The Agony of Choice—Where to Place the Wave of BCMA-Targeted Therapies in the Multiple Myeloma Treatment Puzzle in 2022 and Beyond
Abstract
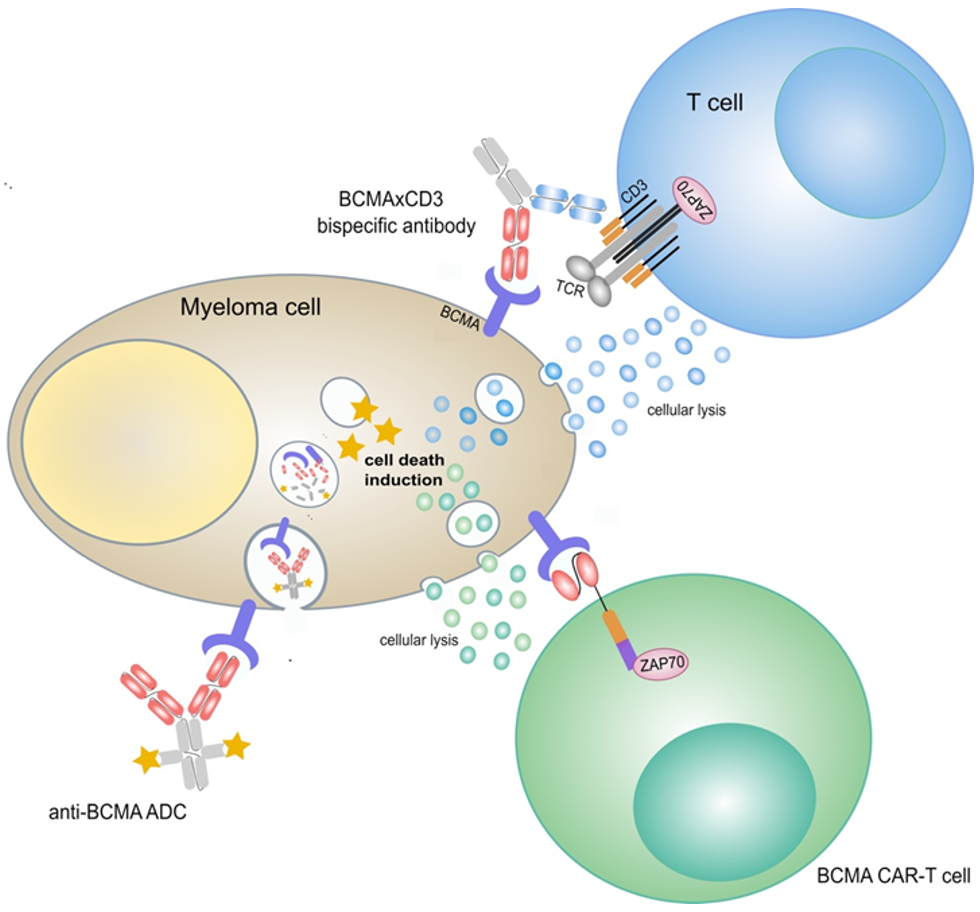
:Simple Summary
Abstract
1. Introduction
2. CAR-T Cells
2.1. Idecabtagene Vicleucel
2.2. Ciltacabtagene Autoleucel
2.3. ALLO-715
2.4. Other BCMA-Targeting CAR-T Cell Constructs
3. Bispecific Antibodies
3.1. AMG420 and AMG701
3.2. Teclistamab
3.3. REGN5458
3.4. TNB-383B
3.5. Elranatamab
3.6. CC-93269
3.7. Other Constructs
4. Antibody Drug Conjugates
4.1. Belantamab Mafodotin
4.2. Other BCMA-Targeting ADCs
5. Drug Targets beyond BCMA in Clinical Development
5.1. FcRH5
5.2. GPRC5D
5.3. SLAMF7
5.4. CD38
5.5. Dual Targeted CAR-T Cells
5.6. Other Targets
6. Who Is Who—How to Find the Right BCMA-Targeted Drug for the Right Patient? Potential Key Patient Selection Criteria for ADCs vs. Bispecific Antibodies vs. CAR-T Cells
6.1. Disease Based Decision Factors
6.2. Patient Based Decision Factors
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 11 July 2021).
