A Principal Component of Quality of Life Measures Is Associated with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.2. Clinical Data
2.3. Quality of Life Questionnaires
2.4. Statistical Analysis
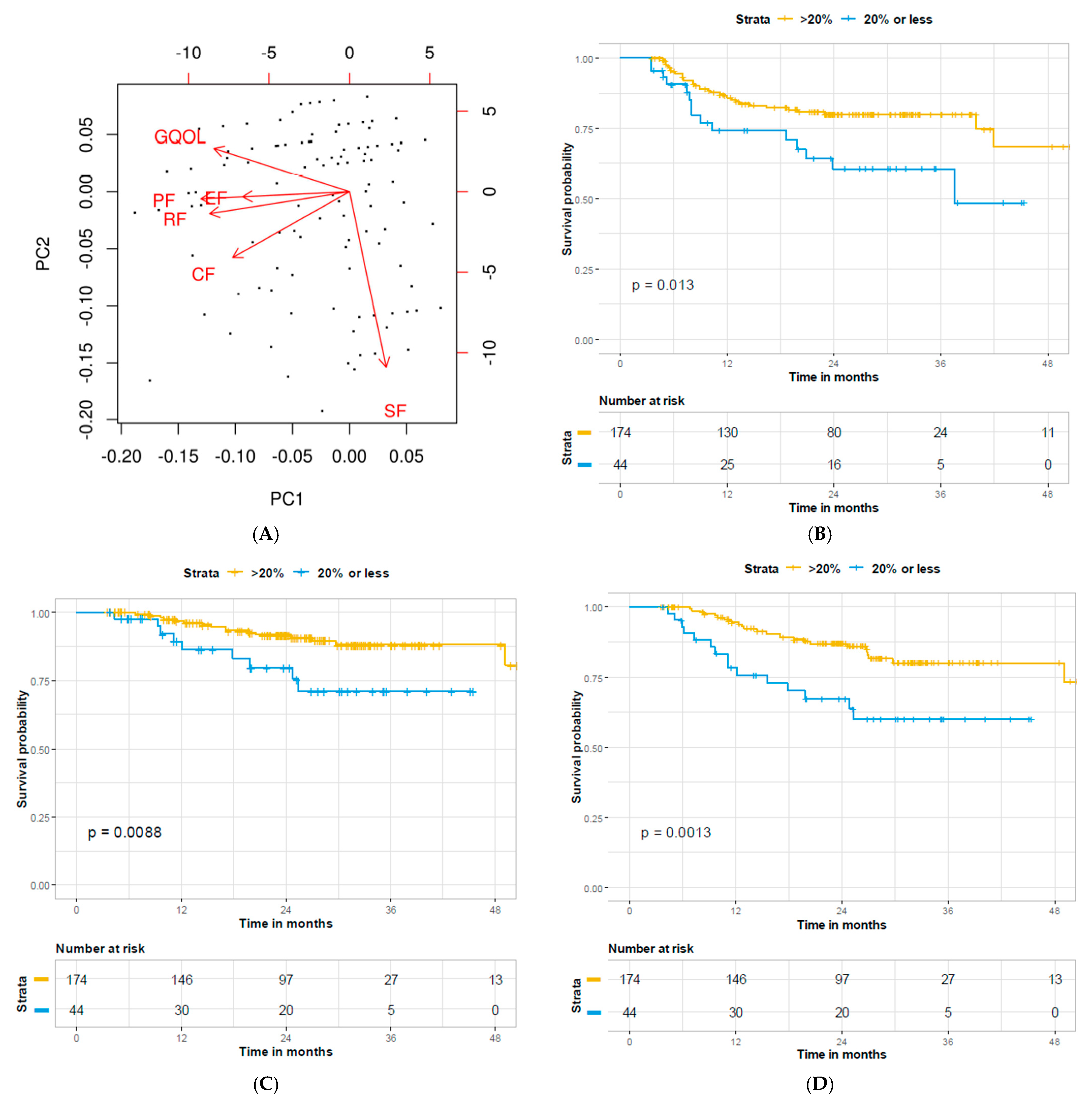
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- U.S. Cancer Statistics Data Visualizations Tool, Based on November 2017 Submission Data (1999–2015). 2018. Available online: www.cdc.gov/cancer/dataviz (accessed on 8 February 2021).
