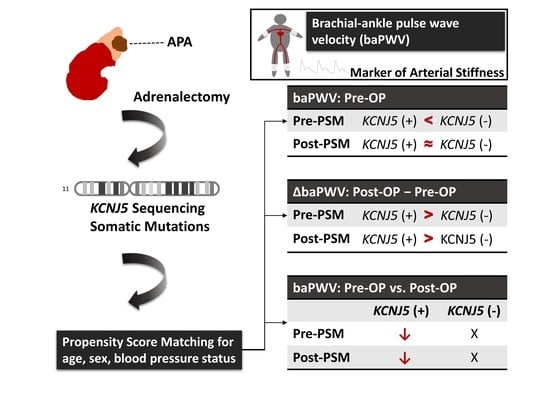
KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated with a Greater Recovery of Arterial Stiffness
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Measurements
2.3. Diagnostic Criteria for Aldosterone-Producing Adenomas
2.4. Arterial Stiffness Evaluation
2.5. Adrenalectomy
2.6. Histopathologic Studies
2.7. Genomic DNA Extraction and Sequencing of the KCNJ5 Gene
2.8. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Data of All APA Patients before and after Matching
3.2. baPWV of All APA Patients before and after Matching
3.3. The Change in Clinical Data after Adrenalectomy before and after Matching
3.4. Paired Comparisons of Clinical Data in All Patients before PSM before and after Adrenalectomy, and Comparisons of Parameters 1 Year after Surgery between the APA Patients with and without Mutations
3.5. Paired Comparisons of Clinical Data in Matched Patients before and after Adrenalectomy
3.6. Correlation of KCNJ5 Mutations with Baseline log baPWV and the Change in log baPWV before and after PSM
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar]
- Käyser, S.C.; Dekkers, T.; Groenewoud, H.J.; van der Wilt, G.J.; Carel Bakx, J.; van der Wel, M.C.; Hermus, A.R.; Lenders, J.W.; Deinum, J. Study heterogeneity and estimation of prevalence of primary aldosteronism: A systematic review and meta-regression analysis. J. Clin. Endocrinol. Metab. 2016, 101, 2826–2835. [Google Scholar]
- Buffolo, F.; Monticone, S.; Burrello, J.; Tetti, M.; Veglio, F.; Williams, T.A.; Mulatero, P. Is primary aldosteronism still largely unrecognized? Horm. Metab. Res. 2017, 49, 908–914. [Google Scholar]
- Lacolley, P.; Labat, C.; Pujol, A.; Delcayre, C.; Benetos, A.; Safar, M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: Effects of eplerenone. Circulation 2002, 106, 2848–2853. [Google Scholar]
- Strauch, B.; Petrák, O.; Wichterle, D.; Zelinka, T.; Holaj, R.; Widimský, J., Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am. J. Hypertens. 2006, 19, 909–914. [Google Scholar]
- Strauch, B.; Petrák, O.; Zelinka, T.; Wichterle, D.; Holaj, R.; Kasalický, M.; Safarík, L.; Rosa, J.; Widimský, J., Jr. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am. J. Hypertens. 2008, 21, 1086–1092. [Google Scholar]
- Lin, Y.H.; Lin, L.Y.; Chen, A.; Wu, X.M.; Lee, J.K.; Su, T.C.; Wu, V.C.; Chueh, S.C.; Lin, W.C.; Lo, M.T.; et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 2012, 221, 154–159. [Google Scholar]
- Amar, L.; Plouin, P.F.; Steichen, O. Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism. Orphanet J. Rare Dis. 2010, 5, 9. [Google Scholar]
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess ldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002, 40, 23–27. [Google Scholar]
- Choi, M.; Scholl, U.I.; Yue, P.; Bjorklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011, 331, 768–772. [Google Scholar]
- El Zein, R.M.; Boulkroun, S.; Fernandes-Rosa, F.L.; Zennaro, M.C. Molecular genetics of conn adenomas in the era of exome analysis. Presse Med. 2018, 47, e151–e158. [Google Scholar]
