Proteasomes in Patient Rectal Cancer and Different Intestine Locations: Where Does Proteasome Pool Change?
Abstract
:Simple Summary
Abstract
1. Introduction
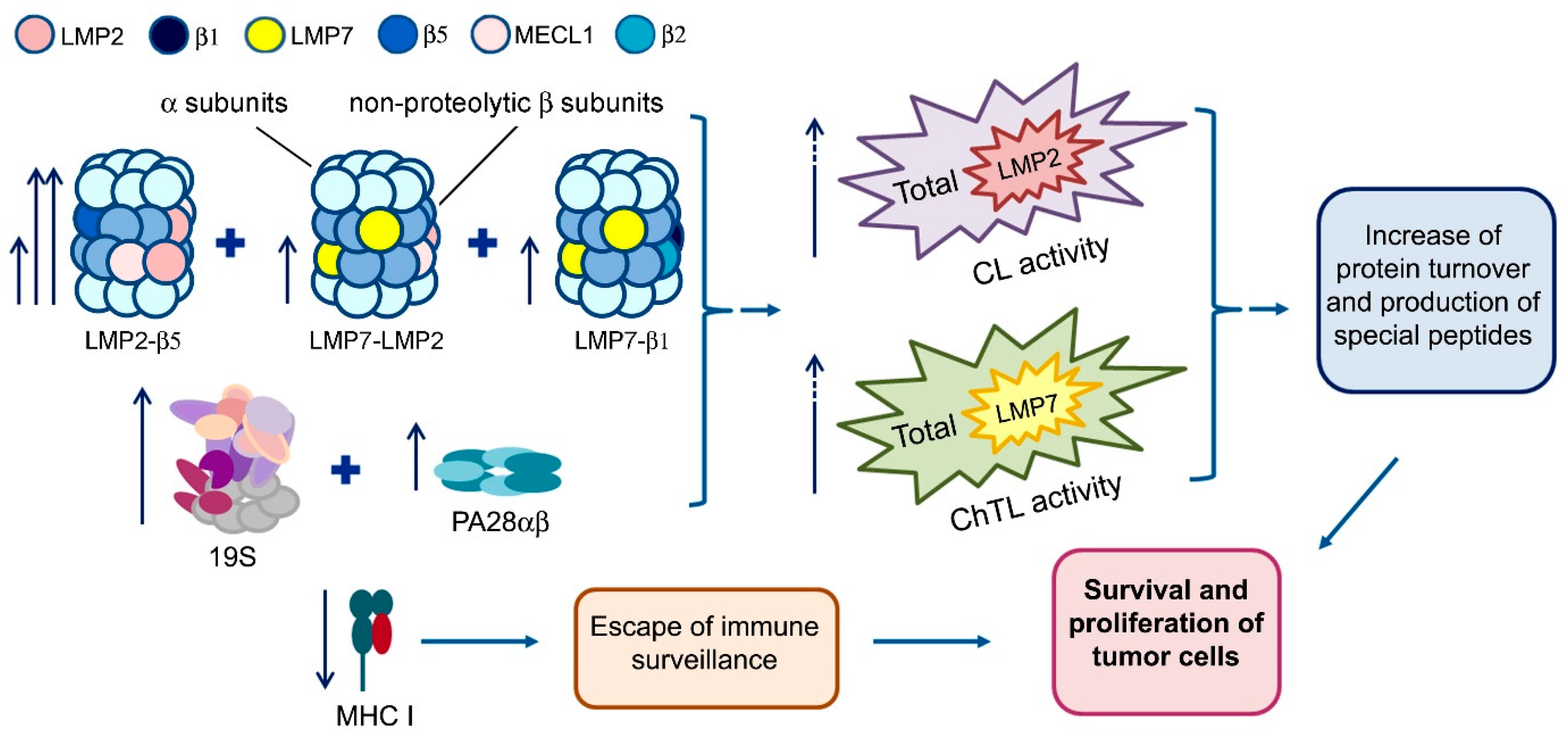
2. Results and Discussion
2.1. Proteasome Activities in Rectal Cancer and Different Intestine Locations In Vitro
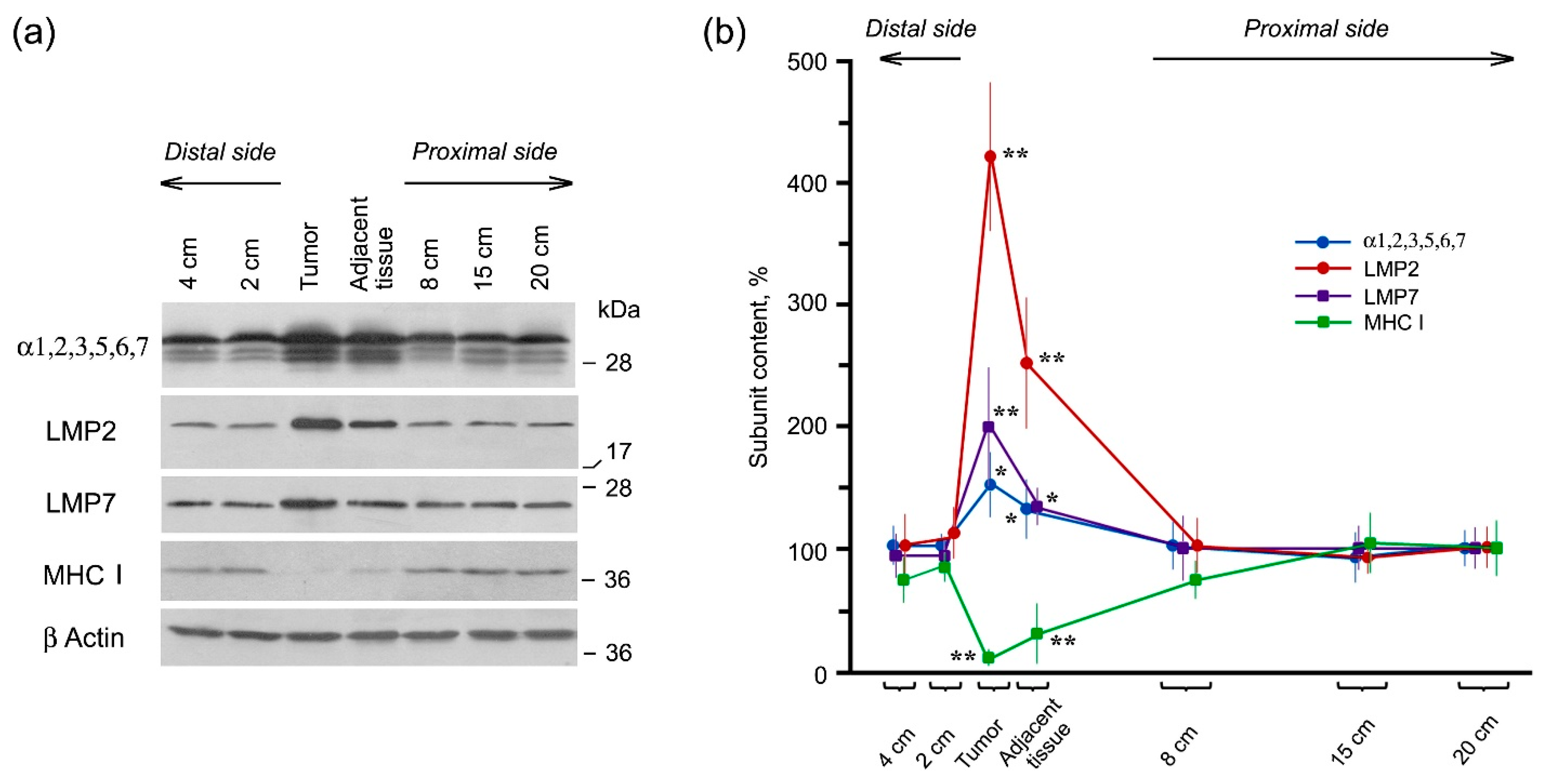
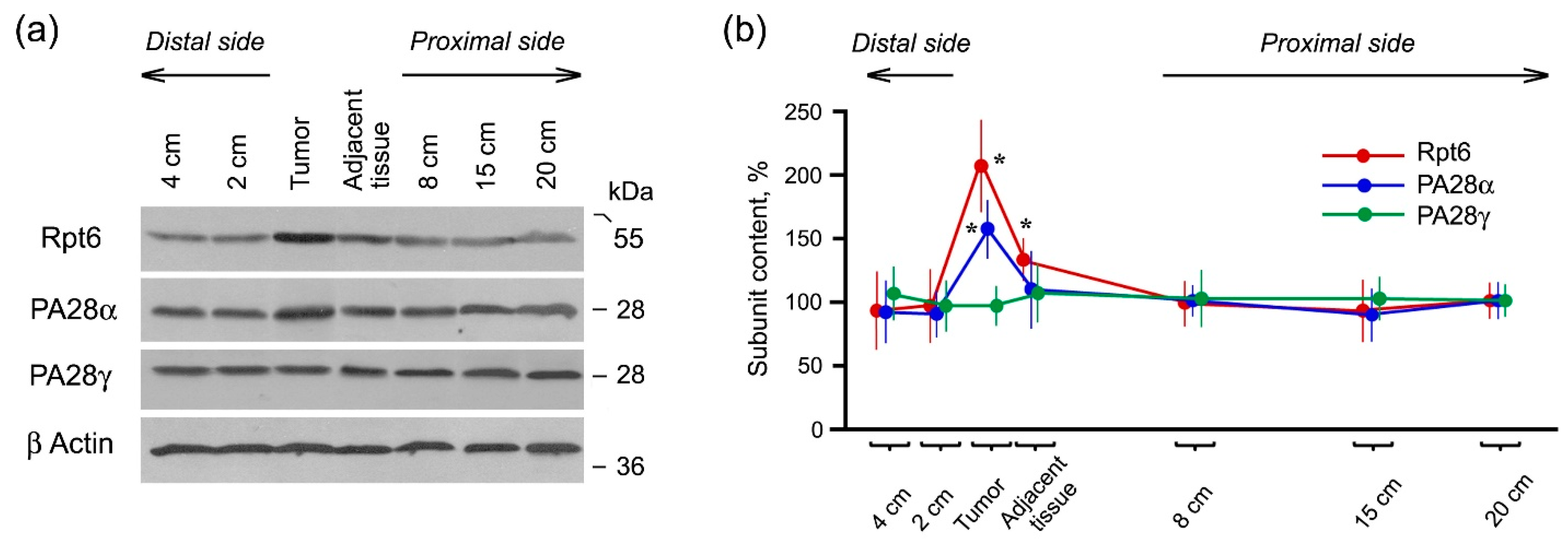
2.2. Expression of Proteasome Pool Components and MHC Class I Molecules in Rectal Cancer and Different Intestine Locations
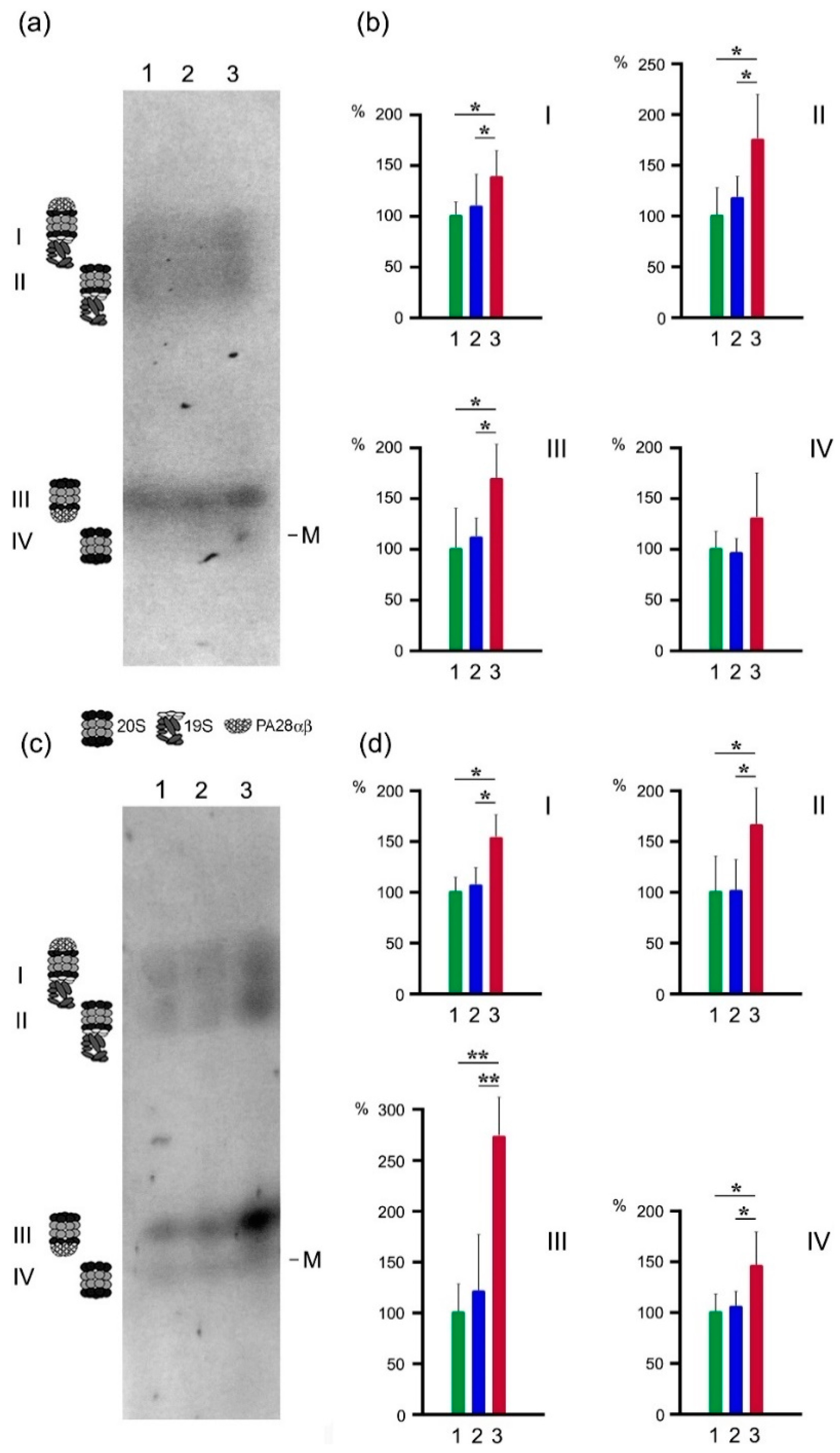
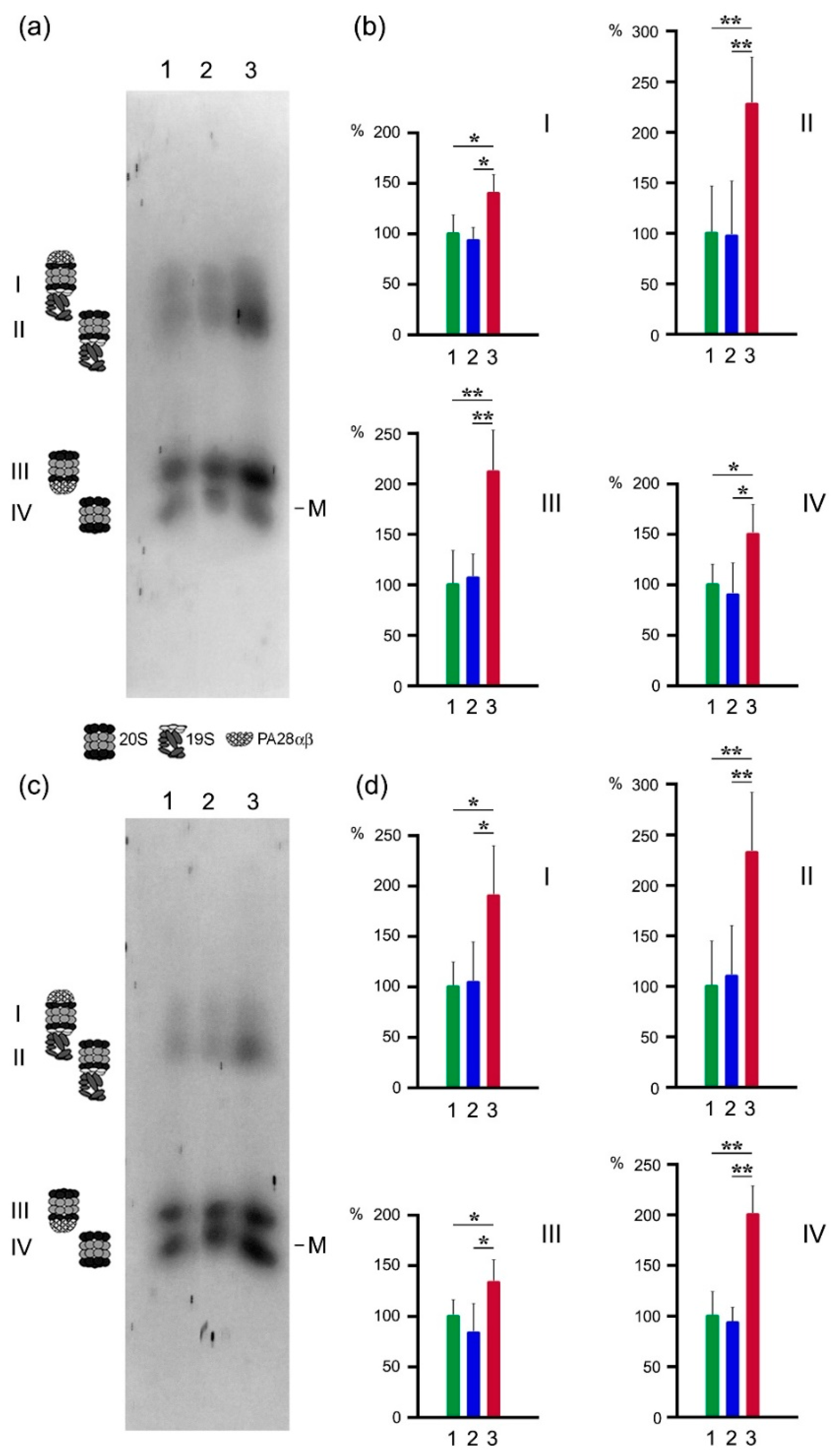
2.3. Proteasome Activities in Native Gel
3. Materials and Methods
3.1. Patient Tissue Samples
3.2. Antibodies and Main Reagents
3.3. Preparation of Clarified Tissue Homogenates
3.4. Detection of Proteasome Activities In Vitro
3.5. Western Blotting
3.6. Detection of Proteasome Activities in Native Gel
3.7. Technical Remarks
- (1)
- We investigated the expression of LMP2 and LMP7 immune subunits, but not MECL1 subunit, since generally MECL1 and LMP2 subunits are mutually required for incorporation into 20S structure, with incorporation of MECL1 depends on LMP2 and incorporation of LMP2 is facilitated by MECL1 [8,9]. Therefore, the change of LMP2 content reflects the change of MECL1 content.