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.H.; Glisson, B.S.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Hansen, C.C.; Egleston, B.; Leachman, B.K.; Churilla, T.M.; De Mora, L.; Ebersole, B.; Bauman, J.R.; Liu, J.C.; Ridge, J.A.; Galloway, T.J. Patterns of Multidisciplinary Care of Head and Neck Squamous Cell Carcinoma in Medicare Patients. JAMA Otolaryngol. Neck Surg. 2020, 146, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ringash, J. Quality of Life in Head and Neck Cancer: Where We Are, and Where We Are Going. Int. J. Radiat. Oncol. 2017, 97, 662–666. [Google Scholar] [CrossRef][Green Version]
- Donaldson, M.S. Taking Stock of Health-Related Quality-of-Life Measurement in Oncology Practice in the United States. J. Natl. Cancer Inst. Monogr. 2004, 2004, 155–167. [Google Scholar] [CrossRef]
- Braun, D.P.; Gupta, D.; Grutsch, J.F.; Staren, E.D. Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health Qual. Life Outcomes 2011, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Innominato, P.F.; Bjarnason, G.; Coens, C.; Humblet, Y.; Tumolo, S.; Genet, D.; Tampellini, M.; Bottomley, A.; Garufi, C.; et al. Validation of patient’s self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: Results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. 2008, 26, 2020–2026. [Google Scholar] [PubMed]
- Gotay, C.C.; Kawamoto, C.T.; Bottomley, A.; Efficace, F. The Prognostic Significance of Patient-Reported Outcomes in Cancer Clinical Trials. J. Clin. Oncol. 2008, 26, 1355–1363. [Google Scholar] [CrossRef]
- Maisey, N.; Norman, A.; Watson, M.; Allen, M.; Hill, M.; Cunningham, D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur. J. Cancer 2002, 38, 1351–1357. [Google Scholar] [CrossRef]
- Mol, L.; Ottevanger, P.; Koopman, M.; Punt, C. The prognostic value of WHO performance status in relation to quality of life in advanced colorectal cancer patients. Eur. J. Cancer 2016, 66, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Oskam, I.M.; Leeuw, I.M.V.-D.; Aaronson, N.K.; Kuik, D.J.; De Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, R.C. Quality of life as predictor of survival: A prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother. Oncol. 2010, 97, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Østhus, A.A.; Aarstad, A.K.H.; Olofsson, J.; Aarstad, H.J. Prediction of Survival by Pretreatment Health-Related Quality-of-Life Scores in a Prospective Cohort of Patients with Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Neck Surg. 2013, 139, 14–20. [Google Scholar] [CrossRef]
- Quinten, C.; Martinelli, F.; Coens, C.; Sprangers, M.A.G.; Ringash, J.; Gotay, C.; Bjordal, K.; Greimel, E.; Reeve, B.B.; Maringwa, J.; et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2014, 120, 302–311. [Google Scholar] [CrossRef]