- De Sousa, K.; Boulkroun, S.; Baron, S.; Nanba, K.; Wack, M.; Rainey, W.E.; Rocha, A.; Giscos-Douriez, I.; Meatchi, T.; Amar, L.; et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension 2020, 75, 1034–1044. [Google Scholar]
- Nanba, K.; Omata, K.; Gomez-Sanchez, C.E.; Stratakis, C.A.; Demidowich, A.P.; Suzuki, M.; Thompson, L.D.R.; Cohen, D.L.; Luther, J.M.; Gellert, L.; et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension 2019, 73, 885–892. [Google Scholar]
- Nanba, K.; Omata, K.; Else, T.; Beck, P.C.C.; Nanba, A.T.; Turcu, A.F.; Miller, B.S.; Giordano, T.J.; Tomlins, S.A.; Rainey, W.E. Targeted molecular characterization of aldosterone-producing adenomas in white americans. J. Clin. Endocrinol. Metab. 2018, 103, 3869–3876. [Google Scholar]
- Fernandes-Rosa, F.L.; Williams, T.A.; Riester, A.; Steichen, O.; Beuschlein, F.; Boulkroun, S.; Strom, T.M.; Monticone, S.; Amar, L.; Meatchi, T.; et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 2014, 64, 354–361. [Google Scholar]
- Boulkroun, S.; Beuschlein, F.; Rossi, G.P.; Golib-Dzib, J.F.; Fischer, E.; Amar, L.; Mulatero, P.; Samson-Couterie, B.; Hahner, S.; Quinkler, M.; et al. Prevalence, clinical, and molecular correlates of kcnj5 mutations in primary aldosteronism. Hypertension 2012, 59, 592–598. [Google Scholar]
- Lenzini, L.; Rossitto, G.; Maiolino, G.; Letizia, C.; Funder, J.W.; Rossi, G.P. A meta-analysis of somatic kcnj5 k(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J. Clin. Endocrinol. Metab. 2015, 100, E1089–E1095. [Google Scholar]
- Wu, V.C.; Wang, S.M.; Chueh, S.J.; Yang, S.Y.; Huang, K.H.; Lin, Y.H.; Wang, J.J.; Connolly, R.; Hu, Y.H.; Gomez-Sanchez, C.E.; et al. The prevalence of ctnnb1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci. Rep. 2017, 7, 39121. [Google Scholar]
- Taguchi, R.; Yamada, M.; Nakajima, Y.; Satoh, T.; Hashimoto, K.; Shibusawa, N.; Ozawa, A.; Okada, S.; Rokutanda, N.; Takata, D.; et al. Expression and mutations of kcnj5 mrna in japanese patients with aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 2012, 97, 1311–1319. [Google Scholar]
- Zheng, F.-F.; Zhu, L.-M.; Nie, A.-F.; Li, X.-Y.; Lin, J.-R.; Zhang, K.; Chen, J.; Zhou, W.-L.; Shen, Z.-J.; Zhu, Y.-C.; et al. Clinical characteristics of somatic mutations in chinese patients with aldosterone-producing adenoma. Hypertension 2015, 65, 622–628. [Google Scholar]
- Hong, A.R.; Kim, J.H.; Song, Y.S.; Lee, K.E.; Seo, S.H.; Seong, M.-W.; Shin, C.S.; Kim, S.W.; Kim, S.Y. Genetics of aldosterone-producing adenoma in korean patients. PLoS ONE 2016, 11, e0147590. [Google Scholar]
- Rossi, G.P.; Cesari, M.; Letizia, C.; Seccia, T.M.; Cicala, M.V.; Zinnamosca, L.; Kuppusamy, M.; Mareso, S.; Sciomer, S.; Iacobone, M.; et al. Kcnj5 gene somatic mutations affect cardiac remodelling but do not preclude cure of high blood pressure and regression of left ventricular hypertrophy in primary aldosteronism. J. Hypertens. 2014, 32, 1514–1521, discussion 1522. [Google Scholar]
