.; Liang, Z.; Wang, X.; Lin, Y.; Mao, Y.; et al. Downregulation of microRNA-182-5p contributes to renal cell carcinoma proliferation via activating the AKT/FOXO3a signaling pathway. Mol. Cancer 2014, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.H.; Wang, W.H.; Wang, J.Q.; Zhang, Y.H.; Li, Y. MicroRNA-122 promotes proliferation, invasion and migration of renal cell carcinoma cells through the PI3K/Akt signaling pathway. Asian Pac. J. Cancer Prev. 2013, 14, 5017–5021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.W.; Li, S.; Yin, F.; Qin, L.L. Expression of miR-205 in renal cell carcinoma and its association with clinicopathological features and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 662–670. [Google Scholar]
- Zhang, Z.; Li, Z.; Li, Y.; Zang, A. MicroRNA and signaling pathways in gastric cancer. Cancer Gene Ther. 2014, 21, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, T.; Yashiro, M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers 2014, 6, 1441–1463. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.H.; Lee, H.S.; Kim, W.H. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab. Investig. 2002, 82, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.M.; Huang, C.; Li, X.F.; Yu, M.Z.; He, Y.; Li, J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 2013, 306, 162–168. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, Y.; Jingjing, D.; Deng, T.; Liu, R.; Zhang, L.; Bai, M.; Li, J.; Zhou, L.; Ning, T.; et al. Integrated analysis of the miRNA, gene and pathway regulatory network in gastric cancer. Oncol. Rep. 2016, 35, 1135–1146. [Google Scholar] [CrossRef]
- Li, N.A.; Wang, W.; Xu, B.; Gong, H. miR-196b regulates gastric cancer cell proliferation and invasion via PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 2016, 11, 1745–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, Y.; Nakada, C.; Noguchi, T.; Tanigawa, M.; Nguyen, L.T.; Uchida, T.; Hujiya, N.; Matsuura, K.; Fujioka, T.; Seto, M.; et al. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010, 70, 2339–2349. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Guo, J.J.; Liu, Y.M.; Wu, X.L. MicroRNA-34 inhibits the growth, invasion, and metastasis of gastric cancer by targeting PDGFR and MET expression. Biosci. Rep. 2014, 34, e00112. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Li, Z.; Zheng, Z.; Wei, J.; Song, D.; Hu, T.; Wu, Q.; Yang, J.Y.; Cai, J.C. miR-15a-3p and miR-16-1-3p negatively regulate twist1 to repress gastric cancer cell invasion and metastasis. Int. J. Biol. Sci. 2017, 13, 122–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, W.; Tong, J.H.M.; Lung, R.W.M.; Dong, Y.; Zhao, J.; Liang, Q.; Zhang, L.; Pan, Y.; Yang, W.; Pang, J.C.S. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol. Cancer 2015, 14, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Chen, J.; Ding, C.; Wei, S.; Zhu, Y.; Yang, W.; Zhang, X.; Wei, X.; Han, D. MicroRNA-137 contributes to dampened tumorigenesis in human gastric cancer by Targeting AKT2. PLoS ONE 2015, 10, e0130124. [Google Scholar] [CrossRef]
- Liang, M.; Shi, B.; Liu, J.; He, L.; Yi, G.; Zhou, L.; Yu, G.; Zhou, X. Downregulation of miR203 induces overexpression of PIK3CA and predicts poor prognosis of gastric cancer patients. Drug Des. Dev. Ther. 2015, 9, 3607–3616. [Google Scholar]