- Vickers, M.; Lee, C.; Tu, D.; Wheatley-Price, P.; Parulekar, W.; Brundage, M.; Moore, M.; Au, H.; O’Callaghan, C.; Jonker, D.; et al. Significance of baseline and change in quality of life scores in predicting clinical outcomes in an international phase III trial of advanced pancreatic cancer: NCIC CTG PA.3. Pancreatology 2016, 16, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, R.C.; Pugh, S.L.; Trotti, A.; Hu, K.; Spencer, S.; Yom, S.S.; Rosenthal, D.; Read, N.; Desai, A.; Gore, E.; et al. Changing functional status within 6 months posttreatment is prognostic of overall survival in patients with head and neck cancer: NRG Oncology Study. Head Neck 2019, 41, 3924–3932. [Google Scholar] [CrossRef]
- Jameson, M.J.; Karnell, L.H.; Christensen, A.J.; Funk, G.F. First-Year Trends in Self-reported General Health Predict Survival in Patients with Head and Neck Cancer. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 958. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, F.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B.; Bairati, I. Health-Related Quality of Life as a Survival Predictor for Patients with Localized Head and Neck Cancer Treated with Radiation Therapy. J. Clin. Oncol. 2009, 27, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Aarstad, H.J.; Østhus, A.A.; Aarstad, H.H.; Lybak, S.; Aarstad, A.K.H. General health-related quality of life scores from head and neck squamous cell carcinoma patients obtained throughout the first year following diagnosis predicted up to 10-year overall survival. Eur. Arch. Otorhinolaryngol. 2017, 275, 207–217. [Google Scholar] [CrossRef]
- Karvonen-Gutierrez, C.A.; Ronis, D.L.; Fowler, K.E.; Terrell, J.E.; Gruber, S.B.; Duffy, S.A. Quality of Life Scores Predict Survival Among Patients with Head and Neck Cancer. J. Clin. Oncol. 2008, 26, 2754–2760. [Google Scholar] [CrossRef]
- Mehanna, H.M.; Morton, R.P. Does Quality of Life Predict Long-term Survival in Patients With Head and Neck Cancer? Arch. Otolaryngol. Head Neck Surg. 2006, 132, 27–31. [Google Scholar] [CrossRef]
- Rogers, S.N.; Waylen, A.E.; Thomas, S.; Penfold, C.; Pring, M.; Waterboer, T.; Pawlita, M.; Hurley, K.; Ness, A.R. Quality of life, cognitive, physical and emotional function at diagnosis predicts head and neck cancer survival: Analysis of cases from the Head and Neck 5000 study. Eur. Arch. Otorhinolaryngol. 2020, 277, 1515–1523. [Google Scholar] [CrossRef]
- Van Nieuwenhuizen, A.J.; Buffart, L.M.; Brug, J.; Leemans, C.R.; Verdonck-de Leeuw, I.M. The association between health related quality of life and survival in patients with head and neck cancer: A systematic review. Oral Oncol. 2015, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-L.; Chen, C.-H.; Sheu, C.-F.; Lang, H.-C.; Hsieh, C.-L. Validating and Improving the Reliability of the EORTC QLQ-C30 Using a Multidimensional Rasch Model. Value Health 2013, 16, 848–854. [Google Scholar] [CrossRef]