- Kitamoto, T.; Suematsu, S.; Matsuzawa, Y.; Saito, J.; Omura, M.; Nishikawa, T. Comparison of cardiovascular complications in patients with and without kcnj5 gene mutations harboring aldosterone-producing adenomas. J. Atheroscler. Thromb. 2015, 22, 191–200. [Google Scholar]
- Wu, V.C.; Huang, K.H.; Peng, K.Y.; Tsai, Y.C.; Wu, C.H.; Wang, S.M.; Yang, S.Y.; Lin, L.Y.; Chang, C.C.; Lin, Y.H.; et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-taiwanese population. Sci. Rep. 2015, 5, 11396. [Google Scholar]
- Chang, Y.Y.; Tsai, C.H.; Peng, S.Y.; Chen, Z.W.; Chang, C.C.; Lee, B.C.; Liao, C.W.; Pan, C.T.; Chen, Y.L.; Lin, L.C.; et al. Kcnj5 somatic mutations in aldosterone-producing adenoma are associated with a worse baseline status and better recovery of left ventricular remodeling and diastolic function. Hypertension 2021, 77, 114–125. [Google Scholar]
- Chang, C.H.; Hu, Y.H.; Tsai, Y.C.; Wu, C.H.; Wang, S.M.; Lin, L.Y.; Lin, Y.H.; Satoh, F.; Wu, K.D.; Wu, V.C. Arterial stiffness and blood pressure improvement in aldosterone-producing adenoma harboring kcnj5 mutations after adrenalectomy. Oncotarget 2017, 8, 29984–29995. [Google Scholar]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar]
- Wu, V.C.; Hu, Y.H.; Er, L.K.; Yen, R.F.; Chang, C.H.; Chang, Y.L.; Lu, C.C.; Chang, C.C.; Lin, J.H.; Lin, Y.H.; et al. Case detection and diagnosis of primary aldosteronism—the consensus of taiwan society of aldosteronism. J. Formos. Med. Assoc. 2017, 116, 993–1005. [Google Scholar]
- Rossi, G.P.; Belfiore, A.; Bernini, G.; Desideri, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; Mallamaci, F.; et al. Comparison of the captopril and the saline infusion test for excluding aldosterone-producing adenoma. Hypertension 2007, 50, 424–431. [Google Scholar]
- Wu, V.C.; Chang, H.W.; Liu, K.L.; Lin, Y.H.; Chueh, S.C.; Lin, W.C.; Ho, Y.L.; Huang, J.W.; Chiang, C.K.; Yang, S.Y.; et al. Primary aldosteronism: Diagnostic accuracy of the losartan and captopril tests. Am. J. Hypertens. 2009, 22, 821–827. [Google Scholar]
- Wu, V.C.; Yang, S.Y.; Lin, J.W.; Cheng, B.W.; Kuo, C.C.; Tsai, C.T.; Chu, T.S.; Huang, K.H.; Wang, S.M.; Lin, Y.H.; et al. Kidney impairment in primary aldosteronism. Clin. Chim. Acta 2011, 412, 1319–1325. [Google Scholar]
- Schwartz, G.L.; Turner, S.T. Screening for primary aldosteronism in essential hypertension: Diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin. Chem. 2005, 51, 386–394. [Google Scholar]
- Chao, C.T.; Wu, V.C.; Kuo, C.C.; Lin, Y.H.; Chang, C.C.; Chueh, S.J.; Wu, K.D.; Pimenta, E.; Stowasser, M. Diagnosis and management of primary aldosteronism: An updated review. Ann. Med. 2013, 45, 375–383. [Google Scholar]
- McDonald, D.A. Regional pulse-wave velocity in the arterial tree. J. Appl. Physiol. 1968, 24, 73–78. [Google Scholar]
- Nomura, K.; Toraya, S.; Horiba, N.; Ujihara, M.; Aiba, M.; Demura, H. Plasma aldosterone response to upright posture and angiotensin ii infusion in aldosterone-producing adenoma. J. Clin. Endocrinol. Metab. 1992, 75, 323–327. [Google Scholar]