- (2)
- For our investigation, the methods requiring proteasome purification, such as quantitative proteomics and similar techniques, were irrelevant, as different steps of proteasome purification lead to partial or total loss of one or another proteasome form and distortion of in-cell state [35,36]. We used the original method of electrophoresis in native gel, specially developed for crude proteasome fractions, to reflect in-cells state of “alive” proteasomes in tissues most correctly.
3.8. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Zhou, H.; Xiong, R.; Lu, Y.; Yan, D.; Xing, T.; Dong, L.; Tang, E.; Yang, H. Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res. Treat. 2007, 104, 21–30. [Google Scholar] [CrossRef]
- Sharova, N.P.; Astakhova, T.M.; Karpova, Y.D.; Lyupina, Y.V.; Alekhin, A.I.; Goncharov, N.G.; Sumedi, I.R.; Cherner, V.A.; Rodoman, G.V.; Kuznetsov, N.A.; et al. Changes in proteasome pool in human papillary thyroid carcinoma development. Cent. Eur. J. Biol. 2011, 6, 486–496. [Google Scholar] [CrossRef]
- Zakharova, L.A.; Khegai, I.I.; Sharova, N.P.; Melnikova, V.I.; Karpova, Y.D.; Astakhova, T.M.; Popova, N.A.; Ivanova, L.N. Pattern of MHC class I and immune proteasome expression in Walker 256 tumor during growth and regression in Brattleboro rats with the hereditary defect of arginine-vasopressin synthesis. Cell. Immunol. 2011, 271, 385–391. [Google Scholar] [CrossRef]