- Van Steen, K.; Curran, D.; Kramer, J.; Molenberghs, G.; Van Vreckem, A.; Bottomley, A.; Sylvester, R. Multicollinearity in prognostic factor analyses using the EORTC QLQ-C30: Identification and impact on model selection. Stat. Med. 2002, 21, 3865–3884. [Google Scholar] [CrossRef] [PubMed]
- Han, H.R.; Hermann, G.M.; Ma, S.J.; Iovoli, A.J.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; Markiewicz, M.R.; et al. Matched pair analysis to evaluate the impact of hospitalization during radiation therapy as an early marker of survival in head and neck cancer patients. Oral Oncol. 2020, 109, 104854. [Google Scholar] [CrossRef] [PubMed]
- Platek, A.J.; Jayaprakash, V.; Merzianu, M.; Platek, M.E.; Cohan, D.M.; Hicks, W.L., Jr.; Marimuthu, S.P.; Winslow, T.B.; Gupta, V.; Arshad, H.; et al. Smoking cessation is associated with improved survival in oropharynx cancer treated by chemoradiation. Laryngoscope 2016, 126, 2733–2738. [Google Scholar] [CrossRef]
- Fung-Kee-Fung, S.D.; Hackett, R.; Hales, L.; Warren, G.; Singh, A.K. A prospective trial of volumetric intensity-modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World J. Clin. Oncol. 2012, 3, 57–62. [Google Scholar] [CrossRef]
- Platek, M.E.; McCloskey, S.A.; Cruz, M.; Burke, M.S.; Reid, M.E.; Wilding, G.E.; Rigual, N.R.; Popat, S.R.; Loree, T.R.; Gupta, V.; et al. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck 2013, 35, 684–688. [Google Scholar] [CrossRef]
- Singer, S.; Wollbrück, D.; Wulke, C.; Dietz, A.; Klemm, E.; Oeken, J.; Meister, E.F.; Gudziol, H.; Bindewald, J.; Schwarz, R. Validation of the EORTC QLQ-C30 and EORTC QLQ-H&N35 in patients with laryngeal cancer after surgery. Head Neck 2009, 31, 64–76. [Google Scholar] [CrossRef]
- Ringner, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Atkinson, T.M.; Andreotti, C.F.; Roberts, K.E.; Saracino, R.M.; Hernandez, M.; Basch, E. The level of association between functional performance status measures and patient-reported outcomes in cancer patients: A systematic review. Support. Care Cancer 2015, 23, 3645–3652. [Google Scholar] [CrossRef] [PubMed]
- Iovoli, A.J.; Hermann, G.M.; Ma, S.J.; Platek, A.J.; Farrugia, M.K.; Yau, E.; Wooten, K.E.; Arshad, H.; Gupta, V.; Kuriakose, M.A.; et al. Association of Nonsteroidal Anti-inflammatory Drug Use with Survival in Patients With Squamous Cell Carcinoma of the Head and Neck Treated With Chemoradiation Therapy. JAMA Netw. Open 2020, 3, e207199. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 218) | ||||
|---|---|---|---|---|
| Median | n | % | ||
| Age | 61.40 | |||