- Riquelme, I.; Tapia, O.; Leal, P.; Sandoval, A.; Varga, M.G.; Letelier, P.; Buchegger, K.; Bizama, C.; Espinoza, J.A.; Peek, R.M.; et al. miR-101-2, miR-125b-2 and miR-451a act as potential tumor suppressors in gastric cancer through regulation of the PI3K/AKT/mTOR pathway. Cell. Oncol. (Dordr.) 2016, 39, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Bai, H.; Hu, H. rs11671784 G/A variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem. Biophys. Res. Commun. 2015, 458, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Homami, A.; Ghazi, F. MicroRNAs as biomarkers associated with bladder cancer. Med. J. Islamic Repub. Iran 2016, 30, 475. [Google Scholar]
- Magee, P.; Shi, L.; Garofalo, M. Role of microRNAs in chemoresistance. Ann. Transl. Med. 2015, 3, 332. [Google Scholar] [PubMed]
- Zhao, X.; Yang, L.; Hu, J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J. Exp. Clin. Cancer Res. 2011, 30, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Hu, S.; Wang, J.; Cai, J.; Xiao, L.; Yu, L.; Wang, Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol. Oncol. 2010, 119, 125–130. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhang, H.; Zhang, P.; Zheng, Y.S.; Zhang, X.J.; Han, B.W.; Luo, X.Q.; Xu, L.; Zhou, H.; Qu, L.H.; et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell. Mol. Med. 2011, 15, 2164–2175. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ma, T.; Huang, C.; Zhang, L.; Lv, X.; Xu, T.; Hu, T.; Li, J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/β-catenin pathway in hepatocellular carcinoma cells. Cell. Signal. 2013, 25, 2693–2701. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, W.; Fu, Y.; Cui, X.; Jin, L.; Chao, J.; Yun, X.; Gao, P.; Shan, S.; Li, J.; et al. MicroRNA-27a-3p Reverses Adriamycin Resistance by Targeting BTG2 and Activating PI3K/Akt Pathway in Breast Cancer Cells. OncoTargets Ther. 2020, 13, 6873–6884. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Song, H.; Zhao, Q.; Dong, K.; Pu, Q.; Gao, H.; Lv, Y. miR-520h Stimulates Drug Resistance to Paclitaxel by Targeting the OTUD3-PTEN Axis in Breast Cancer. Biomed. Res. Int. 2020, 9512793. [Google Scholar] [CrossRef]
- Cretella, D.; Digiacomo, G.; Giovannetti, E.; Cavazzoni, A. PTEN alterations as a potential mechanism for tumor cell escape from PD-1/PD-L1 inhibition. Cancers 2019, 11, 1318. [Google Scholar] [CrossRef] [Green Version]
- Luongo, F.; Colonna, F.; Calapa, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN tumor-suppressor: The dam of stemness in cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maehama, T.; Taylor, G.S.; Dixon, J.E. PTEN and myotubularin: Novel phosphoinositide phosphatases. Annu. Rev. Biochem. 2001, 70, 247–279. [Google Scholar] [CrossRef]
- Sugiyama, M.G.; Fairn, G.D.; Antonescu, C.N. Akt-ing Up Just About Everywhere: Compartment-Specific Akt Activation and Function in Receptor Tyrosine Kinase Signaling. Front. Cell Dev. Biol. 2019, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Downes, C.P. PTEN function: How normal cells control it and tumour cells lose it. Biochem. J. 2004, 382, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Huang, Y.; Ge, C.; Li, Z.; Tian, H.; Li, Q.; Li, H.; Li, R.; Tao, X.; Xue, Y.; et al. SHIP1 inhibits cell growth, migration, and invasion in non-small cell lung cancer through the PI3K/AKT pathway. Oncol. Rep. 2019, 41, 2337–2350. [Google Scholar] [CrossRef]
- Eramo, M.J.; Mitchell, C.A. Regulation of PtdIns (3,4,5) P3/Akt signalling by inositol polyphosphate 5-phosphatases. Biochem. Soc. Trans. 2016, 44, 240–252. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.; Silveira, G.G.; Soave, D.F.; Costa, J.P.O.; Silva, A.R. The role of the LY294002—A non-selective inhibitor of phosphatidylinositol 3-kinase (PI3K) pathway—in cell survival and proliferation in cell line SCC-25. Asian Pac. J. Cancer Prev. 2019, 20, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Bavelloni, A.; Focaccia, E.; Piazzi, M.; Orsini, A.; Ramazzotti, G.; Cocco, L.; Blalock, W.; Faenza, I. Therapeutic potential of nvp-bkm120 in human osteosarcomas cells. J. Cell. Physiol. 