- Usmani, S.; Ahmadi, T.; Ng, Y.; Lam, A.; Desai, A.; Potluri, R.; Mehra, M. Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients With ≥3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD. Oncologist 2016, 21, 1355–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple mye-loma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Acharya, C.; Xiaoyan, F.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; Van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell Maturation Antigen Is a Promising Target for Adoptive T-cell Therapy of Multiple Myeloma. Clin. Cancer Res. 2013, 19, 2048–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Donk, N.; Usmani, S.Z.; Yong, K. CAR-T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021, 8, e446–e461. [Google Scholar] [CrossRef]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for target-ing and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refrac-tory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti-B-cell maturation antigen chimeric an-tigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.L.; Schmitt, M.; Wang, L.; Ramos, C.A.; Jordan, K.; Müller-Tidow, C.; Dreger, P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 2021, 32, 34–48. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2018, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakoub-Agha, I.; Chabannon, C.; Bader, P.; Basak, G.W.; Bonig, H.; Ciceri, F.; Corbacioglu, S.; Duarte, R.F.; Einsele, H.; Hudecek, M.; et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2019, 105, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy–assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Raje, N.S.; Berdeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene Vicleucel (ide-cel, bb2121), a BCMA-Directed CAR T Cell Therapy, in Patients with Relapsed and Refractory Multiple Myeloma: Updated Results from Phase 1 CRB-401 Study. Blood 2020, 136, 26–27. [Google Scholar] [CrossRef]
- Larry, D.; Anderson, J.; Munshi, N.C.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.; Lin, Y.; et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. J. Clin. Oncol. 2021, 39, 8016. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson L.D., J.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Raje, N.S.; Siegel, D.S.; Jagannath, S.; Lonial, F.S.; Munshi, N.C.; Moreau, P.; Goldschmidt, H.; Cavo, M.; Truppel-Hartmann, A.; Rowe, E.; et al. Idecabtagene Vicleucel (ide-cel, bb2121) in Relapsed and Refractory Multiple Myeloma: Analyses of High-Risk Subgroups in the KarMMa Study. Blood 2020, 136, 37–38. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Raje, N.S.; Siegel, D.S.; Lin, Y.; Anderson, J.L.D.; Rodriguez-Otero, P.; Manier, S.; Einsele, H.; Cavo, M.; Truppel-Hartmann, A.; et al. Efficacy and Safety of Idecabtagene Vicleucel (ide-cel, bb2121) in Elderly Patients with Relapsed and Refractory Multiple Myeloma: KarMMa Subgroup Analysis. Blood 2020, 136, 16–17. [Google Scholar] [CrossRef]
- Alsina, M.; Shah, N.; Raje, N.S.; Jagannath, S.; Madduri, D.; Kaufman, J.L.; Siegel, D.S.; Munshi, N.C.; Rosenblatt, J.; Lin, Y.; et al. Updated Results from the Phase I CRB-402 Study of Anti-Bcma CAR-T Cell Therapy bb21217 in Patients with Relapsed and Refractory Multiple Myeloma: Correlation of Expansion and Duration of Response with T Cell Phenotypes. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhao, W.-H.; Liu, J.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Long-Term Follow-up of a Phase 1, First-in-Human Open-Label Study of LCAR-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (BCMA), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 579. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTI-TUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, P314–P324. [Google Scholar] [CrossRef]
- Lin, Y.; Martin, T.; Cohen, A.D.; Jakubowiak, A.; Jasielec, J.; Usmani, S.Z.; Madduri, D.; Agha, M.; Stewart, A.K.; Singh, I.; et al. Cytokine Release Syndrome in Patients with Relapsed/Refractory Multiple Myeloma Treated with Ciltacabtagene Autoleucel in the Phase 1b/2 CARTITUDE-1 Study. Blood 2020, 136, 45–46. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Berdeja, J.G.; Madduri, D.; Jakubowiak, A.J.; Agha, M.E.; Cohen, A.D.; Hari, P.; Yeh, T.-M.; Olyslager, Y.; Banerjee, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed/refractory multiple myeloma (R/R MM): Updated results from CARTITUDE-1. J. Clin. Oncol. 2021, 39, 8005. [Google Scholar] [CrossRef]
- Agha, M.E.; Cohen, A.D.; Madduri, D.; Cohen, Y.C.; Delforge, M.; Hillengass, J.; Goldschmidt, H.; Weisel, K.; Raab, M.-S.; Scheid, C.; et al. CARTITUDE-2: Efficacy and safety of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR T-cell therapy, in patients with progressive multiple myeloma (MM) after one to three prior lines of therapy. J. Clin. Oncol. 2021, 39, 8013. [Google Scholar] [CrossRef]