- Novitsky, Y.W.; Kercher, K.W.; Rosen, M.J.; Cobb, W.S.; Jyothinagaram, S.; Heniford, B.T. Clinical outcomes of laparoscopic adrenalectomy for lateralizing nodular hyperplasia. Surgery 2005, 138, 1009–1017. [Google Scholar]
- Azizan, E.A.B.; Murthy, M.; Stowasser, M.; Gordon, R.; Kowalski, B.; Xu, S.; Brown, M.J.; O’Shaughnessy, K.M. Somatic mutations affecting the selectivity filter of kcnj5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension 2012, 59, 587–591. [Google Scholar]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001, 37, 1236–1241. [Google Scholar]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar]
- Cortez-Cooper, M.Y.; Supak, J.A.; Tanaka, H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am. J. Cardiol. 2003, 91, 1519–1522. [Google Scholar]
- Wang, J.W.; Zhou, Z.Q.; Hu, D.Y. Prevalence of arterial stiffness in north china, and associations with risk factors of cardiovascular disease: A community-based study. BMC Cardiovasc. Disord. 2012, 12, 119. [Google Scholar]
- Ninomiya, T.; Kojima, I.; Doi, Y.; Fukuhara, M.; Hirakawa, Y.; Hata, J.; Kitazono, T.; Kiyohara, Y. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general japanese population: The hisayama study. J. Hypertens. 2013, 31, 477–483, discussion 483. [Google Scholar]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar]
- Sheng, C.S.; Li, Y.; Li, L.H.; Huang, Q.F.; Zeng, W.F.; Kang, Y.Y.; Zhang, L.; Liu, M.; Wei, F.F.; Li, G.L.; et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly chinese. Hypertension 2014, 64, 1124–1130. [Google Scholar]
- Snijder, M.B.; Stronks, K.; Agyemang, C.; Busschers, W.B.; Peters, R.J.; van den Born, B.J. Ethnic differences in arterial stiffness the helius study. Int. J. Cardiol. 2015, 191, 28–33. [Google Scholar]
- Callera, G.E.; Touyz, R.M.; Tostes, R.C.; Yogi, A.; He, Y.; Malkinson, S.; Schiffrin, E.L. Aldosterone activates vascular p38map kinase and nadph oxidase via c-src. Hypertension 2005, 45, 773–779. [Google Scholar]
- Ambrosino, P.; Lupoli, R.; Tortora, A.; Cacciapuoti, M.; Lupoli, G.A.; Tarantino, P.; Nasto, A.; Di Minno, M.N. Cardiovascular risk markers in patients with primary aldosteronism: A systematic review and meta-analysis of literature studies. Int. J. Cardiol. 2016, 208, 46–55. [Google Scholar]
- Park, S.; Kim, J.B.; Shim, C.Y.; Ko, Y.G.; Choi, D.; Jang, Y.; Chung, N. The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J. Hypertens. 2007, 25, 1279–1283. [Google Scholar]
- Cecelja, M.; Chowienczyk, P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 2009, 54, 1328–1336. [Google Scholar]
- Peng, K.Y.; Liao, H.W.; Chan, C.K.; Lin, W.C.; Yang, S.Y.; Tsai, Y.C.; Huang, K.H.; Lin, Y.H.; Chueh, J.S.; Wu, V.C. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension 2020, 76, 1537–1544. [Google Scholar]
- Tang, L.; Li, X.; Wang, B.; Ma, X.; Li, H.; Gao, Y.; Gu, L.; Nie, W.; Zhang, X. Clinical characteristics of aldosterone- and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int. J. Endocrinol. 2018, 2018, 4920841. [Google Scholar]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in atp1a1 and atp2b3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 440–444. [Google Scholar]