| Gender | Male | 173 | 79.4% | |
| Female | 45 | 20.6% | ||
| KPS | 60 | 2 | 0.9% | |
| 70 | 11 | 5.0% | ||
| 80 | 41 | 18.8% | ||
| 90 | 98 | 45.0% | ||
| 100 | 66 | 30.3% | ||
| Smoking status | Never | 61 | 28.0% | |
| Former | 143 | 65.6% | ||
| Current | 14 | 6.4% | ||
| Primary | Oral Cavity | 16 | 7.3% | |
| Pharynx | 126 | 57.8% | ||
| Larynx | 48 | 22.0% | ||
| Salivary Glands | 5 | 2.3% | ||
| Unknown 10 | 19 | 8.7% | ||
| Other | 4 | 1.8% | ||
| T stage | T0 | 18 | 8.3% | |
| T1 | 34 | 15.7% | ||
| T2 | 63 | 29.0% | ||
| T3 | 65 | 30.0% | ||
| T4 | 37 | 17.1% | ||
| N stage | N0 | 42 | 19.4% | |
| N1 | 27 | 12.5% | ||
| N2 | 126 | 58.3% | ||
| N3 | 21 | 9.7% | ||
| M stage | M0 | 216 | 99.1% | |
| M1 | 2 | 0.9% | ||
| Histologic grade (differentiation) | Well | 12 | 5.5% | |
| Moderate | 64 | 29.4% | ||
| Poorly | 66 | 30.3% | ||
| Unknown | 76 | 34.9% | ||
| HPV status | Negative | 42 | 19.4% | |
| Positive | 108 | 49.8% | ||
| Unknown | 68 | 31.2% | ||
| Induction chemotherapy | No | 206 | 94.5% | |
| Yes | 12 | 5.5% | ||
| Concurrent chemotherapy | No | 22 | 10.1% | |
| Yes | 196 | 89.9% | ||
| Prior surgery | No | 167 | 76.6% | |
| Yes | 51 | 23.4% | ||
| Nutritional support | No | 111 | 50.9% | |
| Yes | 107 | 49.1% | ||
| Hospitalized | No | 165 | 75.6% | |
| Yes | 53 | 24.3% | ||
| Relapse | No | 170 | 78.0% | |
| Yes | 48 | 22.0% | ||
| Vital status | Alive | 176 | 80.7% | |
| Dead | 42 | 19.3% | ||
| Follow up (months) | 24.78 | |||
| Pre-Treatment (Median, IQR) | Post-Treatment (Median, IQR) | p-Value | Recovery (Complete, More Than 80%, <80%) | Recovery Mean (Std. Dev.) | |
|---|---|---|---|---|---|
| Functional Domains | |||||
| Physical | 93.3 (80–100) | 86.7 (73.3–100) | 0.0001 | 114 (52.3%), 66 (30.3%), 38 (17.4%) | 0.651 (0.760) |
| Role | 100 (66.7–100) | 83.3 (66.7–100) | 0.001 | 134 (61.5%), 24 (11.1%), 60 (27.5%) | 0.660 (0.882) |
| Social | 100 (66.7–100) | 83.3 (66.7–100) | 0.34 | 152 (69.7%), 21 (9.6%), 45 (20.6%) | 0.509 (0.816) |
| Cognitive | 100 (83.3–100) | 100 (83.3–100) | 0.99 | 169 (77.5%), 31 (14.2%), 18 (8.3%) | 0.307 (0.616) |
| Emotional | 83.3 (66.7–91.7) | 83.3 (66.7–91.7) | 0.34 | 149 (68.3%), 34 (15.6%), 35 (16.1%) | 0.477 (0.757) |
| Global Health Status | 75 (58.3–83.3) | 70.8 (58.3–83.3) | 0.1 | 121 (55.5%), 53 (24.3%), 43 (19.7%) | 0.647 (0.797) |
| Univariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RFS | DSS | OS | |||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1 | 0.97–1.03 | 0.91 | 1.01 | 0.97–1.1 | 0.616 | 1.024 | 0.99–1.0 | 0.157 |
| Gender | 1.08 | 0.54–2.2 | 0.836 | 0.57 | 0.17–1.9 | 0.368 | 1 | 0.46–2.2 | 0.992 |
| KPS | 0.84 | 0.61–1.2 | 0.288 | 0.78 | 0.51–1.2 | 0.240 | 0.66 | 0.49–0.89 | 0.006 |
| Primary | |||||||||