- Mailankody, S.; Matous, J.V.; Liedtke, M.; Sidana, S.; Malik, S.; Nath, R.; Oluwole, O.O.; Karski, E.E.; Lovelace, W.; Zhou, X.; et al. Universal: An Allogeneic First-in-Human Study of the Anti-Bcma ALLO-715 and the Anti-CD52 ALLO-647 in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 24–25. [Google Scholar] [CrossRef]
- Costello, C.L.; Cohen, A.D.; Patel, K.K.; Ali, S.S.; Berdeja, J.G.; Shah, N.; Ganguly, S.; Kocoglu, M.H.; Abedi, M.; Ostertag, E.M.; et al. Phase 1/2 Study of the Safety and Response of P-BCMA-101 CAR-T Cells in Patients with Relapsed/Refractory (r/r) Multiple Myeloma (MM) (PRIME) with Novel Therapeutic Strategies. Blood 2020, 136, 29–30. [Google Scholar] [CrossRef]
- Mailankody, S.; Jakubowiak, A.J.; Htut, M.; Costa, L.J.; Lee, K.; Ganguly, S.; Kaufman, J.L.; Siegel, D.S.D.; Bensinger, W.; Cota, M.; et al. Orvacabtagene autoleucel (orva-cel), a B-cell matu-ration antigen (BCMA)-directed CAR-T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study (NCT03430011). J. Clin. Oncol. 2020, 38, 8504. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Cohen, A.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.K.; Baz, R.C.; Orlowski, R.Z.; Anderson, L.D., Jr.; Ma, H.; Shrewsbury, A.; Croghan, K.A.; Bilgi, M.; Kansagra, A.; Kapoor, P.; et al. Results from Lummicar-2: A Phase 1b/2 Study of Fully Human B-Cell Maturation Antigen-Specific CAR-T Cells (CT053) in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- An, G.; Sui, W.; Wang, T.; Qu, X.; Zhang, X.; Yang, J.; Zhang, Y.; Zhang, L.; Zhu, J.; Zheng, C.; et al. An Anti-Bcma CAR T-Cell Therapy (C-CAR088) Shows Promising Safety and Efficacy Profile in Relapsed or Refractory Multiple Myeloma. Blood 2020, 136, 29–30. [Google Scholar] [CrossRef]
- Lejeune, M.; Köse, M.C.; Duray, E.; Einsele, H.; Beguin, Y.; Caers, J. Bispecific, T-Cell-Recruiting Antibodies in B-Cell Malignancies. Front. Immunol. 2020, 11, 762. [Google Scholar] [CrossRef]
- Caraccio, C.; Krishna, S.; Phillips, D.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11, 501. [Google Scholar] [CrossRef]
- Kumar, S.; Rajkumar, S.V. BiTEing the Tumor. J. Clin. Oncol. 2020, 38, 2077–2079. [Google Scholar] [CrossRef]
- Verkleij, C.P.; Frerichs, K.A.; Broekmans, M.; Absalah, S.; Maas-Bosman, P.W.; Kruyswijk, S.; Nijhof, I.S.; Mutis, T.; Zweegman, S.; Van De Donk, N.W. T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget 2020, 11, 4076–4081. [Google Scholar] [CrossRef]
- Kontermann, R.E.; Brinkmann, U. Bispecific antibodies. Drug Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, M.P.; Bonifant, C.L.; Gottschalk, S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood 2018, 131, 30–38. [Google Scholar] [CrossRef]
- Sanchez, L.; Dardac, A.; Madduri, D.; Richard, S.; Richter, J. B-cell maturation antigen (BCMA) in multiple myeloma: The new frontier of targeted therapies. Ther. Adv. Hematol. 2021, 12, 2040620721989585. [Google Scholar] [CrossRef]
- Zhou, X.; Einsele, H.; Danhof, S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J. Clin. Med. 2020, 9, 2166. [Google Scholar] [CrossRef]
- Geis, M.; Nowotny, B.; Bohn, M.-D.; Kouhestani, D.; Einsele, H.; Bumm, T.; Stuhler, G. Combinatorial targeting of multiple myeloma by complementing T cell engaging antibody fragments. Commun. Biol. 2021, 4, 44. [Google Scholar] [CrossRef]
- Hipp, S.; Tai, Y.T.; Blanset, D.; Deegen, P.; Wahl, J.; Thomas, O.; Rattel, B.; Adam, P.J.; Adam, P.J.; Friedrich, M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017, 31, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.L.; Goyos, A.; Li, C.-M.; Deegen, P.; Bogner, P.; Sternjak, A.; Thomas, O.; Klinger, M.; Wahl, J.; Friedrich, M.; et al. AMG 701 induces cytotoxicity of multiple myeloma cells and depletes plasma cells in cynomolgus monkeys. Blood Adv. 2020, 4, 4180–4194. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.H.; Kuo, T.C.; Zhang, Y.; Chen, A.; Geng, T.; Aschenbrenner, L.; Kamperschroer, C.; Pascua, E.; Chen, W.; Delaria, K.; et al. Preclinical Efficacy and Safety Comparison of CD3 Bispecific and ADC Modalities Targeting BCMA for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2019, 18, 2008–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiLillo, D.J.; Olson, K.; Mohrs, K.; Meagher, T.C.; Bray, K.; Sineshchekova, O.; Startz, T.; Kuhnert, J.; Retter, M.W.; Godin, S.; et al. A BCMAxCD3 bispecific T cell-engaging anti-body demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR-T cells. Blood Adv. 2021, 5, 1291–1304. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Powers, G.; Luistro, L.; Babich, A.; Baldwin, E.; Li, Y.; Zhang, X.; Mendonça, M.; Majewski, N.; Nanjunda, R.; et al. Teclistamab is an active T cell-redirecting bispecific anti-body against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020, 4, 4538–4549. [Google Scholar] [CrossRef]