2019, 234, 10907–10917. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Becker, K.P.; Mekhail, T.; Chowdhary, S.A.; Eakle, J.F.; Wright, D.; Langdon, R.M.; Yost, K.J.; Padula, G.D.A.; West-Osterfield, K.; et al. Phase I/II study of bevacizumab with BKM120, an oral PI3K inhibitor, in patients with refractory solid tumors (phase I) and relapsed/refractory glioblastoma (phase II). J. Neurooncol. 2019, 144, 303–311. [Google Scholar] [CrossRef]
- Weinberg, M.A. RES-529: A PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anticancer Drugs 2016, 27, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Kaley, T.J.; Panageas, K.S.; Mellinghoff, I.K.; Nolan, C.; Gavrilovic, I.T.; De Angelis, L.M.; Abrey, L.E.; Holland, E.C.; Lassman, A.B. Phase II trial of an AKT inhibitor (perifosine) for recurrent glioblastoma. J. Neurooncol. 2019, 144, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Fan, X.; Li, Q.; Wang, Y.; Chen, X.; Xiao, P.; Passerini, A.G.; Simon, S.I.; Sun, C. IRF-1 mediates the suppressive effects of mTOR inhibition on arterial endothelium. J. Mol. Cell. Cardiol. 2020, 140, 30–41. [Google Scholar] [CrossRef]
- Mendez-Gomez, M.; Castro-Mercado, E.; Pena-Uribe, C.A.; Reyes-de la Cruz, H.; Lopez-Bucio, J.; Garcia-Pineda, E. TARGET OF RAPAMYCIN signaling plays a role in Arabidopsis growth promotion by Azospirillum brasilense Sp245. Plant Sci. 2020, 293, 110416. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Napolitano, F.; Rosa, R.; D’Amato, V.; Servetto, A.; Marciano, R.; De Placido, P.; Bianco, C.; Bianco, R. Mechanisms of resistance to mTOR inhibitors. Crit. Rev. Oncol. Hematol. 2020, 147, 102886. [Google Scholar] [CrossRef]
- Brakemeier, S.; Arns, W.; Lehner, F.; Witzke, O.; Vonend, O.; Sommerer, C.; Muhlfeld, A.; Rath, T.; Schuhmann, R.; Zukunft, B.; et al. Everolimus in de novo kidney transplant recipients participating in the Eurotransplant senior program: Results of a prospective randomized multicenter study (SENATOR). PLoS ONE 2019, 14, e0222730. [Google Scholar] [CrossRef]
- Schotz, U.; Balzer, V.; Brandt, F.W.; Ziemann, F.; Subtil, F.S.B.; Rieckmann, T.; Kocher, S.; Engenhart-Cabillic, R.; Dikomey, E.; Wittig, A.; et al. Dual PI3K/mTOR inhibitor NVP-BEZ235 enhances radiosensitivity of head and neck squamous cell carcinoma (HNSCC) cell lines due to suppressed double-strand break (DSB) repair by non-homologous end joining. Cancers 2020, 12, 467. [Google Scholar] [CrossRef] [Green Version]
- Soltani, A.; Torki, S.; Ghahfarokhi, M.S.; Jami, M.S. & Ghatrehsamani; M. Targeting the phosphoinositide 3-kinase/AKT pathways by small molecules and natural compounds as a therapeutic approach for breast cancer cells. Mol. Biol. Rep. 2019, 46, 4809–4816. [Google Scholar]
- Chen, Y.; Wang, T.; Du, J.; Li, Y.; Wang, X.; Zhou, Y.; Yu, X.; Fan, W.; Zhu, Q.; Tong, X.; et al. The Critical Role of PTEN/PI3K/AKT Signaling Pathway in Shikonin-Induced Apoptosis and Proliferation Inhibition of Chronic Myeloid Leukemia. Cell. Physiol. Biochem. 2018, 47, 981–993. [Google Scholar] [CrossRef]
- Wu, D.M.; Zhang, T.; Liu, Y.B.; Deng, S.H.; Han, R.; Liu, T.; Li, J.; Xu, Y. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziaeemehr, A.; Shahidsales, S.; Gharib, M.; Khazaei, M.; Ferns, G.A.; Ryzhikov, M.; Avan, A.; Hassanian, S.M. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J. Cell. Physiol. 2020, 235, 4146–4152. [Google Scholar] [CrossRef] [PubMed]