- Scholl, U.I.; Goh, G.; Stolting, G.; de Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.W.; et al. Somatic and germline cacna1d calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar]
- Scholl, U.I.; Healy, J.M.; Thiel, A.; Fonseca, A.L.; Brown, T.C.; Kunstman, J.W.; Horne, M.J.; Dietrich, D.; Riemer, J.; Kücükköylü, S.; et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin. Endocrinol. 2015, 83, 779–789. [Google Scholar]
- Shahin, Y.; Khan, J.A.; Chetter, I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: A meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis 2012, 221, 18–33. [Google Scholar]
- Frimodt-Møller, M.; Kamper, A.L.; Strandgaard, S.; Kreiner, S.; Nielsen, A.H. Beneficial effects on arterial stiffness and pulse-wave reflection of combined enalapril and candesartan in chronic kidney disease—A randomized trial. PLoS ONE 2012, 7, e41757. [Google Scholar]
- Anan, F.; Takahashi, N.; Ooie, T.; Yufu, K.; Hara, M.; Nakagawa, M.; Yonemochi, H.; Saikawa, T.; Yoshimatsu, H. Effects of valsartan and perindopril combination therapy on left ventricular hypertrophy and aortic arterial stiffness in patients with essential hypertension. Eur. J. Clin. Pharmacol. 2005, 61, 353–359. [Google Scholar]

| Variables | Before Propensity Score Matching | After Propensity Score Matching * | ||||
|---|---|---|---|---|---|---|
| Patient Characteristics | KCNJ5 (+) (n = 126) | KCNJ5 (−) (n = 87) | p | KCNJ5 (+) (n = 66) | KCNJ5 (−) (n = 66) | p |
| Age, years | 47.3 ± 10.3 | 55.3 ± 10.5 | <0.001 | 50.3 ± 9.6 | 52.5 ± 10.1 | 0.190 |
| Sex, male | 53(42%) | 36(36%) | 0.921 | 27(41%) | 27(41%) | 1.000 |
| Height, cm | 164 ± 8 | 163 ± 9 | 0.213 | 163 ± 8 | 163 ± 9 | 0.763 |
| Weight, kg | 66 ± 13 | 67 ± 14 | 0.774 | 67 ± 12 | 66 ± 14 | 0.930 |
| Body mass index, kg/m2 | 24.5 ± 3.5 | 25.1 ± 3.8 | 0.248 | 24.9 ± 3.7 | 24.8 ± 3.7 | 0.914 |
| Duration of hypertension, years | 6.5 ± 6.0 | 9.0 ± 8.6 | 0.018 | 7.0 ± 6.1 | 8.4 ± 8.5 | 0.281 |
| SBP, mm Hg | 156 ± 22 | 151 ± 21 | 0.114 | 153 ± 22 | 152 ± 21 | 0.930 |
| DBP, mm Hg | 94 ± 15 | 89 ± 13 | 0.003 | 90 ± 14 | 90 ± 13 | 0.923 |
| Diabetes mellitus | 10(8%) | 12(14%) | 0.189 | 8(12%) | 7(11%) | 0.786 |
| Dyslipidemia | 21(17%) | 20(23%) | 0.321 | 15(23%) | 15(23%) | 1.000 |
| Number of anti-hypertensive drugs | 2.1 ± 1.1 | 1.9 ± 1.1 | 0.251 | 1.9 ± 1.0 | 2.0 ± 1.0 | 0.670 |
| Hypertension medication type | ||||||
| ACEI/ARB | 50(40%) | 44(50%) | 0.172 | 26(39%) | 38(58%) | 0.037 |
| α -Blocker | 34(27%) | 15(17%) | 0.097 | 15(23%) | 13(20%) | 0.673 |
| β -Blocker | 44(35%) | 30(34%) | 0.858 | 20(30%) | 22(33%) | 0.711 |
| CCB | 93(74%) | 57(65%) | 0.183 | 45(68%) | 44(67%) | 0.854 |
| Diuretics except aldosterone antagonist | 8(6%) | 9(10%) | 0.210 | 3(5%) | 8(12%) | 0.118 |
| Aldosterone antagonist | 37(29%) | 18(21%) | 0.203 | 19(29%) | 12(18%) | 0.153 |
| Vasodilator | 9(7%) | 6(7%) | 0.963 | 3(5%) | 4(6%) | 0.700 |