| Oral cavity | ref | ref | ref | ||||||
| Pharynx | 0.43 | 0.18–1.0 | 0.061 | 0.24 | 0.08–0.75 | 0.014 | 0.289 | 0.08–0.72 | 0.008 |
| Larynx | 0.58 | 0.22–1.5 | 0.274 | 0.69 | 0.22–2.2 | 0.534 | 0.645 | 0.25–1.7 | 0.371 |
| Salivary glands | 0 | 0-inf | 0.966 | 0 | 0-inf | 0.978 | 0.186 | 0.04–0.93 | 0.040 |
| Unknown 10 | 0.47 | 0.06–3.9 | 0.484 | 0 | 0-inf | 0.990 | 0 | 0-inf | 0.978 |
| Other | 0 | 0-inf | 0.989 | 0 | 0-inf | 0.994 | 0 | 0-inf | 0.986 |
| T stage | |||||||||
| T0 | 0 | 0-inf | 0.967 | 0 | 0-inf | 0.977 | 0.19 | 0.04–0.82 | 0.026 |
| T1 | 0.43 | 0.13–1.4 | 0.156 | 0.1 | 0.01–0.82 | 0.032 | 0.17 | 0.05–0.58 | 0.005 |
| T2 | 0.88 | 0.39–2.0 | 0.763 | 0.34 | 0.12–0.98 | 0.046 | 0.25 | 0.11–0.59 | 0.001 |
| T3 | 1.13 | 0.51–2.5 | 0.763 | 0.51 | 0.20–1.3 | 0.161 | 0.4 | 0.19–0.83 | 0.014 |
| T4 | ref | ref | ref | ||||||
| N stage | |||||||||
| N0 | ref | ref | |||||||
| N1 | 0.5 | 0.26–2.8 | 0.792 | 1.2 | 0.35–4.4 | 0.750 | 1.2 | 0.42–3.3 | 0.764 |
| N2 | 1.17 | 0.53–2.6 | 0.705 | 0.72 | 0.27–1.9 | 0.495 | 0.78 | 0.36–1.7 | 0.514 |
| N3 | 2.55 | 0.95–6.8 | 0.062 | 0.37 | 0.04–3.1 | 0.359 | 0.96 | 0.29–3.1 | 0.940 |
| M stage | 0.05 | 0-inf | 0.674 | 0.05 | 0-inf | 0.948 | 0.05 | 0-inf | 0.926 |
| Grade | |||||||||
| Well | 0 | 0-inf | 0.966 | 0 | 0-inf | 0.177 | 0.78 | 0.10–6.1 | 0.814 |
| Moderate | 1.36 | 0.69–2.7 | 0.379 | 1.9 | 0.73–5.0 | 0.190 | 1.72 | 0.78–3.8 | 0.180 |
| Poorly | 1.3 | 0.55–2.2 | 0.775 | 1.3 | 0.50–3.7 | 0.583 | 1.72 | 0.80–3.7 | 0.160 |
| Unknown | ref | ref | ref | ||||||
| HPV status | |||||||||
| Negative | 1.1 | 0.55–2.4 | 0.724 | 0.8 | 0.29–2.2 | 0.651 | 0.97 | 0.47–2.0 | 0.930 |
| Positive | 0.6 | 0.31–1.2 | 0.124 | 0.37 | 0.15–0.92 | 0.032 | 0.34 | 0.16–0.70 | 0.004 |
| Unknown | ref | ref | ref | ||||||
| Induction chemo | 0.88 | 0.21–3.6 | 0.854 | 0.87 | 0.12–6.5 | 0.893 | 2.2 | 0.77–6.1 | 0.140 |
| Concurrent chemo | 1.2 | 0.37–3.9 | 0.758 | 1.7 | 0.22–12.4 | 0.617 | 0.68 | 0.24–1.9 | 0.470 |
| Smoking | |||||||||
| Never | 0.32 | 0.12–0.88 | 0.28 | 0.15 | 0.03–0.66 | 0.013 | 0.26 | 0.09–0.76 | 0.014 |
| Former | 0.48 | 0.20–1.1 | 0.097 | 0.42 | 0.14–1.3 | 0.125 | 0.43 | 0.18–1.1 | 0.064 |
| Current | ref | ref | |||||||
| Anticoagulant use | 0.45 | 0.11–1.9 | 0.212 | 0.04 | 0.2–21.5 | 0.321 | 1.44 | 0.56–3.7 | 0.447 |
| NSAID use | 1.08 | 0.61–1.9 | 0.79 | 1 | 0.47–2.3 | 0.945 | 0.96 | 0.52–1.8 | 0.899 |
| Prior surgery | 1.25 | 0.64–2.5 | 0.517 | 1.4 | 0.57–3.6 | 0.450 | 1.2 | 0.57–2.5 | 0.620 |
| Nutritional support | 1.7 | 0.93–2.9 | 0.09 | 2.6 | 1.1–6.0 | 0.028 | 2.6 | 1.4–5.1 | 0.004 |
| Hospitalized | 1.2 | 0.66–2.3 | 0.508 | 1.4 | 0.60–3.2 | 0.452 | 1.6 | 0.83–3.2 | 0.141 |
| Financial toxicity | 1.2 | 0.56–2.6 | 0.632 | 1.6 | 0.59–4.2 | 0.365 | 1.5 | 0.69–3.2 | 0.308 |
