Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
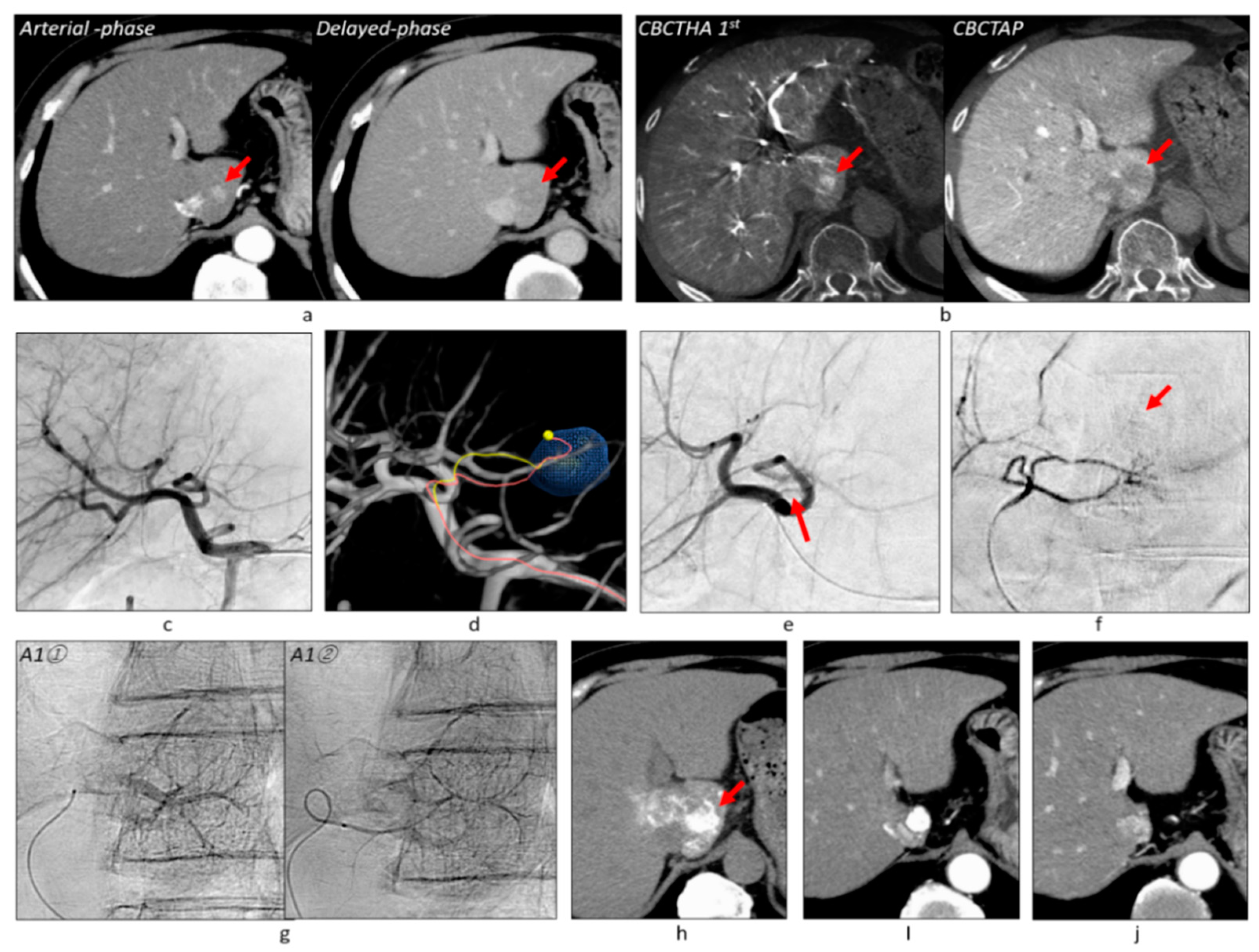
2.2. Protocol of CBCT and Angiography
2.3. Tumor-Feeder Detection Using TACE Guidance Software
2.4. TACE Procedure
2.5. Endpoint of the TACE Procedure
2.6. Follow-Up
2.7. Assessment
2.7.1. Tumor Hypervascularity on DSA and CBCTHA
2.7.2. Detectability of Tumor-Feeders by AFD
2.7.3. Grades of Portal Vein Visualization with Iodized Oil
2.7.4. Technical Success of cTACE
2.7.5. Complications
2.7.6. Tumor Response, Tumor Recurrence, and Prognosis
2.7.7. Statistical Analysis
3. Results
3.1. Tumor Hypervascularity on DSA and CBCTHA
3.2. Embolized Branches
3.3. Grades of Portal Vein Visualization
3.4. Technical Success of TACE
3.5. Complications
3.6. Tumor Response and Local Tumor Progression
3.7. Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc. Interv. Radiol. 2010, 33, 41–52. [Google Scholar] [CrossRef]
- Sacco, R.; Bargellini, I.; Bertini, M.; Bozzi, E.; Romano, A.; Petruzzi, P.; Tumino, E.; Gianni, B.; Federici, G.; Cioni, R.; et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2011, 22, 1545–1552. [Google Scholar] [CrossRef]
- Golifieri, R.; Giampalma, E.; Renzulli, M.; Cioni, R.; Bargellini, I.; Bartolozzi, C.; Breatta, A.D.; Gandini, G.; Nani, R.; Gasparini, D.; et al. PRECISION Italia Study Group. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br. J. Cancer 2014, 111, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cucchetti, A.; Trevisani, F.; Cappelli, A.; Mosconi, C.; Renzulli, M.; Pinna, A.D.; Golfieri, R. Cost-effectiveness of doxorubicin-eluting beads versus conventional trans-arterial chemo-embolization for hepatocellular carcinoma. Dig. Liver Dis. 2016, 48, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Inaba, Y.; Tanaka, T.; Sugawara, S.; Kodama, Y.; Aramaki, T.; Anai, H.; Morita, S.; Tsukahara, Y.; Seki, H.; et al. A prospective, randomized, controlled trial of selective DEB-TACE vs. selective cTACE with epirubicinfor hepatocellular carcinoma: JIVROSG-1302 PRESIDENT study. J. Clin. Oncol. 2020, 38. suppl abstr 4518. [Google Scholar] [CrossRef]