- Shashova, E.E.; Lyupina, Y.V.; Glushchenko, S.A.; Slonimskaya, E.M.; Savenkova, O.V.; Kulikov, A.M.; Gornostaev, N.G.; Kondakova, I.V.; Sharova, N.P. Proteasome functioning in breast cancer: Connection with clinical-pathological factors. PLoS ONE 2014, 9, e109933. [Google Scholar] [CrossRef]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef] [Green Version]
- Unno, M.; Mizushima, T.; Morimoto, Y.; Tomisugi, Y.; Tanaka, K.; Yasuoka, N.; Tsukihara, T. The structure of the mammalian 20S proteasome at 2.75 E resolution. Structure 2002, 10, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Sharova, N.P. Immune Proteasomes and Immunity. Russ. J. Dev. Biol. 2006, 37, 139–145. [Google Scholar] [CrossRef]
- Groettrup, M.; Standera, S.; Stohwasser, R.; Kloetzel, P.-M. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc. Natl. Acad. Sci. USA 1997, 94, 8970–8975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; van Kaer, L.; Monaco, J.J.; Colbert, A. Immunoproteasome assembly: Cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaume, B.; Chapiro, J.; Stroobant, V.; Colau, D.; van Holle, B.; Parvizi, G.; Bousquet-Dubouch, M.P.; Théate, I.; Parmentier, N.; van