- Trinklein, N.D.; Pham, D.; Schellenberger, U.; Buelow, B.; Boudreau, A.; Choudhry, P.; Clarke, S.C.; Dang, K.; Harris, K.E.; Iyer, S.; et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. mAbs 2019, 11, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti–B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Minnema, M.C.; Lee, H.C.; Spencer, A.; Kapoor, P.; Madduri, D.; Larsen, J.; Ailawadhi, S.; Kaufman, J.L.; Raab, M.S.; et al. A Phase 1 First in Human (FIH) Study of AMG 701, an Anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE® (bispecific T-cell engager) Molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM). Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Mateos, M.-V.; Nahi, H.; Krishnan, A.Y.; Van De Donk, N.W.; Miguel, J.S.; Oriol, A.; Rosiñol, L.; Chari, A.; Adams, H.; et al. Phase I study of teclistamab, a humanized B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed/refractory multiple myeloma (R/R MM). J. Clin. Oncol. 2020, 38, 100. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Rosinol, L.; Chari, A.; Bhutani, M.; Karlin, L.; et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. Lancet 2021, 398, 665–674. [Google Scholar] [CrossRef]
- Garfall, A.L.; Usmani, S.Z.; Mateos, M.-V.; Nahi, H.; Van De Donk, N.W.; San-Miguel, J.F.; Rocafiguera, A.O.; Rosinol, L.; Chari, A.; Bhutani, M.; et al. Updated Phase 1 Results of Teclistamab, a B-Cell Maturation Antigen (BCMA) × CD3 Bispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 27. [Google Scholar] [CrossRef]
- Krishnan, A.Y.; Garfall, A.L.; Mateos, M.-V.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Rosiñol, L.; Chari, A.; Bhutani, M.; et al. Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM). J. Clin. Oncol. 2021, 39, 8007. [Google Scholar] [CrossRef]
- Madduri, D.; Rosko, A.; Brayer, J.; Zonder, J.; Bensinger, W.I.; Li, J.; Xu, L.; Adriaens, L.; Chokshi, D.; Zhang, W.; et al. REGN5458, a BCMA x CD3 Bispecific Monoclonal Antibody, Induces Deep and Durable Responses in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 41–42. [Google Scholar] [CrossRef]
- Rodriguez, C.; D’Souza, A.; Shah, N.; Voorhees, P.M.; Buelow, B.; Vij, R.; Kumar, S.K. Initial Results of a Phase I Study of TNB-383B, a BCMA × CD3 Bispecific T-Cell Redirecting Antibody, in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 43–44. [Google Scholar] [CrossRef]
- Bahlis, N.J.; Raje, N.S.; Costello, C.; Dholaria, B.R.; Solh, M.M.; Levy, M.Y.; Tomasson, M.H.; Dube, H.; Liu, F.; Liao, K.H.; et al. Efficacy and safety of elranatamab (PF-06863135), a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MM). J. Clin. Oncol. 2021, 39, 8006. [Google Scholar] [CrossRef]
- Costa, L.J.; Wong, S.W.; Bermúdez, A.; De La Rubia, J.; Mateos, M.-V.; Ocio, E.M.; Rodríguez-Otero, P.; San-Miguel, J.; Li, S.; Sarmiento, R.; et al. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood 2019, 134, 143. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Mauser, M.; Einsele, H. Outcome of BCMA Bite (AMG420) Therapy in Relapse and Refractory Multiple Myeloma (RRMM) Patients. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Cho, S.-F.; Lin, L.; Xing, L.; Li, Y.; Wen, K.; Yu, T.; Hsieh, P.A.; Munshi, N.; Wahl, J.; Matthes, K.; et al. The immunomodulatory drugs lenalidomide and pomalidomide enhance the potency of AMG 701 in multiple myeloma preclinical models. Blood Adv. 2020, 4, 4195–4207. [Google Scholar] [CrossRef]
- Frerichs, K.A.; Broekmans, M.E.C.; Marin Soto, J.A.; van Kessel, B.; Heymans, M.W.; Holthof, L.C.; Verkleij, C.P.M.; Boominathan, R.; Vaidya, B.; Sendecki, J.; et al. Preclinical Activity of JNJ-7957, a Novel BCMA×CD3 Bispecific Antibody for the Treatment of Multiple Myeloma, Is Potentiated by Daratumumab. Clin. Cancer Res. 2020, 26, 2203–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raje, N.; Jakubowiak, A.; Gasparetto, C.; Cornell, R.; Krupka, H.; Navarro, D.; Forgie, A.J.; Udata, C.; Basu, C.; Chou, J.; et al. Safety, Clinical Activity, Pharmacokinetics, and Pharmacodynamics from a Phase I Study of PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 1869. [Google Scholar] [CrossRef]
- Cho, S.-F.; Anderson, K.C.; Tai, Y.-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef]
- Schade, H.; Madan, S.; Medvedova, E.; Nath, R.; Knapp, L.; Lemon, B.; Sun, L.L. HPN217-3001: A Phase 1/2 Open-Label, Multicenter, Dose Escalation and Dose Expansion Study of the Safety, Tolerability, and Pharmacokinetics of HPN217, a Bcma-Targeting T-Cell Engager, in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 10. [Google Scholar] [CrossRef]
- Younes, A.; Bartlett, N.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.; Forero-Torres, A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sievers, E.; Larson, R.; Stadtmauer, E.A.; Estey, E.; Löwenberg, B.; Dombret, H.; Karanes, C.; Theobald, M.; Bennett, J.M.; Sherman, M.L.; et al. Efficacy and Safety of Gemtuzumab Ozogamicin in Patients With CD33-Positive Acute Myeloid Leukemia in First Relapse. J. Clin. Oncol. 2001, 19, 3244–3254. [Google Scholar] [CrossRef]