- Miyayama, S.; Matsui, O.; Yamashiro, M.; Ryu, Y.; Kaito, K.; Ozaki, K.; Takeda, T.; Yoneda, N.; Notsumata, K.; Toya, D.; et al. Ultraselective transcatheter arterial chemoembolization with a 2-F tip microcatheter for small hepatocellular carcinomas: Relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J. Vasc. Interv. Radiol. 2007, 18, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Mitsui, T.; Zen, Y.; Sudo, Y.; Yamashiro, M.; Okuda, M.; Yoshie, Y.; Sanada, T.; Notsumata, K.; Tanaka, N.; et al. Histopathological findings after ultraselective transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol. Res. 2009, 39, 374–381. [Google Scholar] [CrossRef]
- Miyayama, S.; Matsui, O.; Yamashiro, M.; Ryu, Y.; Takata, H.; Takeda, T.; Aburano, H.; Shigenari, N. Iodized oil accumulation in the hypovascular tumor portion of early-stage hepatocellular carcinoma after ultraselective transcatheter arterial chemoembolization. Hepatol. Int. 2007, 1, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Matsui, O. Superselective conventional transarterial chemoembolization for hepatocellular carcinoma: Rationale, technique, and outcome. J. Vasc. Interv. Radiol. 2016, 27, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S. Ultraselective conventional transarterial chemoembolization: When and how? Clin. Mol. Hepatol. 2019, 25, 344–353. [Google Scholar] [CrossRef] [PubMed]
- eUpdate Hepatocellular Carcinoma Algorithm Published. ESMO Guidelines Committee. Available online: https://www.esmo.org/guidelines/gastrointestinal-cancers/hepatocellular-carcinoma/eupdate-hepatocellular-carcinoma-algorithm (accessed on 19 June 2020).