- Rittler, D.; Baranyi, M.; Molnár, E.; Garay, T.; Jalsovszky, I.; Varga, I.K.; Hegedűs, L.; Aigner, C.; Tóvári, J.; Tímár, J.; et al. The Antitumor Effect of Lipophilic Bisphosp honate BPH1222 in Melanoma Models: The Role of the PI3K/Akt Pathway and the Small G Protein Rheb. Int. J. Mol. Sci. 2019, 20, 4917. [Google Scholar] [CrossRef] [Green Version]
- Rocha Gda, G.; Oliveira, R.R.; Kaplan, M.A.; Gattass, C.R. 3β-Acetyl tormentic acid reverts MRP1/ABCC1 mediated cancer resistance through modulation of intracellular levels of GSH and inhibition of GST activity. Eur. J. Pharmacol. 2014, 741, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.; Kim, E.; Kim, J.H.; Yoon, K.; Kim, S.; Lee, J.; Cho, J.Y. AKT1-targeted proapoptotic activity of compound K in human breast cancer cells. J. Ginseng Res. 2019, 43, 692–698. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.C. Bcl-2 on the brink of breakthroughs in cancer treatment. Cell Death Differ. 2018, 25, 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Aranda, M.; Pérez-Ruiz, E.; Redondo, M. Bcl-2 Inhibition to Overcome Resistance to Chemo- and Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Q.; Li, S.; Cheng, P.; Deng, M.; He, X.; Wang, Z.; Yang, C.H.; Zhao, X.Y.; Huang, J. High expression of anti-apoptotic protein Bcl-2 is a good prognostic factor in colorectal cancer: Result of a meta-analysis. World J. Gastroenterol. 2017, 23, 5018. [Google Scholar] [CrossRef] [PubMed]
- Itoi, T.; Yamana, K.; Bilim, V.; Takahashi, K.; Tomita, F. Impact of frequent Bcl-2 expression on better prognosis in renal cell carcinoma patients. Br. J. Cancer. 2004, 90, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Hidaka, S.; Tagawa, Y.; Nagayasu, T. Bcl-2 protein expression correlates with better prognosis in patients with advanced non-small cell lung cancer. Anticancer Res. 2004, 24, 1925–1928. [Google Scholar]
- Huang, X.; Wu, Z.; Mei, Y.; Wu, M. XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling. EMBO J. 2013, 32, 2204–2216. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.R.; Bu, R.; Ahmed, M.; Jehan, Z.; Beg, S.; Al-Sobhi, S.; Al-Dayel, F.; Siraj, A.K.; Uddin, S.; Al-Kuraya, K.S. Role of X-linked inhibitor of apoptosis as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2015, 100, E974–E985. [Google Scholar] [CrossRef] [Green Version]
- Xiang, G.; Wen, X.; Wang, H.; Chen, K.; Liu, H. Expression of X-linked inhibitor of apoptosis protein in human colorectal cancer and its correlation with prognosis. J. Surg. Oncol. 2009, 100, 708–712. [Google Scholar]
- Augello, C.; Caruso, L.; Maggioni, M.; Donadon, M.; Montorsi, M.; Santambrogio, R.; Torzilli, G.; Vaira, V.; Pellegrini, C.; Roncalli, M.; et al. Inhibitors of apoptosis proteins (IAPs) expression and their prognostic significance in hepatocellular carcinoma. BMC Cancer 2009, 9, 125. [Google Scholar] [CrossRef]
- Esposito, I.; Kleeff, J.; Abiatari, I.; Shi, X.; Giese, N.; Bergmann, F.; Roth, W.; Friess, H.; Schirmacher, P. Overexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancer. J. Clin. Pathol. 2007, 60, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Che, X.; Yang, D.; Zong, H.; Wang, J.; Li, X.; Chen, F.; Chen, X.; Song, X. Nuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patients. Urol. Oncol. 2012, 30, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Siraj, A.K.; Ahmed, M.; Bu, R.; Pratheeshkumar, P.; Alrashed, A.M.; Qadri, Z.; Ajarim, D.; Al-Dayel, F.; Beg, S.; et al. XIAP over-expression is an independent poor prognostic marker in Middle Eastern breast cancer and can be targeted to induce efficient apoptosis. BMC Cancer 2017, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Chalasani, P.; Rensvold, D.; Kurtin, S.; Pretzinger, C.; Jolivet, J.; Ramanathan, R.K.; Von Hoff, D.D.; Weiss, G.J. Phase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. Am. J. Clin. Oncol. 