- Burris, H.A.; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2) –Positive Breast Cancer After Prior HER2-Directed Therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.R.W.; Gliddon, L.; Fieles, W.; et al. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Kinneer, K.; Flynn, M.; Thomas, S.B.; Meekin, J.; Varkey, R.; Xiao, X.; Zhong, H.; Breen, S.; Hynes, P.G.; Fleming, R.; et al. Preclinical assessment of an antibody–PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia 2018, 33, 766–771. [Google Scholar] [CrossRef]
- Singh, R.K.; Jones, R.J.; Hong, S.; Shirazi, F.; Wang, H.; Kuiatse, I.; Pahl, A.; Orlowski, R.Z. HDP101, a Novel B-Cell Maturation Antigen (BCMA)-Targeted Antibody Conjugated to α-Amanitin, Is Active Against Myeloma with Preferential Efficacy Against Pre-Clinical Models of Deletion 17p. Blood 2018, 132, 593. [Google Scholar] [CrossRef]
- Lee, H.C.; Raje, N.S.; Landgren, O.; Upreti, V.V.; Wang, J.; Avilion, A.A.; Hu, X.; Rasmussen, E.; Ngarmchamnanrith, G.; Fujii, H.; et al. Phase 1 study of the anti-BCMA antibody-drug conjugate AMG 224 in patients with relapsed/refractory multiple myeloma. Leukemia 2021, 35, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2019, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.A.; Callander, N.S.; Sborov, D.W.; Suvannasankha, A.; et al. Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs). J. Clin. Oncol. 2020, 38, 8536. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.; Chari, A.; Abdallah, A.-O.; Callander, N.S.; Sborov, D.; Suvannasankha, A.; et al. DREAMM-2: Single-Agent Belantamab Mafodotin (Belamaf) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) – 1-Year Outcomes By Prior Therapies. Blood 2020, 136, 1417. [Google Scholar] [CrossRef]
- Cohen, A.D.; Trudel, S.; Lonial, S.; Libby, E.N.; Lee, H.C.; Besemer, B.; Facon, T.; Nooka, A.K.; Callander, N.S.; Chari, A.; et al. DREAMM-2: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) and high-risk (HR) cytogenetics. J. Clin. Oncol. 2020, 38, 8541. [Google Scholar] [CrossRef]
- Farooq, A.V.; Degli Esposti, S.; Popat, R.; Thulasi, P.; Lonial, S.; Nooka, A.K.; Jakubowiak, A.; Sborov, D.; Zaugg, B.E.; Badros, A.Z.; et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody-Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef] [PubMed]
- Lonial, F.S.; Nooka, M.A.; Thulasi, P.; Badros, A.Z.; Jeng, B.H.; Callander, N.S.; Sborov, M.D.; Zaugg, B.E.; Popat, R.; Degli Esposti, S.; et al. Recovery of Ocular Events with Longer-Term Follow-up in the DREAMMM-2 Study of Single-Agent Belantamab Mafodotin (Belamaf) in Patients with Relapsed or Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 26–27. [Google Scholar] [CrossRef]
- Richardson, P.G.; Lee, H.C.; Abdallah, A.-O.; Cohen, A.D.; Kapoor, P.; Voorhees, P.M.; Hoos, A.; Wang, K.; Baron, J.; Piontek, T.; et al. Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: Analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J. 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; McCurdy, A.; Sutherland, H. Part 1 Results of a Dose Finding Study of Belantamab Mafodotin (GSK2857916) in Combination with Pomalidomide (POM) and Dexamethasone (DEX) for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2021, 136, 25. [Google Scholar]
- Trudel, S.; Davis, R.; Lewis, N.M.; Bakshi, K.K.; Chopra, B.; De Oca, R.M.; Ferron-Brady, G.; Eliason, L.; Kremer, B.E.; Gupta, I.; et al. DREAMM-8: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin with Pomalidomide and Dexamethasone (B-Pd) Vs Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 4. [Google Scholar] [CrossRef]
- Popat, R.; Nooka, M.A.; Stockerl-Goldstein, K.; Abonour, R.; Ramaekers, R.; Khot, A.; Forbes, A.; Lee, C.; Augustson, M.F.F.B.; Spencer, A.; et al. DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 19–20. [Google Scholar] [CrossRef]
- Rifkin, R.M.; Boyd, M.B.K.; Grosicki, S.; Kim, K.; Di Raimondo, F.; A Dimopoulos, M.; Weisel, K.; Arnulf, B.; Hajek, R.; Hungria, V.T.M.; et al. DREAMM-7: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin (Belamaf) with Bortezomib, and Dexamethasone (B-Vd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 53–54. [Google Scholar] [CrossRef]
- Nooka, A.K.; Weisel, K.; van de Donk, N.W.; Routledge, D.; Otero, P.R.; Song, K.; Quach, H.; Callander, N.; Minnema, M.C.; Trudel, S.; et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Futur. Oncol. 2021, 17, 1987–2003. [Google Scholar] [CrossRef]
- Kumar, S.K.; Migkou, M.; Bhutani, M.; Spencer, A.; Ailawadhi, S.; Kalff, A.; Walcott, F.; Pore, N.; Gibson, D.; Wang, F.; et al. Phase 1, First-in-Human Study of MEDI2228, a BCMA-Targeted ADC in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 26–27. [Google Scholar] [CrossRef]
- Strassz, A.; Raab, M.S.; Orlowski, M.R.Z.; Kulke, M.; Schiedner, G.; Pahl, A. A First in Human Study Planned to Evaluate Hdp-101, an Anti-BCMA Amanitin Antibody-Drug Conjugate with a New Payload and a New Mode of Action, in Multiple Myeloma. Blood 2020, 136, 34. [Google Scholar] [CrossRef]