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.; et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology 2021, 73, S1. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Okuda, M.; Yoshie, Y.; Sugimori, N.; Igarashi, S.; Nakashima, Y.; Matsui, O. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc. Interv. Radiol. 2009, 32, 255–264. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc. Interv. Radiol. 2014, 37, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, C.; Sakurai, M.; Monden, M.; Marukawa, T.; Hosoki, T.; Tokunaga, K.; Wakasa, K.; Okamura, J.; Kozuka, T. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer 1991, 67, 81–86. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Hattori, Y.; Orito, N.; Matsui, K.; Tsuji, K.; Yoshida, M.; Matsui, O. Efficacy of cone-beam computed tomography during transcatheter arterial chemoembolization for hepatocellular carcinoma. Jpn. J. Radiol. 2011, 29, 371–377. [Google Scholar] [CrossRef]
- Deschamps, F.; Solomon, S.B.; Thornton, R.H.; Rao, P.; Hakime, A.; Kuoch, V.; de Baere, T. Computed analysis of three-dimensional cone-beam computed tomography angiography for determination of tumor-feeding vessels during chemoembolization of liver tumor: A pilot study. Cardiovasc. Interv. Radiol. 2010, 33, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Iwazawa, J.; Ohue, S.; Hashimoto, N.; Muramoto, O.; Mitani, T. Clinical utility and limitations of tumor-feeder detection software for liver cancer embolization. Eur. J. Radiol. 2013, 82, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Hashimoto, M.; Hashimoto, N.; Ikuno, M.; Okumura, K.; Yoshida, M.; Matsui, O. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2013, 24, 501–508. [Google Scholar] [CrossRef]
- Minami, Y.; Yagyu, Y.; Murakami, T.; Kudo, M. Tracking navigation imaging of transcatheter arterial chemoembolization for hepatocellular carcinoma using three-dimensional cone-beam CT angiography. Liver Cancer 2014, 3, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Ikuno, M.; Okumura, K.; Yoshida, M. Ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinoma guided by automated tumor-feeders detection software: Technical success and short-term tumor response. Abdom. Imaging 2014, 39, 645–656. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Nagai, K.; Tohyama, J.; Kawamura, K. Efficacy of automated tumor-feeder detection software using cone-beam computed tomography technology in transarterial targeted therapy. Interv. Radiol. 2016, 1, 28–38. [Google Scholar] [CrossRef][Green Version]
- Miyayama, S.; Yamashiro, M.; Sugimori, N.; Ikeda, R.; Okimura, K.; Sakuragawa, N. Outcomes of patients with hepatocellular carcinoma treated with conventional transarterial chemoembolization using guidance software. J. Vasc. Interv. Radiol. 2019, 30, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S. Interventions for hepatocellular carcinoma. Innervision 2016, 31, 15–18. (In Japanese) [Google Scholar]
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal lesions in the cirrhotic liver: Their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig. Dis. 2014, 32, 696–704. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Nagai, K.; Tohyama, J.; Kawamura, K.; Yoshida, M.; Sakuragawa, N. Evaluation of tumor recurrence after superselective conventional transcatheter arterial chemoembolization for hepatocellular carcinoma: Comparison of computed tomography and gadoxetate disodium-enhanced magnetic resonance imaging. Hepatol. Res. 2016, 46, 890–898. [Google Scholar] [CrossRef]
- Sasaki, A.; Kai, S.; Iwashita, Y.; Hirano, S.; Ohta, M.; Kitano, S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer 2005, 103, 299–306. [Google Scholar] [CrossRef]
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 27 November 2017).
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Concejero, A.; Chen, C.L.; Wang, C.C.; Wang, S.H.; Lin, C.C.; Liu, Y.W.; Yang, C.H.; Yong, C.C.; Lin, T.S.; Jawan, B.; et al. Living donor liver transplantation for hepatocellular carcinoma: A single-center experience in Taiwan. Transplantation 2008, 85, 398–406. [Google Scholar] [CrossRef]
- Golfieri, R.; Renzulli, M.; Mosconi, C.; Forlani, L.; Giampalma, E.; Piscaglia, F.; Trevisani, F.; Bolondi, L.; Bologna Liver Oncology Group (BLOG). Hepatocellular carcinoma responding to superselective transarterial chemoembolization: An issue of nodule dimension? J. Vasc. Interv. Radiol. 2013, 24, 509–517. [Google Scholar] [CrossRef]