| Recovery | |||||||||
| PF | 0.61 | 0.76–1.6 | 0.608 | 1.3 | 0.78–2.2 | 0.310 | 1.3 | 0.91–2.0 | 0.142 |
| RF | 1.3 | 0.96–1.8 | 0.095 | 1.4 | 0.91–2.1 | 0.128 | 1.6 | 1.1–2.2 | 0.009 |
| SF | 0.73 | 0.48–1.1 | 0.135 | 0.61 | 0.31–1.2 | 0.147 | 1 | 0.69–1.5 | 0.956 |
| CF | 1.4 | 0.94–2.1 | 0.097 | 1.4 | 0.8–2.6 | 0.225 | 1.4 | 0.92–2.2 | 0.115 |
| EF | 1.3 | 0.89–1.8 | 0.191 | 1.6 | 0.98–2.5 | 0.063 | 1.8 | 1.3–2.6 | 0.001 |
| GQOL | 1.3 | 0.92–1.8 | 0.149 | 1.8 | 1.1–2.9 | 0.015 | 1.7 | 1.2–2.4 | 0.006 |
| PC1 | 0.83 | 0.70–0.99 | 0.039 | 0.74 | 0.59–0.94 | 0.013 | 0.74 | 0.62–0.89 | 0.001 |
| Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RFS | DSS | OS | |||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| KPS | 0.76 | 0.53–1.1 | 0.148 | ||||||
| Primary | |||||||||
| Oral cavity | ref | ref | ref | ||||||
| Pharynx | 0.48 | 0.17–1.3 | 0.153 | 0.24 | 0.08–0.75 | 0.014 | 0.58 | 0.19–1.7 | 0.325 |
| Larynx | 0.76 | 0.26–2.3 | 0.624 | 0.69 | 0.22–2.2 | 0.534 | 0.61 | 0.20–1.9 | 0.381 |
| Salivary glands | 0 | 0-inf | 0.969 | 0 | 0-inf | 0.978 | 0.34 | 0.06–2.1 | 0.239 |
| Unknown 10 | 0.69 | 0.08–6.3 | 0.74 | 0 | 0-inf | 0.990 | 0 | 0-inf | 0.979 |
| Other | 0 | 0-inf | 0.993 | 0 | 0-inf | 0.994 | 0 | 0-inf | 0.989 |
| T stage | |||||||||
| T0 | * | * | * | * | * | * | |||
| T1 | 0.184 | 0.02–1.6 | 0.125 | 0.27 | 0.07–0.99 | 0.049 | |||
| T2 | 0.54 | 0.17–1.7 | 0.297 | 0.43 | 0.17–1.1 | 0.080 | |||
| T3 | 0.681 | 0.25–1.9 | 0.452 | 0.61 | 0.28–1.4 | 0.228 | |||
| T4 | ref | ref | |||||||
| N stage | |||||||||
| N0 | ref | ||||||||
| N1 | 1.7 | 0.48–6.1 | 0.407 | ||||||
| N2 | 1.8 | 0.75–4.4 | 0.187 | ||||||
| N3 | 4.1 | 1.4–11.8 | 0.010 | ||||||
| HPV status | |||||||||
| Negative | 0.89 | 0.30–2.6 | 0.825 | 1 | 0.47–2.3 | 0.348 | |||
| Positive | 0.85 | 0.24–3.0 | 0.798 | 0.58 | 0.19–1.5 | 0.224 | |||
| Unknown | ref | ref | |||||||
| Smoking | |||||||||
| Never | 0.42 | 0.14–1.2 | 0.112 | 0.34 | 0.67–1.8 | 0.199 | 0.57 | 0.18–1.8 | 0.348 |
| Former | 0.56 | 0.21–1.5 | 0.24 | 0.6 | 0.16–2.2 | 0.434 | 0.53 | 0.19–1.5 | 0.224 |
| Current | ref | ref | ref | ||||||
| Nutritional support | 1.1 | 0.62–2.1 | 0.67 | 1.5 | 0.60–3.6 | 0.396 | 1.7 | 0.83–3.5 | 0.144 |
| PC1 | 0.84 | 0.69–1.0 | 0.068 | 0.77 | 0.61–0.98 | 0.036 | 0.76 | 0.63–0.91 | 0.004 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| Age | −0.017 | 0.011 | 0.117 | |||
| Gender | −0.106 | 0.259 | 0.684 | |||
| KPS | 0.068 | 0.119 | 0.569 | |||
| Primary | ||||||
| Oral cavity | ref | ref | ||||
| Pharynx | 0.361 | 0.41 | 0.378 | 0.335 | 0.401 | 0.404 |
| Larynx | 0.176 | 0.446 | 0.693 | 0.134 | 0.432 | 0.755 |
| Salivary glands | 1.012 | 0.524 | 0.545 | 0.819 | 1.521 | 0.591 |
| Unknown 10 | −0.217 | 0.791 | 0.784 | −0.564 | 0.764 | 0.461 |