- Polson, A.G.; Zheng, B.; Elkins, K.; Chang, W.; Du, C.; Dowd, P.; Yen, L.; Tan, C.; Hongo, J.-A.; Koeppen, H.; et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int. Immunol. 2006, 18, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkins, K.; Zheng, B.; Go, M.; Slaga, D.; Du, C.; Scales, S.J.; Yu, S.-F.; McBride, J.; De Tute, R.; Rawstron, A.; et al. FcRL5 as a Target of Antibody–Drug Conjugates for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2012, 11, 2222–2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Stagg, N.; Johnston, J.; Harris, M.; Menzies, S.; DiCara, D.; Clark, V.; Hristopoulos, M.; Cook, R.; Slaga, D.; et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synpase Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell 2017, 31, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.K.; Krishnan, A.Y.; Singhal, S.; Boccia, R.V.; Patel, M.R.; Niesvizky, R.; Chanan-Khan, A.A.; Ailawadhi, S.; Brumm, J.; Mundt, K.E.; et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.D.; Harrison, S.J.; Krishnan, A.; Fonseca, R.; Forsberg, P.A.; Spencer, A.; Berdeja, J.G.; Laubach, J.P.; Li, M.; Choeurng, V.; et al. Initial Clinical Activity and Safety of BFCR4350A, a FcRH5/CD3 T-Cell-Engaging Bispecific Antibody, in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 42–43. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Gleiss, A.; Porpaczy, E.; Kainz, B.; Grunt, T.W.; Raderer, M.; Hilgarth, B.; Drach, J.; Ludwig, H.; Gisslinger, H.; et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur. J. Clin. Investig. 2012, 42, 953–960. [Google Scholar] [CrossRef]
- Inoue, S.; Nambu, T.; Shimomura, T. The RAIG Family Member, GPRC5D, Is Associated with Hard-Keratinized Structures. J. Investig. Dermatol. 2004, 122, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long, T.J.; Ng, K.Y.; Ghoddusi, M.; Purdon, T.J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019, 11, eaau7746. [Google Scholar] [CrossRef]
- Fernández de Larrea, C.; Staehr, M.; Lopez, A.; Ng, K.; Chen, Y.; Godfrey, W.; Purdon, T.J.; Ponomarev, V.; Wendel, H.G.; Brentjens, R.J.; et al. Defining an Optimal Dual-Targeted CAR-T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov. 2020, 1, 146–154. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Edavettal, S.; Mendonça, M.; Li, Y.; Tornetta, M.; Babich, A.; Majewski, N.; Husovsky, M.; Reeves, D.; Walsh, E.; et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 2020, 135, 1232–1243. [Google Scholar] [CrossRef]
- Chari, A.; Berdeja, J.; Oriol, A.; Van de Donk, N.; Rodriguez, P.; Askari, E.; Mateos, M.-V.; Minnema, M.C.; Verona, R.; Girgis, C.; et al. A Phase 1, First-in-Human Study of Talquetamab, a G Protein-Coupled Recetor Family C Group 5 Member D (GPRC5D) × CD3 Bispecific Antibody, in Patients with Relapsed and/or Refractory Multiple Myeloma (RRMM). Blood Adv. 2020, 136, 40–41. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Krishnan, A.Y.; Oriol, A.; Van De Donk, N.W.C.J.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Minnema, M.C.; Costa, L.J.; Verona, R.; et al. Updated results of a phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM). J. Clin. Oncol. 2021, 39, 8008. [Google Scholar] [CrossRef]
- His, E.D.; Steinle, R.; Balasa, B.; Szmania, S.; Draksharapu, A.; Shum, B.P.; Huseni, M.; Powers, D.; Nanisetti, A.; Zhang, Y.; et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008, 14, 2775–2784. [Google Scholar]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; Leblanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Nath, R.; Afar, D.E.H.; Mateos, M.V.; Berdeja, J.G.; Raab, M.S.; Guenther, A.; Martínez-López, J.; Jakubowiak, A.J.; Leleu, X.; et al. First-in-Human Phase I Study of ABBV-838, an Anti-body-Drug Conjugate Targeting SLAMF7/CS1 in Patients with Relapsed and Refractory Multiple Myeloma. Clin. cancer Res. 2020, 26, 2308–2317. [Google Scholar] [CrossRef] [Green Version]
- Gogishvili, T.; Danhof, S.; Prommersberger, S.; Rydzek, J.; Schreder, M.; Brede, C.; Einsele, H.; Hudecek, M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 2017, 130, 2838–2847. [Google Scholar] [CrossRef] [Green Version]
- Radocha, J.; van de Donk, N.; Weisel, K. Monoclonal Antibodies and Antibody Drug Conjugates in Multiple Myeloma. Cancers 2021, 13, 1571. [Google Scholar] [CrossRef]
- Drent, E.; Groen, R.W.; Noort, W.A.; Themeli, M.; Van Bueren, J.J.L.; Parren, P.W.; Kuball, J.; Sebestyen, Z.; Yuan, H.; De Bruijn, J.; et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica 2016, 101, 616–625. [Google Scholar] [CrossRef] [Green Version]
- Bruins, M.W.S.; Zheng, M.W.; Higgins, J.P.; Willert, E.K.; Newcomb, J.; Dash, A.B.; Van De Donk, N.W.; Zweegman, M.S.; Mutis, T. TAK-169, a Novel Recombinant Immunotoxin Specific for CD38, Induces Powerful Preclinical Activity Against Patient-Derived Multiple Myeloma Cells. Blood 2020, 136, 11–12. [Google Scholar] [CrossRef]