den Eynde, B.J. Two abundant proteasome subtypes that uniquely process some antigens presented by HLA class I molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 18599–18604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlmann, B. Mammalian proteasome subtypes: Their diversity in structure and function. Arch. Biochem. Biophys. 2016, 591, 132–140. [Google Scholar] [CrossRef]
- Raynes, R.; Pomatto, L.C.; Davies, K.J. Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Asp. Med. 2016, 50, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, Y.J.; Tak, K.H.; Kim, C.W.; Roh, S.A.; Choi, E.K.; Cho, D.H.; Kim, J.H.; Kim, S.K.; Kim, S.Y.; Kim, Y.S.; et al. PSMB8 as a candidate marker of responsiveness to preoperative radiation therapy in rectal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, X.; Huang, L.; Chi, P. The expression and clinical significance of PA28 γ in colorectal cancer. J. Investig. Med. 2013, 61, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Boland, K.; Flanagan, L.; McCawley, N.; Pabari, R.; Kay, E.W.; McNamara, D.A.; Murray, F.; Byrne, A.T.; Ramtoola, Z.; Concannon, C.G.; et al. Targeting the 19S proteasomal subunit, Rpt4, for the treatment of colon cancer. Eur. J. Pharmacol. 2016, 780, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Astakhova, T.M.; Ivanova, E.V.; Rodoman, G.V.; Sumedi, I.R.; Afanas’ev, S.G.; Goncharov, A.L.; Kondakova, I.V.; Sharova, N.P. Effect of neoadjuvant chemoradiation therapy on proteasome pool in rectal cancer. Bull. Exp. Biol. Med. 2017, 164, 191–194. [Google Scholar] [CrossRef]
- Lindman, H.R. Analysis of Variance in Complex Experimental Designs; W.H. Freeman and Co.: New York, NY, USA, 1974; 352p. [Google Scholar]
- Wang, S.; Wang, T.; Wang, L.; Zhong, L.; Li, K. Overexpression of RNF126 promotes the development of colorectal cancer via enhancing p53 ubiquitination and degradation. Onco Targets Ther. 2020, 13, 10917–10929. [Google Scholar] [CrossRef] [PubMed]
- Astakhova, T.M.; Bozhok, G.A.; Alabedal’karim, N.M.; Karpova, Y.D.; Lyupina, Y.V.; Ushakova, E.M.; Legach, E.I.; Bondarenko, T.P.; Sharova, N.P. Proteasome expression in ovarian heterotopic allografts of Wistar and August rats under induction of donor specific tolerance. Russ. J. Dev. Biol. 2019, 50, 261–267. [Google Scholar] [CrossRef]
- Erokhov, P.A.; Lyupina, Y.V.; Radchenko, A.S.; Kolacheva, A.A.; Nikishina, Y.O.; Sharova, N.P. Detection of active proteasome structures in brain extracts: Proteasome features of August rat brain with violations in monoamine metabolism. Oncotarget 2017, 