| Laboratory parameters | ||||||
| Creatinine, mg/dL | 0.87 ± 0.34 | 0.89 ± 0.29 | 0.694 | 0.89 ± 0.42 | 0.92 ± 0.31 | 0.718 |
| Potassium, mmol/L | 3.3 ± 0.6 | 3.8 ± 0.4 | <0.001 | 3.3 ± 0.6 | 3.8 ± 0.5 | <0.001 |
| PAC †, ng/dL | 51(45) | 34(23) | <0.001 | 46(42) | 34(22) | 0.012 |
| PRA †, ng/mL/h | 0.17(0.52) | 0.28(0.70) | 0.171 | 0.17(0.45) | 0.36(0.78) | 0.090 |
| ARR †, ng/dL per ng/mL/h | 271(737) | 134(429) | 0.003 | 254(619) | 118(361) | 0.017 |
| Log PAC | 1.69 ± 0.28 | 1.53 ± 0.27 | <0.001 | 1.66 ± 0.29 | 1.55 ± 0.27 | 0.026 |
| Log PRA | −0.74 ± 0.73 | −0.60 ± 0.82 | 0.167 | −0.75 ± 0.71 | 0.54 ± 0.89 | 0.133 |
| Log ARR | 2.44 ± 0.78 | 2.12 ± 0.80 | 0.005 | 2.41 ± 0.73 | 2.08 ± 0.86 | 0.022 |
| baPWV †, cm/s | 1554(428) | 1661(445) | 0.088 | 1559(414) | 1571(422) | 0.831 |
| log baPWV | 3.20 ± 0.07 | 3.22 ± 0.08 | 0.046 | 3.21 ± 0.07 | 3.21 ± 0.08 | 0.530 |
| Variables | Before Propensity Score Matching | After Propensity Score Matching * | ||||
|---|---|---|---|---|---|---|
| Patient Characteristics | KCNJ5 (+) (n = 106) | KCNJ5 (−) (n = 74) | p | KCNJ5 (+) (n = 58) | KCNJ5 (−) (n = 58) | p |
| ∆SBP, mmHg | −26 ± 22 | −16 ± 22 | 0.002 | −24 ± 23 | −16 ± 23 | 0.085 |
| ∆DBP, mmHg | −13 ± 16 | −7 ± 13 | 0.003 | −10 ± 14 | −7 ± 14 | 0.142 |
| ∆Number of hypertensive drugs | −1.7 ± 1.1 | −1.2 ± 1.1 | 0.001 | −1.6 ± 1.1 | −1.3 ± 1.1 | 0.052 |
| ∆Creatinine, mg/dL | 0.19 ± 0.36 | 0.07 ± 0.22 | 0.009 | 0.18 ± 0.38 | 0.06 ± 0.23 | 0.427 |
| ∆Potassium, mmol/L | 1.1 ± 0.7 | 0.4 ± 0.6 | <0.001 | 1.1 ± 0.8 | 0.5 ± 0.6 | <0.001 |
| ∆log PAC | −0.24 ± 0.35 | −0.05 ± 0.35 | <0.001 | −0.19 ± 0.36 | −0.04 ± 0.35 | 0.033 |
| ∆log PRA | 0.99 ± 0.95 | 0.68 ± 0.99 | 0.034 | 0.99 ± 0.93 | 0.67 ± 1.03 | 0.082 |
| ∆log ARR | −1.23 ± 1.04 | −0.72 ± 1.00 | 0.001 | −1.18 ± 1.02 | −0.71 ± 1.00 | 0.015 |
| Variables | KCNJ5 (+) | KCNJ5 (−) | |||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | Baseline (n = 106) | Post-OP 1Y (n = 106) | p | Baseline (n = 74) | Post-OP 1Y (n = 74) | p | p § |
| SBP, mm Hg | 157 ± 22 | 130 ± 16 | <0.001 | 152 ± 21 | 136 ± 19 | <0.001 | 0.044 |
| DBP, mm Hg | 95 ± 15 | 82 ± 11 | <0.001 | 89 ± 12 | 82 ± 11 | <0.001 | 0.862 |
| Number of hypertensive drugs | 2.1 ± 1.1 | 0.4 ± 0.8 | <0.001 | 1.9 ± 1.1 | 0.7 ± 1.0 | <0.001 | 0.010 |
| Creatinine, mg/dL | 0.88 ± 0.36 | 1.07 ± 0.61 | 0.001 | 0.90 ± 0.31 | 0.96 ± 0.33 | 0.014 | 0.171 |
| Potassium, mmol/L | 3.3 ± 0.7 | 4.4 ± 0.4 | <0.001 | 3.8 ± 0.5 | 4.2 ± 0.4 | <0.001 | 0.081 |
| Log PAC | 1.71 ± 0.26 | 1.47 ± 0.23 | <0.001 | 1.53 ± 0.27 | 1.49 ± 0.26 | 0.262 | 0.589 |
| Log PRA | −0.74 ± 0.78 | 0.99 ± 0.95 | <0.001 | −0.55 ± 0.84 | 0.68 ± 0.99 | <0.001 | 0.248 |
| Log ARR | 2.45 ± 0.82 | 1.21 ± 0.65 | <0.001 | 2.08 ± 0.81 | 1.36 ± 0.73 | <0.001 | 0.182 |
| Log baPWV * | 3.20 ± 0.07 | 3.16 ± 0.08 | <0.001 | 3.22 ± 0.08 | 3.21 ± 0.09 | 0.127 | <0.001 |
| Variables | KCNJ5 (+) | KCNJ5 (−) | |||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | Baseline (n = 58) | Post-OP 1Y (n = 58) | p | Baseline (n = 58) | Post-OP 1Y (n = 58) | p | p § |