- Doucey, M.-A.; Pouleau, B.; Estoppey, C.; Stutz, C.; Croset, A.; Laurendon, A.; Monney, T.; Pluess, M.; Ries-Fecourt, C.; Macoin, J.; et al. ISB 1342: A first-in-class CD38 T cell engager for the treatment of relapsed refractory multiple myeloma. J. Clin. Oncol. 2021, 39, 8044. [Google Scholar] [CrossRef]
- Li, C.; Mei, H.; Hu, Y.; Guo, T.; Liu, L.; Jiang, H.; Tang, L.; Wu, Y.H.; Ai, L.; Deng, L.; et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood 2019, 134, 930. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, B.; Gao, L.; Liu, L.; Ge, J.; He, A.; Du, J.J.; Li, L.; Lu, J.; Chen, X.; et al. Clinical Results of a Multicenter Study of the First-in-Human Dual BCMA and CD19 Targeted Novel Platform Fast CAR-T Cell Therapy for Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Yan, L.; Qu, S.; Shang, J.; Shi, X.; Kang, L.; Xu, N.; Zhu, M.; Zhou, J.; Jin, S.; Yao, W.; et al. Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med. 2020, 10, 563–574. [Google Scholar] [CrossRef]
- Sun, C.; Mahendravada, A.; Ballard, B.; Kale, B.; Ramos, C.; West, J.; Maguire, T.; McKay, K.; Lichtman, E.; Tuchman, S.; et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget 2019, 10, 2369–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, S.; Leutkens, T.; Scherer, S.; Davis, P.; Erica, V.M.; Olson, M. CD229 CAR-T cells eliminate multiple myeloma and tumor propagating cells without fratricide. Nat. Commun. 2020, 11, 798. [Google Scholar] [CrossRef]
- Casucci, M.; Di Robilant, B.N.; Falcone, L.; Camisa, B.; Norelli, M.; Genovese, P.; Gentner, B.; Gullotta, F.; Ponzoni, M.; Bernardi, M.; et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 2013, 122, 3461–3472. [Google Scholar] [CrossRef]
- Sherbenou, D.; Aftab, B.T.; Su, Y.; Behrens, C.R.; Wiita, A.; Logan, A.C.; Acosta-Alvear, D.; Hann, B.C.; Walter, P.; Shuman, M.A.; et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J. Clin. Investig. 2016, 126, 4640–4653. [Google Scholar] [CrossRef]
- Guo, B.; Chen, M.; Han, Q.; Hui, F.; Dai, H.; Zhang, W. CD138-directed adoptive immunotherapy of chimeric antigen recep-tor (CAR)-modified T cells for multiple myeloma. Transplant Cell Ther. 2016, 2, 28–35. [Google Scholar]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Timmers, M.; Roex, G.; Wang, Y.; Campillo-Davo, D.; Van Tendeloo, V.F.I.; Chu, Y.; Berneman, Z.; Luo, F.; Van Acker, H.H.; Anguille, S. Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front. Immunol. 2019, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, E.; Veitonmäki, N.; Norlén, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2018, 6, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, D. Antibody-drug conjugates in clinical trials for lymphoid malignancies and multiple myeloma. J. Hematol. Oncol. 2019, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Chung, T.H.; Kumar, S.; Usmani, S.; Munshi, N.; Avet-Loiseau, H.; Goldschmidt, H.; Durie, B.; Durie, B. Gene signature combinations improve prognos-tic stratification of multiple myeloma patients. Leukemia 2016, 30, 1071–1078. [Google Scholar] [CrossRef]
- Samur, M.K.; Aktas Samur, A.; Fulciniti, M.; Szalat, R.; Han, T.; Shammas, M.; Richardson, P.; Magrangeas, F.; Minvielle, S.; Corre, J.; et al. Genome-Wide Somatic Alterations in Multiple Myeloma Reveal a Superior Outcome Group. J. Clin. Oncol. 2020, 38, 3107–3118. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Olyslager, Y.; Banerjee, A.; Goldberg, J.D.; et al. Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen (BCMA)-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38, 8505. [Google Scholar] [CrossRef]
- Munshi, N.C.; Larry, D.; Anderson, J.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.D.; Lin, Y.; et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR-T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J. Clin. Oncol. 2020, 38, 8503. [Google Scholar] [CrossRef]
- Cohen, A.D.; Hari, M.P.; Htut, M.; Berdeja, J.G.; Madduri, D.; Usmani, M.S.Z.; Allred, A.J.; Olyslager, M.Y.; Banerjee, A.; Goldberg, J.D.; et al. Patient Expectations and Perceptions of Treatment in CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel in Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 13–15. [Google Scholar] [CrossRef]
- Shah, N.; Delforge, M.; San-Miguel, J.F.; Bertin, K.B.; Tahir, M.J.; Lewis, H.B.; Wang, J.; Braverman, J.; Campbell, T.B.; Dhanda, D.; et al. Secondary Quality-of-Life Domains in Patients with Relapsed and Refractory Multiple Myeloma Treated with the Bcma-Directed CAR T Cell Therapy Idecabtagene Vicleucel (ide-cel; bb2121): Results from the Karmma Clinical Trial. Blood 2020, 136, 28–29. [Google Scholar] [CrossRef]

| CAR-T Cell Construct (Name) | Study Name and/or Phase | Number of Patients | Triple-Class Refractory, % | High-Risk Cytogenetics/EMD, % | Median PFS, Months (95% CI) | Median OS, Months (95% CI) | ≥CR, % | CRS, All Grades, % | Neurotoxicity, All Grades, % | NCT Number | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ide-cel (bb2121) | CRB-401, Phase 1 | 62 | 69 | 27/37 | 8.8 (5.9–11.9) | 34.2 (19.2–NE) | 39 | 76 | 36 | NCT02658929 | [18,19] |
