Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Quality Control Standards for MSCs
2.2. Animals
2.3. Intranasal Cell Administration and Biodistribution
2.4. Welfare Assessing
2.5. Behavioral Tests
2.6. Blood Sample Collection and Biochemical Determinations
2.7. Cytokines Bio-Plex Immunoassay
2.8. Magnetic Resonance Imaging and 1H Magnetic Resonance Spectroscopy
2.9. Tissue Collection
2.10. RNA Extraction and Quantitative Reverse Transcription PCR
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
3.1. MSC Culture Expansion in Compliance with Quality Control Standards
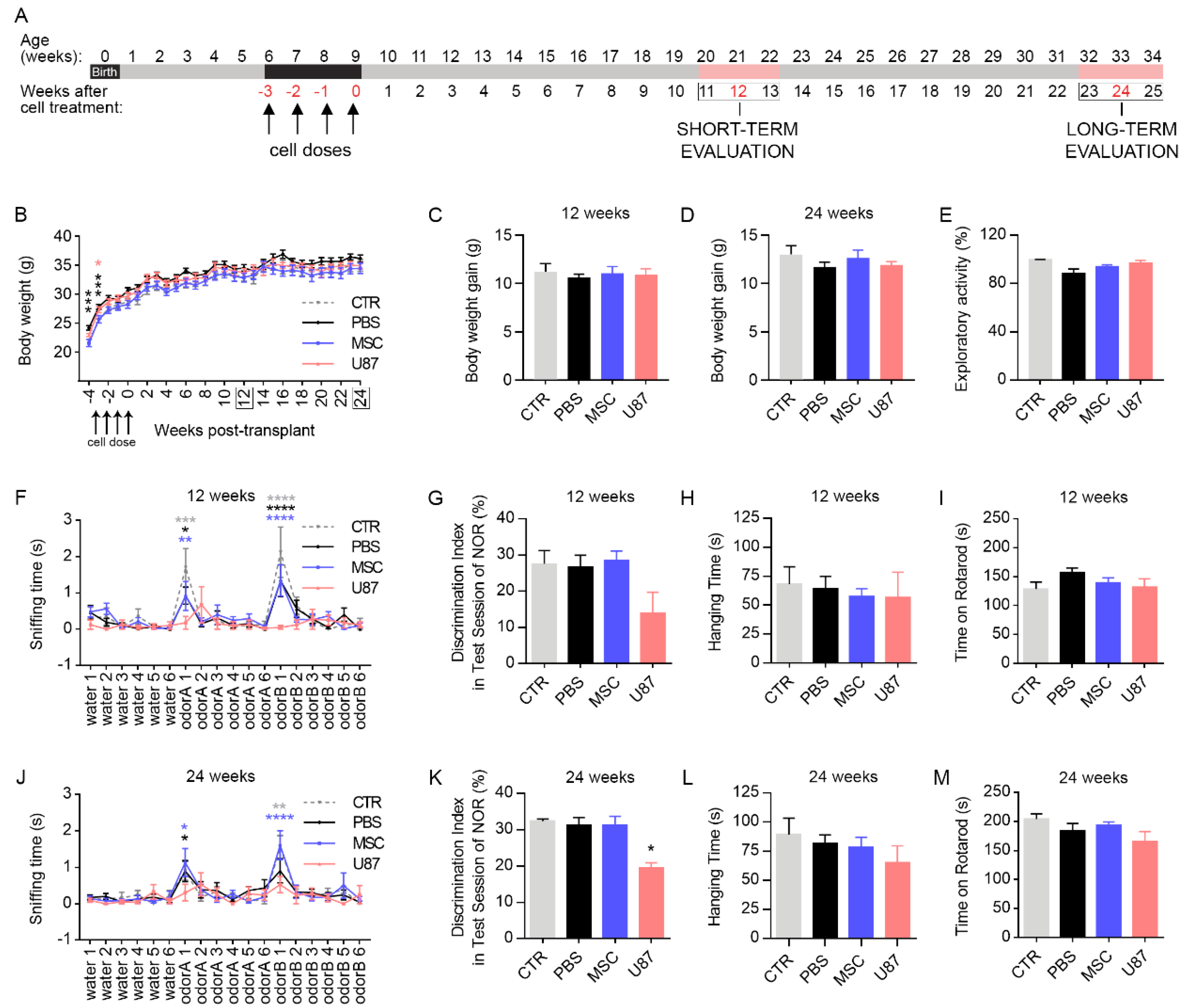
3.2. Intranasal Delivery of MSCs Does Not Affect Mice Welfare and Functional Performance
3.3. Biochemistry Analysis and Determination of Oxidative Stress-Related Parameters in Blood Samples
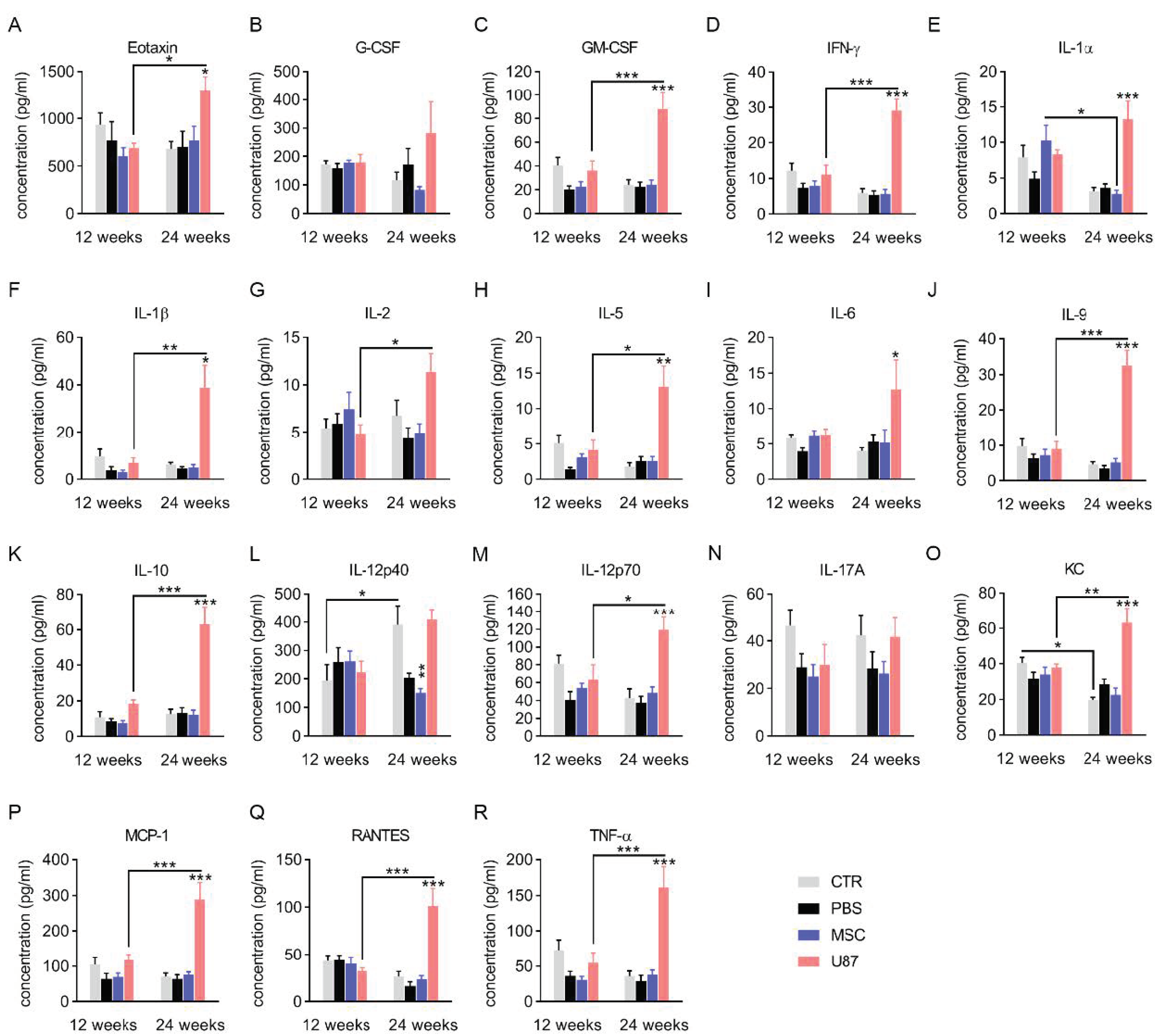
3.4. Analysis of the Cytokine Profile in Blood Plasma
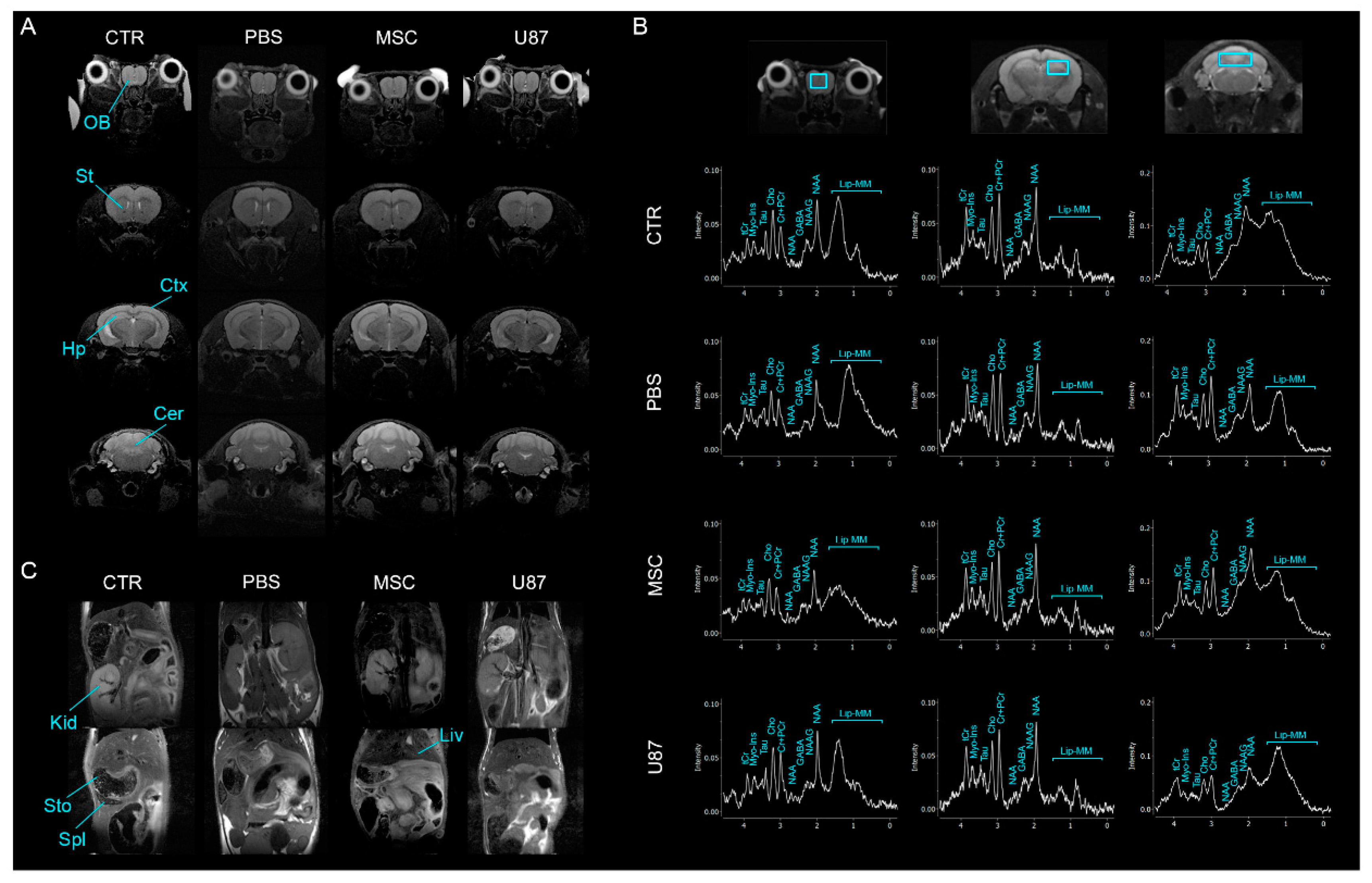
3.5. Intranasal Administration of MSCs Does Not Induce Anomalies in the Brain and Other Organs In Vivo
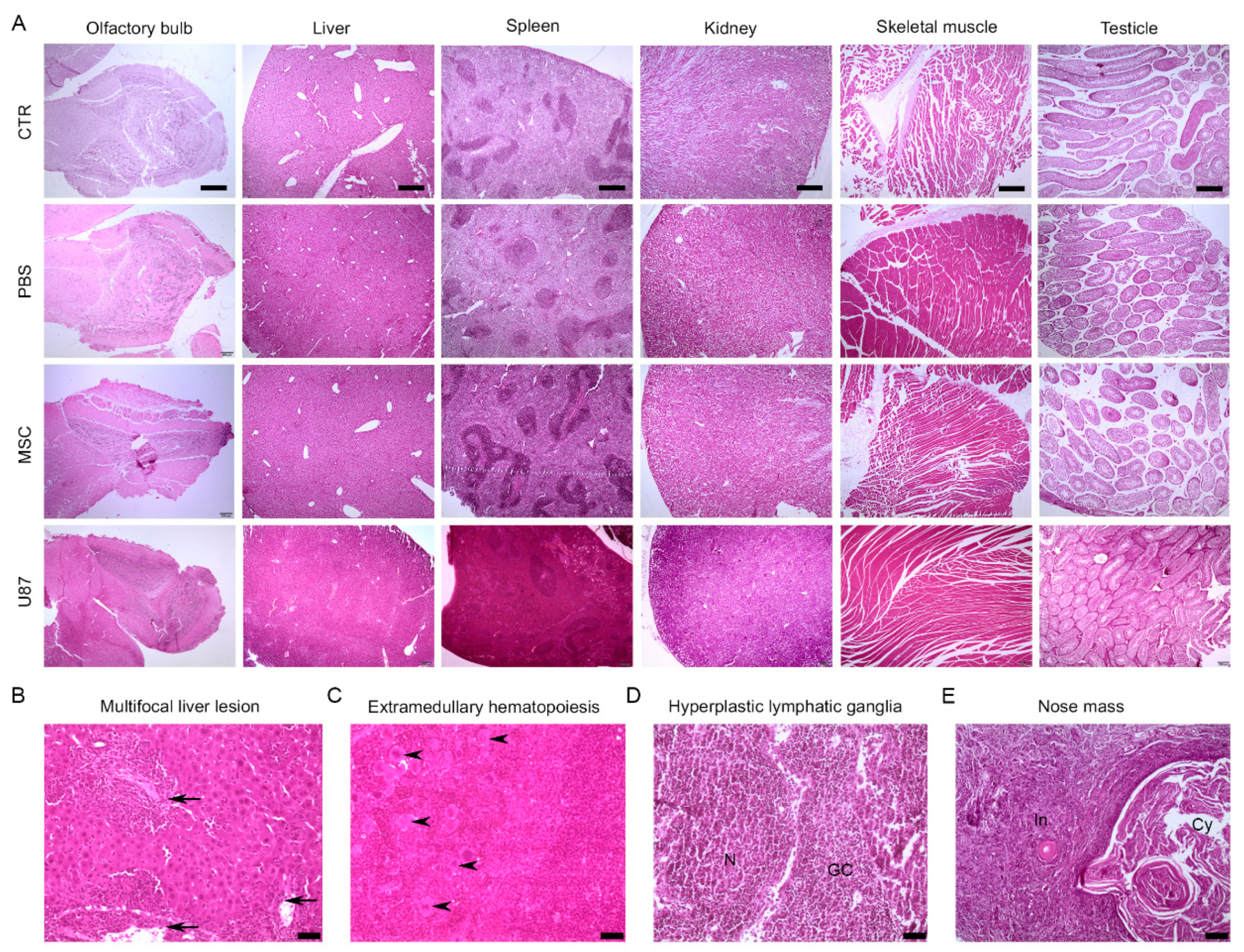
3.6. Long-Term Histological Analysis of the Major Organs Does Not Evidence Lesions after Intranasal Administration of MSCs
3.7. Biodistribution Suggests a Progressive Disappearance of Transplanted Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rushkevich, Y.N.; Kosmacheva, S.M.; Zabrodets, G.V.; Ignatenko, S.I.; Goncharova, N.V.; Severin, I.N.; Likhachev, S.A.; Potapnev, M.P. The Use of Autologous Mesenchymal Stem Cells for Cell Therapy of Patients with Amyotrophic Lateral Sclerosis in Belarus. Bull. Exp. Biol. Med. 2015, 159, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Gotherstrom, C.; Westgren, M.; Shaw, S.W.; Astrom, E.; Biswas, A.; Byers, P.H.; Mattar, C.; Graham, G.; Taslimi, J.; Uwe, U.; et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: A two-center experience. Stem Cells Transl. Med. 2014, 3, 255–264. [Google Scholar] [CrossRef]
- Vega, A.; Martin-Ferrero, M.A.; Del Canto, F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.-Q.; Luan, S.-L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- Thakkar, U.G.; Trivedi, H.L.; Vanikar, A.V.; Dave, S.D. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow–derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 2015, 17, 940–947. [Google Scholar] [CrossRef]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef] [PubMed]
- Karantalis, V.; DiFede, D.L.; Gerstenblith, G.; Pham, S.; Symes, J.; Zambrano, J.; Fishman, J.; Pattany, P.; McNiece, I.; Conte, J.; et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ. Res. 2014, 114, 1302–1310. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.; Soria, B.; Capilla-Gonzalez, V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Ramos-Zuriga, R.; Gonzalez-Perez, O.; Macedas-Ornelas, A.; Capilla-Gonzalez, V.; Quinones-Hinojosa, A. Ethical Implications in the Use of Embryonic and Adult Neural Stem Cells. Stem Cells Int. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Escacena, N.; Quesada-Hernandez, E.; Capilla-Gonzalez, V.; Soria, B.; Hmadcha, A. Bottlenecks in the Efficient Use of Advanced Therapy Medicinal Products Based on Mesenchymal Stromal Cells. Stem Cells Int. 2015, 2015, 895714. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Sheldon, R.A.; Kavelaars, A.; Derugin, N.; Vexler, Z.S.; Willemen, H.; Mass, M.; Heijnen, C.; Ferriero, D. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke 2013, 44, 1426–1432. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef]