| Other | 0.445 | 0.863 | 0.607 | −1.34 | 1.14 | 0.241 |
| T stage | ||||||
| T0 | ref | ref | ||||
| T1 | −0.722 | 0.439 | 0.1 | 0.194 | 1.54 | 0.899 |
| T2 | −0.918 | 0.401 | 0.023 | 0.079 | 1.51 | 0.958 |
| T3 | −0.557 | 0.399 | 0.165 | 0.648 | 1.53 | 0.672 |
| T4 | −1.063 | 0.433 | 0.015 | 0.303 | 1.54 | 0.844 |
| N stage | ||||||
| N0 | ref | ref | ||||
| N1 | 0.406 | 0.377 | 0.283 | 0.504 | 0.39 | 0.198 |
| N2 | 0.103 | 0.27 | 0.704 | 0.378 | 0.302 | 0.212 |
| N3 | 0.718 | 0.409 | 0.081 | 1.07 | 0.429 | 0.013 |
| M stage | −1.344 | 1.097 | 0.222 | |||
| HPV status | ||||||
| Negative | ref | |||||
| Positive | 0.223 | 0.282 | 0.43 | |||
| Unknown | 0.225 | 0.304 | 0.46 | |||
| Induction chemo | −0.352 | 0.46 | 0.444 | |||
| Concurrent chemo | −0.34 | 0.348 | 0.329 | |||
| Smoking | ||||||
| Never | ref | |||||
| Former | −0.268 | 0.235 | 0.257 | |||
| Current | 0.219 | 0.457 | 0.632 | |||
| Anticoagulant use | 0.228 | 0.363 | 0.531 | |||
| NSAID use | −0.372 | 0.208 | 0.076 | −0.354 | 0.2 | 0.083 |
| Prior surgery | 0.073 | 0.086 | 0.392 | |||
| Nutritional support | −0.578 | 0.206 | 0.006 | −0.38 | 0.218 | 0.079 |
| Hospitalized | −0.997 | 0.235 | <0.001 | −0.997 | 0.244 | <0.001 |
| Financial toxicity | −0.008 | 0.305 | 0.718 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrugia, M.; Yu, H.; Ma, S.J.; Iovoli, A.J.; Attwood, K.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; et al. A Principal Component of Quality of Life Measures Is Associated with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy. Cancers 2021, 13, 1155. https://doi.org/10.3390/cancers13051155
Farrugia M, Yu H, Ma SJ, Iovoli AJ, Attwood K, Wooten KE, Arshad H, Gupta V, McSpadden RP, Kuriakose MA, et al. A Principal Component of Quality of Life Measures Is Associated with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy. Cancers. 2021; 13(5):1155. https://doi.org/10.3390/cancers13051155
Chicago/Turabian StyleFarrugia, Mark, Han Yu, Sung Jun Ma, Austin J. Iovoli, Kristopher Attwood, Kimberly E. Wooten, Hassan Arshad, Vishal Gupta, Ryan P. McSpadden, Moni A. Kuriakose, and et al. 2021. "A Principal Component of Quality of Life Measures Is Associated with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy" Cancers 13, no. 5: 1155. https://doi.org/10.3390/cancers13051155
APA StyleFarrugia, M., Yu, H., Ma, S. J., Iovoli, A. J., Attwood, K., Wooten, K. E., Arshad, H., Gupta, V., McSpadden, R. P., Kuriakose, M. A., Markiewicz, M. R., Chan, J. M., Hicks, W. L., Jr., Platek, M. E., Ray, A. D., Repasky, E. A., & Singh, A. K. (2021). A Principal Component of Quality of Life Measures Is Associated with Survival for Head and Neck Cancer Patients Treated with Radiation Therapy. Cancers, 13(5), 1155. https://doi.org/10.3390/cancers13051155