| SBP, mm Hg | 154 ± 23 | 131 ± 15 | <0.001 | 152 ± 20 | 136 ± 19 | <0.001 | 0.092 |
| DBP, mm Hg | 92 ± 14 | 81 ± 10 | <0.001 | 90 ± 12 | 84 ± 11 | 0.001 | 0.194 |
| Number of hypertensive drugs | 2.0 ± 1.0 | 0.4 ± 0.8 | <0.001 | 2.0 ± 1.0 | 0.7 ± 1.0 | <0.001 | 0.013 |
| Creatinine, mg/dL | 0.91 ± 0.45 | 1.09 ± 0.75 | 0.001 | 0.94 ± 0.32 | 1.01 ± 0.35 | 0.083 | 0.469 |
| Potassium, mmol/L | 3.3 ± 0.7 | 4.3 ± 0.4 | <0.001 | 3.8 ± 0.5 | 4.3 ± 0.4 | <0.001 | 0.472 |
| Log PAC | 1.68 ± 0.30 | 1.49 ± 0.22 | <0.001 | 1.55 ± 0.27 | 1.50 ± 0.26 | 0.339 | 0.836 |
| Log PRA | −0.75 ± 0.77 | 0.24 ± 0.62 | <0.001 | −0.52 ± 0.91 | 0.15 ± 0.75 | <0.001 | 0.462 |
| Log ARR | 2.42 ± 0.79 | 1.24 ± 0.64 | <0.001 | 2.07 ± 0.86 | 1.35 ± 0.72 | <0.001 | 0.390 |
| log baPWV † | 3.21 ± 0.06 | 3.17 ± 0.07 | <0.001 | 3.22 ± 0.08 | 3.20 ± 0.09 | 0.154 | 0.045 |
| Pre-PSM | Post-PSM | |||
|---|---|---|---|---|
| Model | Pre-OP log baPWV | ∆ log baPWV | Pre-OP log baPWV | ∆ log baPWV |
| Model 1 | β = −0.137, p = 0.046 (−0.043, 0.000) | β = −0.190, p = 0.014 (−0.046, −0.005) | β = −0.055, p = 0.530 (−0.036, 0.019) | β = −0.199, p = 0.040 (−0.051, −0.001) |
| Model 2 | β= 0.065, p = 0.293 (−0.009, 0.029) | β= −0.194, p = 0.017 (−0.047, −0.005) | β= 0.004, p = 0.959 (−0.023, 0.024) | β= −0.191, p = 0.049 (−0.050, 0.000) |
| Model 3 | β= 0.025, p = 0.644 (−0.013, 0.021) | β= −0.161, p = 0.043 (−0.043, −0.001) | β= −0.002, p = 0.980 (−0.021, 0.020) | β= −0.194, p = 0.036 (−0.049, −0.002) |
| Model 4 | β = 0.020, p = 0.721 (−0.014, 0.020) | β = −0.166, p = 0.039 (−0.043, −0.001) | β= −0.001, p = 0.982 (−0.021, 0.021) | β = −0.187, p = 0.043 (−0.048, −0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Pan, C.-T.; Chen, Z.-W.; Tsai, C.-H.; Peng, S.-Y.; Chang, C.-C.; Lee, B.-C.; Liao, C.-W.; Peng, K.-Y.; Chiu, Y.-W.; et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated with a Greater Recovery of Arterial Stiffness. Cancers 2021, 13, 4313. https://doi.org/10.3390/cancers13174313
Chang Y-Y, Pan C-T, Chen Z-W, Tsai C-H, Peng S-Y, Chang C-C, Lee B-C, Liao C-W, Peng K-Y, Chiu Y-W, et al. KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated with a Greater Recovery of Arterial Stiffness. Cancers. 2021; 13(17):4313. https://doi.org/10.3390/cancers13174313
Chicago/Turabian StyleChang, Yi-Yao, Chien-Ting Pan, Zheng-Wei Chen, Cheng-Hsuan Tsai, Shih-Yuan Peng, Chin-Chen Chang, Bo-Ching Lee, Che-Wei Liao, Kang-Yung Peng, Yu-Wei Chiu, and et al. 2021. "KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated with a Greater Recovery of Arterial Stiffness" Cancers 13, no. 17: 4313. https://doi.org/10.3390/cancers13174313
APA StyleChang, Y.-Y., Pan, C.-T., Chen, Z.-W., Tsai, C.-H., Peng, S.-Y., Chang, C.-C., Lee, B.-C., Liao, C.-W., Peng, K.-Y., Chiu, Y.-W., Chou, C.-H., Wu, V.-C., Liu, L.-Y. D., Hung, C.-S., & Lin, Y.-H. (2021). KCNJ5 Somatic Mutations in Aldosterone-Producing Adenoma Are Associated with a Greater Recovery of Arterial Stiffness. Cancers, 13(17), 4313. https://doi.org/10.3390/cancers13174313