- Jones, J.; Estirado, A.; Redondo, C.; Pacheco-Torres, J.; Sirerol-Piquer, M.-S.; García-Verdugo, J.M.; Martinez, S. Mesenchymal Stem Cells Improve Motor Functions and Decrease Neurodegeneration in Ataxic Mice. Mol. Ther. 2015, 23, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Menge, T.; Zhao, Y.; Zhao, J.; Wataha, K.; Zhang, J.; Letourneau, P.; Rebell, J.; Shen, L.; Wang, J.; Peng, Z.; et al. Mesenchymal Stem Cells Regulate Blood Brain Barrier Integrity in Traumatic Brain Injury Through Production of the Soluble Factor TIMP3. Sci. Transl. Med. 2012, 4, 161ra150. [Google Scholar] [CrossRef]
- Zhu, L.H.; Bai, X.; Zhang, N.; Wang, S.-Y.; Li, W.; Jiang, J. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014, 1563, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 2019, 50, 2835–2841. [Google Scholar] [CrossRef]
- Mello, T.G.; Rosado-De-Castro, P.H.; Campos, R.M.P.; Vasques, J.F.; Junior, W.S.R.; Mattos, R.S.D.A.R.D.; Puig-Pijuan, M.T.; Foerster, B.U.; Gutfilen, B.; Souza, S.A.L.; et al. Intravenous Human Umbilical Cord-Derived Mesenchymal Stromal Cell Administration in Models of Moderate and Severe Intracerebral Hemorrhage. Stem Cells Dev. 2020, 29, 586–598. [Google Scholar] [CrossRef]
- Lee, N.K.; Yang, J.; Chang, E.H.; Park, S.; Lee, J.; Choi, S.; Oh, W.; Chang, J.; Na, D. Intra-Arterially Delivered Mesenchymal Stem Cells Are Not Detected in the Brain Parenchyma in an Alzheimer’s Disease Mouse Model. PLoS ONE 2016, 11, e0155912. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Roch, M.; Holtkamp, N.; Kurtz, A. Systemically administered human bone marrow-derived mesenchymal stem home into peripheral organs but do not induce neuroprotective effects in the MCAo-mouse model for cerebral ischemia. Neurosci. Lett. 2012, 513, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Sinden, J.; Miljan, E.; Dunn, L. Intracranial Delivery of Stem Cells. Transl. Stroke Res. 2011, 2, 266–271. [Google Scholar] [CrossRef]
- Li, Y.-H.; Feng, L.; Zhang, G.-X.; Ma, C.-G. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef]
- Soria, B.; Martin-Montalvo, A.; Aguilera, Y.; Mellado-Damas, N.; López-Beas, J.; Herrera-Herrera, I.; López, E.; Barcia, J.A.; Alvarez-Dolado, M.; Hmadcha, A.; et al. Human Mesenchymal Stem Cells Prevent Neurological Complications of Radiotherapy. Front. Cell. Neurosci. 2019, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.; Fisher, P.G. Neurological complications following treatment of children with brain tumors. J. Pediatr. Rehabil. Med. 2011, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Szentes, A.; Eros, N.; Kekecs, Z.; Jakab, Z.; Torok, S.; Schiler, D.; Hauser, P.; Garami, M. Cognitive deficits and psychopathological symptoms among children with medulloblastoma. Eur. J. Cancer Care (Engl.) 2018, 27, e12912. [Google Scholar] [CrossRef]
- Williams, N.L.; Rotondo, R.L.; Bradley, J.A.; Pincus, D.W.; Fort, J.A.; Wynn, T.; Morris, C.G.; Mendenhall, N.P.; Indelicato, D.J. Late Effects After Radiotherapy for Childhood Low-grade Glioma. Am. J. Clin. Oncol. 2018, 41, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Zureick, A.H.; Evans, C.L.; Niemierko, A.; Grieco, J.; Nichols, A.; Fullerton, B.; Hess, C.; Goebel, C.; Gallotto, S.; Weyman, E.; et al. Left hippocampal dosimetry correlates with visual and verbal memory outcomes in survivors of pediatric brain tumors. Cancer 2018, 124, 2238–2245. [Google Scholar] [CrossRef]