8, 70941–70957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astakhova, T.M.; Delone, G.V.; Lupina, Y.V.; Abramova, E.B.; Uryvaeva, I.V.; Sharova, N.P. Changes in the proteasome pool during malignant transformation of mouse liver cells. Acta Nat. 2010, 2, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Astakhova, T.M.; Moiseeva, E.V.; Sharova, N.P. Features of the proteasome pool in spontaneously occurring malignant tumors of the mammary gland in mice. Russ. J. Dev. Biol. 2020, 51, 317–322. [Google Scholar] [CrossRef]
- Raule, M.; Cerruti, F.; Benaroudj, N.; Migotti, R.; Kikuchi, J.; Bachi, A.; Navon, A.; Dittmar, G.; Cascio, P. PA28αβ reduces size and increases hydrophilicity of 20S immunoproteasome peptide products. Chem. Biol. 2014, 21, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hungria, V.T.M.; Crusoé, E.Q.; Bittencourt, R.I.; Maiolino, A.; Magalhães, R.J.P.; Sobrinho, J.D.N.; Pinto, J.V.; Fortes, R.C.; Moreira, E.D.S.; Tanaka, P.Y. New proteasome inhibitors in the treatment of multiple myeloma. Hematol. Transfus. Cell Ther. 2019, 41, 76–83. [Google Scholar] [CrossRef]
- Sassin, W. Deja Vue? Beac. J. Stud. Ideol. Ment. Dimens. 2019, 2, 020210216. Available online: http://hdl.handle.net/20.500.12656/thebeacon.2.020210216 (accessed on 14 July 2020).