| Ide-cel (bb2121) | KarMMa, Phase 2 | 128 | 84 | 35/39 | 8.8 (5.6–11.6) | 19.4 (18.2–NE) | 33 | 84 | 18 | NCT03361748 | [20,21] |
| Cilta-cel | LEGEND-2, Phase 1/2 | 57 | NR | NR | 20 (10–28) | Not reached, 18-month OS 68% (54–79%) | 74 | 90 | 1 | NCT03090659 | [26] |
| Cilta-cel | CARTITUDE-1, Phase 1b/2 | 97 | 88 | 24/13 | 22.8 (22.8–NE) | Not reached, 18-month OS 80.9% (71.4–87.6%) | 80 | 95 | 21 | NCT03548207 | [27,29] |
| Orva-cel | EVOLVE, Phase 1/2 | 62 | 94 | 41/23 | 9.3 in the 300 × 106 group (n = 19), not reached in the other groups | NR | 36 | 89 | 13 | NCT03430011 | [33] |
| bb21217 | CRB-402, Phase 1 | 69 | 64 | 33/NR | NR, mDOR 17.0 (9.4–NE) | NR | 29 | 70 | 16 | NCT03274219 | [24] |
| NCI CAR-BCMA | Phase 1 | 24 | NR | 46/NR | NR, mEFS 31 weeks | NR | 8 | 71 | NR | NCT02215967 | [13,34] |
| UPenn CART-BCMA | Phase 1 | 25 | 72 | 96/28 | 65/57/125 days in cohort 1/2/3 | NR | 8 | 88 | 32 | NCT02546167 | [35] |
| P-BCMA-101 | PRIME, Phase 1/2 | 55 | 60 | NR | NR | NR | NR, ≥VGPR: 50 (n = 6) * | 17 (n = 53) | 4 (n = 53) | NCT03288493 | [32] |
| CT053 | LUMMICAR STUDY 2, Phase 1 | 20 | 85 | 55/25 | NR | NR | 25 | 79 | 16 | NCT03915184 | [36] |
| ALLO-715 | UNIVERSAL, Phase 1 | 31 | NR | 48/23 | NR | NR | ≥VGPR: 40 | 45 | 0 | NCT04093596 | [31] |
| C-CAR088 | Phase 1 | 23 | NR | 81/NR | Not reached, 6-month PFS 65.1% (47–90) | NR | 44 | 91 | 4 | NCT03751293 NCT03815383 NCT04322292 NCT04295018 | [37] |
| Agent | Drug Design | Trial Phase | Drug Status | Best ORR % | CRS % (n) | NCT Number | References |
|---|---|---|---|---|---|---|---|
| AMG420 | BiTE | 1 | No further development | 70% (at MTD, n = 10) | 38% (16/42) | NCT03836053 | [53] |
| AMG701 | Half-life extended BiTE | 1/2 | Phase 1/2 study ongoing | 83% (last evaluated dose expansion cohort, n = 6) | 61% (46/75) | NCT03287908 | [54] |
| Teclistamab (JNJ-64007957) | BsAb, IgG4 Fc region (DuoBody) | 1/2 | Several phase 1/2 studies ongoing, monotherapy and combinations | 69% (most active IV and SC doses, n = 68) | 55% (82/149) | NCT04557098 NCT03145181 | [55,56,57,58] |
| REGN5458 | BsAb, IgG4 Fc region (VelociBi) | 1/2 | Phase 1/2 study ongoing | 62.5% (highest tested dose level, n = 8) | 39% (19/49) | NCT03761108 | [59] |
| TNB-383B | BsAb, IgG4 Fc region, dual BCMA binding domains | 1 | Phase 1 study ongoing | 80% (highest tested dose levels, n = 15) | 45% (26/58) | NCT03933735 | [60] |
| Elranatamab (PF-06863135) | BsAb, IgG2a Fc region | 2 | Phase 2 study ongoing (MAGNETISMM-3) | 83.3% (RP2D SC, n = 6) | 73% (22/30) | NCT04649359 | [61] |
| CC-93269 | BsAb, IgG1 Fc region, bivalent anti-BCMA arm | 1 | Phase 1 study ongoing | 89% (highest tested dose level, n = 9) | 77% (23/30) | NCT03486067 | [62] |
| Agent | Cytotoxic Conjugate | Trial Phase | Drug Status/Published Results | NCT Number | References |
|---|---|---|---|---|---|
| Belantamab mafodotin (GSK2857916) | Monomethyl auristatin F (MMAF) | 2, 3 | First-in-class approval 2020; several studies with different drug combinations ongoing | NCT02064387 | [73] |
| MEDI2228 | Pyrrolobenzodiazepine (PBD) | 1 | Early phase 1 results published | NCT03489525 | [74] |
| HDP-101 | Amanitin | 1/2 | Phase 1 not yet recruiting | NCT04879043 | [75] |
| AMG-224 | Mertansine (DM1) | 1 | Early phase 1 results published; deprioritized in favor of the development of bispecific antibody constructs by the company | NCT02561962 | [76] |
| CC99712 | Undisclosed | 1 | Recruiting, no results available | NCT04036461 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strassl, I.; Schreder, M.; Steiner, N.; Rudzki, J.; Agis, H.; Künz, T.; Müser, N.; Willenbacher, W.; Petzer, A.; Neumeister, P.; et al. The Agony of Choice—Where to Place the Wave of BCMA-Targeted Therapies in the Multiple Myeloma Treatment Puzzle in 2022 and Beyond. Cancers 2021, 13, 4701. https://doi.org/10.3390/cancers13184701
Strassl I, Schreder M, Steiner N, Rudzki J, Agis H, Künz T, Müser N, Willenbacher W, Petzer A, Neumeister P, et al. The Agony of Choice—Where to Place the Wave of BCMA-Targeted Therapies in the Multiple Myeloma Treatment Puzzle in 2022 and Beyond. Cancers. 2021; 13(18):4701. https://doi.org/10.3390/cancers13184701
Chicago/Turabian StyleStrassl, Irene, Martin Schreder, Normann Steiner, Jakob Rudzki, Hermine Agis, Tina Künz, Nino Müser, Wolfgang Willenbacher, Andreas Petzer, Peter Neumeister, and et al. 2021. "The Agony of Choice—Where to Place the Wave of BCMA-Targeted Therapies in the Multiple Myeloma Treatment Puzzle in 2022 and Beyond" Cancers 13, no. 18: 4701. https://doi.org/10.3390/cancers13184701
APA StyleStrassl, I., Schreder, M., Steiner, N., Rudzki, J., Agis, H., Künz, T., Müser, N., Willenbacher, W., Petzer, A., Neumeister, P., & Krauth, M. T. (2021). The Agony of Choice—Where to Place the Wave of BCMA-Targeted Therapies in the Multiple Myeloma Treatment Puzzle in 2022 and Beyond. Cancers, 13(18), 4701. https://doi.org/10.3390/cancers13184701