- Jacobs, V.L.; Valdes, P.A.; Hickey, W.F.; De Leo, J.A. Current Review of in Vivo GBM Rodent Models: Emphasis on the CNS-1 Tumour Model. ASN Neuro. 2011, 3, AN20110014. [Google Scholar] [CrossRef]
- Spangenberg, E.M.; Keeling, L.J. Assessing the welfare of laboratory mice in their home environment using animal-based measures—A benchmarking tool. Lab. Anim. 2016, 50, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Capilla-Gonzalez, V.; Gil-Perotin, S.; Ferragud, A.; Bonet-Ponce, L.; Canales, J.J.; Garcia-Verdugo, J.M. Exposure to N-ethyl-N-nitrosourea in adult mice alters structural and functional integrity of neurogenic sites. PLoS ONE 2012, 7, e29891. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Klein, S.M.; Vykoukal, J.; Lechler, P.; Zeitler, K.; Gehmert, S.; Schreml, S.; Alt, E.; Bogdahn, U.; Prantl, L. Noninvasive in vivo assessment of muscle impairment in the mdx mouse model--a comparison of two common wire hanging methods with two different results. J. Neurosci. Methods 2012, 203, 292–297. [Google Scholar] [CrossRef]
- Lopez-Noriega, L.; Cobo-Vuilleumier, N.; Narbona-Perez, A.J.; Araujo-Garrido, J.L.; Lorenzo, P.I.; Mellado-Gil, J.M.; Moreno, J.C.; Gauthier, B.R.; Martin-Montalvo, A. Levothyroxine enhances glucose clearance and blunts the onset of experimental type 1 diabetes mellitus in mice. Br. J. Pharmacol. 2017, 174, 3795–3810. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; Von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Vaes, J.E.G.; van Kammen, C.M.; Trayford, C.; van der Toorn, A.; Ruhwedel, T.; Bender, M.; Dijkhuizen, R.; Mobius, W.; van Rijt, S.; Nijboer, C. Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity. Glia 2020. [Google Scholar] [CrossRef]