- Ito, S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [Green Version]
- Lysenko, L.A. Back to anthropology: What does it mean to development studies? Beac. J. Stud. Ideol. Ment. Dimens. 2019, 2, 020000000. Available online: https://hdl.handle.net/20.500.12656/thebeacon.2.020000000 (accessed on 11 June 2020).
- Donskikh, O.A. Horror Zivilisationis, oder Horror der Subjektivität. Beac. J. Stud. Ideol. Ment. Dimens. 2019, 2, 020110205. Available online: http://hdl.handle.net/20.500.12656/thebeacon.2.020110205 (accessed on 1 August 2020).
- Jang, H.H. Regulation of protein degradation by proteasomes in cancer. J. Cancer Prev. 2018, 23, 153–161. [Google Scholar] [CrossRef]
- Sassin, W. Die Grenzen der Ökonomie: Globalisierung—Vom Füllhorn zum Giftbecher? Eur. Crossrd. 2020, 1, 010410216. Available online: http://hdl.handle.net/20.500.12656/eurcrossrd.1.010410216 (accessed on 18 July 2020).
- Sassin, W. Er-Schöpfung der Schöpfung, oder Eine neue Kulturstufe in der Entwicklung des homo. Beac. J. Stud. Ideol. Ment. Dimens. 2019, 2, 020510203. Available online: https://hdl.handle.net/20.500.12656/thebeacon.2.020510203 (accessed on 24 June 2020).
- Trader, D.J.; Simanski, S.; Kodadek, T. A reversible and highly selective inhibitor of the proteasomal ubiquitin receptor rpn13 is toxic to multiple myeloma cells. J. Am. Chem. Soc. 2015, 137, 6312–6319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yakushi, T.; Parlati, F.; Mackinnon, A.L.; Perez, C.; Ma, Y.; Carter, K.P.; Colayco, S.; Magnuson, G.; Brown, B.; et al. Capzimin is a potent and specific inhibitor of proteasome isopeptidase Rpn11. Nat. Chem. Biol. 2017, 13, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astakhova, T.M.; Morozov, A.V.; Erokhov, P.A.; Mikhailovskaya, M.I.; Akopov, S.B.; Chupikova, N.I.; Safarov, R.R.; Sharova, N.P. Combined effect of bortezomib and menadione sodium bisulfite on proteasomes of tumor cells: The dramatic decrease of bortezomib toxicity in a preclinical trial. Cancers 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Tanahashi, N. Preparation of proteasomes. In Cell Biology: A Laboratory Handbook, 2nd ed.; Celis, J.E., Ed.; Academic Press: New York, NY, USA, 1997; pp. 129–134. [Google Scholar]
- Abramova, E.B.; Astakhova, T.M.; Erokhov, P.A.; Sharova, N.P. Multiple forms of proteasomes and approaches to their separation. Biol. Bull. 2004, 31, 115–120. [Google Scholar] [CrossRef]

| Factors | Designation |
|---|---|
| Activities in tumor | (1) |
| Activities in adjacent tissue | (2) |
| Activities in intestine distal side, 4 cm from tumor | (3) |
| Activities in intestine distal side, 2 cm from tumor | (4) |
| Activities in intestine proximal side, 8 cm from tumor | (5) |
| Activities in intestine proximal side, 15 cm from tumor | (6) |
| Activities in intestine proximal side, 20 cm from tumor | (7) |
| Activity | Statistical Indicators | ||
|---|---|---|---|
| df | Fst | p | |
| ChTL | 6 | 276.9 | <0.0001 |
| CL | 6 | 414.2 | <0.0001 |
| LMP7 | 6 | 77.1 | <0.0001 |
| LMP2 | 6 | 58.9 | <0.0001 |