2013, 36, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Fulda, S.; Carosati, E. IAP antagonists: Promising candidates for cancer therapy. Drug Discov. Today 2010, 15, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, J.; Sha, B.; Ma, Y.; Hu, T.; Ma, Y.; Sun, H.; Shi, J.; Dong, Z.; Li, P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget 2017, 8, 27471–27480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.K.; Toker, A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000prime Rep. 2015, 7, 13. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, R.; Wei, J.; Zhou, Y.; Ji, W.; Liu, J.; Zhi, X.; Zhang, J. Effects of PI3K inhibitor NVP-BKM120 on overcoming drug resistance and eliminating cancer stem cells in human breast cancer cells. Cell Death Dis. 2015, 6, e2020. [Google Scholar] [CrossRef] [PubMed]
- Eberle, J. Countering TRAIL Resistance in Melanoma. Cancers 2019, 11, 656. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kushwaha, P.P.; Gupta, S. Emerging targets in cancer drug resistance. Cancer Drug Resist. 2019, 2, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Cong, S.; Dong, J.; Jin, L.; Jiang, D.; Wang, X.; Chen, Q.; Li, F. Paired-related homeobox 1 overexpression promotes multidrug resistance via PTEN/PI3K/AKT signaling in MCF-7 breast cancer cells. Mol. Med. Rep. 2020, 4, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Miled, N.; Yan, Y.; Hon, W.C.; Perisic, O.; Zvelebil, M.; Inbar, Y.; Schneidman-Duhovny, D.; Wolfson, H.J.; Backer, J.M.; Williams, R.L. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science 2007, 317, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Lin, Z.M.; Ge, N.; Zhang, D.L.; Huang, J.; Kong, F. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am. J. Chin. Med. 2015, 43, 1471–1486. [Google Scholar] [CrossRef]
- Dong, C.; Chen, Y.; Ma, J.; Yang, R.; Li, H.; Liu, R.; You, D.; Luo, C.; Li, H.; Yang, S.; et al. Econazole nitrate reversed the resistance of breast cancer cells to Adriamycin through inhibiting the PI3K/AKT signaling pathway. Am. J. Cancer Res. 2020, 10, 263–274. [Google Scholar]
- Annageldiyev, C.; Tan, S.F.; Thakur, S.; Dhanyamraju, P.K.; Ramisetti, S.R.; Bhadauria, P.; Schick, J.; Zeng, Z.; Sharma, V.; Dunton, W.; et al. The PI3K/AKT Pathway Inhibitor ISC-4 Induces Apoptosis and Inhibits Growth of Leukemia in Preclinical Models of Acute Myeloid Leukemia. Front. Oncol. 2020, 10, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, R.; Larisch, S. Targeting XIAP for Promoting Cancer Cell Death—The Story of ARTS and SMAC. Cells 2020, 9, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J. Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. https://doi.org/10.3390/cancers13163949
Rascio F, Spadaccino F, Rocchetti MT, Castellano G, Stallone G, Netti GS, Ranieri E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers. 2021; 13(16):3949. https://doi.org/10.3390/cancers13163949
Chicago/Turabian StyleRascio, Federica, Federica Spadaccino, Maria Teresa Rocchetti, Giuseppe Castellano, Giovanni Stallone, Giuseppe Stefano Netti, and Elena Ranieri. 2021. "The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review" Cancers 13, no. 16: 3949. https://doi.org/10.3390/cancers13163949
APA StyleRascio, F., Spadaccino, F., Rocchetti, M. T., Castellano, G., Stallone, G., Netti, G. S., & Ranieri, E. (2021). The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers, 13(16), 3949. https://doi.org/10.3390/cancers13163949