- Balyasnikova, I.V.; Prasol, M.S.; Ferguson, S.D.; Han, Y.; Ahmed, A.; Gutova, M.; Tobias, A.; Mustafi, D.; Rincon, E.; Zhang, L.; et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 140–148. [Google Scholar] [CrossRef]
- Chau, M.J.; Deveau, T.C.; Gu, X.; Gu, X.; Xu, Y.; Yu, S.P.; Wei, L. Delayed and repeated intranasal delivery of bone marrow stromal cells increases regeneration and functional recovery after ischemic stroke in mice. BMC Neurosci. 2018, 19, 20. [Google Scholar] [CrossRef]
- Ji, G.; Liu, M.; Zhao, X.F.; Liu, X.-Y.; Guo, Q.-L.; Guan, Z.-F.; Zhou, H.-G.; Guo, J.-C. NF-kappaB Signaling is Involved in the Effects of Intranasally Engrafted Human Neural Stem Cells on Neurofunctional Improvements in Neonatal Rat Hypoxic-Ischemic Encephalopathy. CNS Neurosci. Ther. 2015, 21, 926–935. [Google Scholar] [CrossRef]
- Oppliger, B.; Joerger-Messerli, M.; Mueller, M.; Reinhart, U.; Schneider, P.; Surbek, D.V.; Schoeberlein, A. Intranasal Delivery of Umbilical Cord-Derived Mesenchymal Stem Cells Preserves Myelination in Perinatal Brain Damage. Stem Cells Dev. 2016, 25, 1234–1242. [Google Scholar] [CrossRef]
- Wei, Z.Z.; Gu, X.; Ferdinand, A.; Lee, J.H.; Ji, X.; Ji, X.M.; Yu, S.P.; Wei, L. Intranasal Delivery of Bone Marrow Mesenchymal Stem Cells Improved Neurovascular Regeneration and Rescued Neuropsychiatric Deficits after Neonatal Stroke in Rats. Cell Transpl. 