- Ueda, K.; Matsui, O.; Kawamori, Y.; Nakamuma, Y.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Nonomura, A.; Takashima, T. Hypervascular hepatocellular carcinoma: Evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology 1998, 206, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sakon, M.; Nagano, H.; Nakamori, S.; Dono, K.; Ueshita, K.; Murakami, T.; Nakamura, H.; Monden, M. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: Analysis based on tumor hemodynamics. Arch. Surg. 2002, 137, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Uchida, H.; Nishimine, K.; Soda, S.; Oshima, M.; Nakano, H.; Nagano, N.; Nishimura, Y.; Yoshioka, T.; Guo, Q.; et al. Segmental transcatheter hepatic artery chemoembolization with iodized oil for hepatocellular carcinoma: Antitumor effect and influence on normal tissue. J. Vasc. Interv. Radiol. 1993, 4, 543–549. [Google Scholar] [CrossRef]
- Iwazawa, J.; Ohue, S.; Hashimoto, N.; Mitani, T. Comparison of the number of image acquisitions and procedural time required for transarterial chemoembolization of hepatocellular carcinoma with and without tumor-feeder detection software. Radiol. Res. Pract. 2013, 2013, 580839. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Yamashiro, M.; Nagai, K.; Yokka, A.; Yoshida, M.; Sakuragawa, N. Performance of novel virtual parenchymal perfusion software visualizing embolized areas of transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol. Res. 2017, 47, 446–454. [Google Scholar] [CrossRef]
- Miyayama, S.; Yamashiro, M.; Ikeda, R.; Matsumoto, J.; Ogawa, N.; Sakuragawa, N. Usefulness of virtual parenchymal perfusion software visualizing embolized areas to determine optimal catheter position in superselective conventional transarterial chemoembolization for hepatocellular carcinoma. Hepatol. Res. 2021, 51, 313–322. [Google Scholar] [CrossRef]
- Granito, A.; Bolondi, L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017, 18, e101–e112. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, C.C.; Hung, C.H.; Chen, C.L.; Lu, S.N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef]
- Ryu, T.; Tamaki, Y.; Wada, Y.; Saitsu, H. Operative microwave ablation for hepatocellular carcinoma within 3 cm and 3 nodules: Experience in 559 patients. J. Gastrointest. Surg. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, J.H.; Lee, D.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.H.; Kim, H.C.; Lee, J.M.; Chung, J.W.; Yi, N.J.; et al. Small single-nodule hepatocellular carcinoma: Comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014, 271, 909–918. [Google Scholar] [CrossRef]
- Terayama, N.; Matsui, O.; Gabata, T.; Kobayashi, S.; Sanada, J.; Ueda, K.; Kadoya, M.; Kawamori, Y. Accumulation of iodized oil within the nonneoplastic liver adjacent to hepatocellular carcinoma via the drainage routes of the tumor after transcatheter arterial embolization. Cardiovasc. Interv. Radiol. 2001, 24, 383–387. [Google Scholar] [CrossRef]








| Patient | Value |
| Number of patients | 259 |
| Sex, male/female | 154/105 |
| Age, y, mean (range) | 73.4 ± 8.2 (39–91) |
| Child–Pugh Class (score) A (5/6)/B (7/8/9)/C (10) | 202 (144/58)/49 (28/14/7)/8 (8) |
| ECOG performance status 0/1 | 241/18 |
| Etiology | Value |
| Hepatitis C | 147 |
| Hepatitis B | 51 |
| Hepatitis B + C | 8 |
| Alcohol | 14 |
| Hepatitis C + alcohol | 2 |
| Hepatitis B + alcohol | 2 |
| Nonalcoholic steatohepatitis | 10 |
| Primary biliary cirrhosis | 3 |
| Unknown | 22 |
| Tumor | Value |
| Single/2/3 | 167/61/31 |
| Size, mm, mean (range) | 17.2 ± 5.9 mm (5–30) |
| AFP level, ng/mL, mean (range) | 85.7 ± 396.6 (2–3950) |
| PIVKA-II level, mAU/mL, mean (range) | 186.7 ± 1278.0 (7–18,371) |
| Prior Treatment | Value |
| None | 173 |
| Hepatectomy | 16 |
| TACE | 31 |
| RFA | 13 |
| Hepatectomy + TACE/RFA/HAIC/radiation | 9/2/1/1 |
| TACE + RFA | 13 |
| HAIC | 1 |
| HAIC + TACE | 1 |
| Radiation | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyayama, S.; Yamashiro, M.; Ikeda, R.; Matsumoto, J.; Takeuchi, K.; Sakuragawa, N.; Ueda, T.; Sanada, T.; Notsumata, K.; Terada, T. Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm. Cancers 2021, 13, 6370. https://doi.org/10.3390/cancers13246370
Miyayama S, Yamashiro M, Ikeda R, Matsumoto J, Takeuchi K, Sakuragawa N, Ueda T, Sanada T, Notsumata K, Terada T. Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm. Cancers. 2021; 13(24):6370. https://doi.org/10.3390/cancers13246370
Chicago/Turabian StyleMiyayama, Shiro, Masashi Yamashiro, Rie Ikeda, Junichi Matsumoto, Kiyotaka Takeuchi, Naoko Sakuragawa, Teruyuki Ueda, Taku Sanada, Kazuo Notsumata, and Takuro Terada. 2021. "Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm" Cancers 13, no. 24: 6370. https://doi.org/10.3390/cancers13246370
APA StyleMiyayama, S., Yamashiro, M., Ikeda, R., Matsumoto, J., Takeuchi, K., Sakuragawa, N., Ueda, T., Sanada, T., Notsumata, K., & Terada, T. (2021). Efficacy of Superselective Conventional Transarterial Chemoembolization Using Guidance Software for Hepatocellular Carcinoma within Three Lesions Smaller Than 3 cm. Cancers, 13(24), 6370. https://doi.org/10.3390/cancers13246370