| PL | Homogenous Groups of ChTL Activity | PL | Homogenous Groups of CL Activity | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fisher LSD Test | Bonferroni Test | Fisher LSD Test | Bonferroni Test | ||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | ||
| (5) | **** | **** | (4) | **** | **** | ||||||||||||
| (3) | **** | **** | **** | (3) | **** | **** | **** | **** | |||||||||
| (7) | **** | **** | **** | (6) | **** | **** | **** | **** | **** | ||||||||
| (4) | **** | **** | (5) | **** | **** | **** | **** | ||||||||||
| (6) | **** | **** | (7) | **** | **** | ||||||||||||
| (2) | **** | **** | (2) | **** | **** | ||||||||||||
| (1) | **** | **** | (1) | **** | **** | ||||||||||||
| PL | Homogenous Groups of LMP7 Activity | PL | Homogenous Groups of LMP2 Activity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fisher LSD Test | Bonferroni Test | Fisher LSD Test | Bonferroni Test | ||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | ||
| (4) | **** | **** | (3) | **** | **** | ||||||||||
| (6) | **** | **** | (7) | **** | **** | **** | |||||||||
| (3) | **** | **** | **** | (4) | **** | **** | **** | ||||||||
| (7) | **** | **** | **** | (5) | **** | **** | **** | ||||||||
| (5) | **** | **** | (6) | **** | **** | ||||||||||
| (2) | **** | **** | (2) | **** | **** | ||||||||||
| (1) | **** | **** | (1) | **** | **** | ||||||||||
| PL | C and p Indicators for Different Proteasome Locations | ||||||
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
| (1) | 1.0000 | 0.6611 | 0.5466 | 0.6188 | 0.3226 | 0.5058 | 0.4109 |
| (2) | p = 0.001 | 1.0000 | 0.6368 | 0.5780 | 0.4690 | 0.4859 | 0.4611 |
| (3) | p = 0.010 | p = 0.002 | 1.0000 | 0.8615 | 0.5296 | 0.7880 | 0.6174 |
| (4) | p = 0.003 | p = 0.006 | p < 0.001 | 1.0000 | 0.4418 | 0.7709 | 0.6595 |
| (5) | p = 0.154 | p = 0.032 | p = 0.014 | p = 0.045 | 1.0000 | 0.4611 | 0.4058 |
| (6) | p = 0.019 | p = 0.026 | p < 0.001 | p < 0.001 | p = 0.035 | 1.0000 | 0.8048 |
| (7) | p = 0.064 | p = 0.035 | p = 0.003 | p = 0.001 | p = 0.068 | p < 0.001 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erokhov, P.A.; Kulikov, A.M.; Karpova, Y.D.; Rodoman, G.V.; Sumedi, I.R.; Goncharov, A.L.; Razbirin, D.V.; Gorelova, V.S.; Sharova, N.P.; Astakhova, T.M. Proteasomes in Patient Rectal Cancer and Different Intestine Locations: Where Does Proteasome Pool Change? Cancers 2021, 13, 1108. https://doi.org/10.3390/cancers13051108
Erokhov PA, Kulikov AM, Karpova YD, Rodoman GV, Sumedi IR, Goncharov AL, Razbirin DV, Gorelova VS, Sharova NP, Astakhova TM. Proteasomes in Patient Rectal Cancer and Different Intestine Locations: Where Does Proteasome Pool Change? Cancers. 2021; 13(5):1108. https://doi.org/10.3390/cancers13051108
Chicago/Turabian StyleErokhov, Pavel A., Alexey M. Kulikov, Yaroslava D. Karpova, Grigory V. Rodoman, Ilia R. Sumedi, Artem L. Goncharov, Dmitry V. Razbirin, Vera S. Gorelova, Natalia P. Sharova, and Tatiana M. Astakhova. 2021. "Proteasomes in Patient Rectal Cancer and Different Intestine Locations: Where Does Proteasome Pool Change?" Cancers 13, no. 5: 1108. https://doi.org/10.3390/cancers13051108