2015, 24, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Mohammadi, S.; Alani, B.; Karimian, M.; Moradian-Tehrani, R.; Noureddini, M. Intranasal administration of endometrial mesenchymal stem cells as a suitable approach for Parkinson’s disease therapy. Mol. Biol. Rep. 2019, 46, 4293–4302. [Google Scholar] [CrossRef]
- Beigi Boroujeni, F.; Pasbakhsh, P.; Mortezaee, K.; Pirhajati, V.; Alizadeh, R.; Aryanpour, R.; Madadi, S.; Kashani, I. Intranasal delivery of SDF-1alpha-preconditioned bone marrow mesenchymal cells improves remyelination in the cuprizone-induced mouse model of multiple sclerosis. Cell Biol. Int. 2020, 44, 499–511. [Google Scholar] [CrossRef]
- Yu-Taeger, L.; Stricker-Shaver, J.; Arnold, K.; Bambynek-Dziuk, P.; Novati, A.; Singer, E.; Lourhmati, A.; Fabian, C.; Magg, J.; Riess, O.; et al. Intranasal Administration of Mesenchymal Stem Cells Ameliorates the Abnormal Dopamine Transmission System and Inflammatory Reaction in the R6/2 Mouse Model of Huntington Disease. Cells 2019, 8, 595. [Google Scholar] [CrossRef]
- McDonald, C.A.; Djuliannisaa, Z.; Petraki, M.; Paton, M.; Penny, T.; Sutherland, A.; Castillo-Melendez, M.; Novak, I.; Jenkins, G.; Fahey, M.; et al. Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic(-)Ischemic Brain Injury. Int. J. Mol. Sci. 2019, 20, 2449. [Google Scholar] [CrossRef]
- Mangraviti, A.; Tzeng, S.Y.; Gullotti, D.; Kozielski, K.L.; Kim, J.E.; Seng, M.; Abbadi, S.; Schiapparelli, P.; Sarabia-Estrada, R.; Vescovi, A.; et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 2016, 100, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Boström, M.; Ek, C.J.; Li, T.; Xie, C.; Xu, Y.; Sun, Y.; Blomgren, K.; Zhu, C. Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum. Sci. Rep. 2017, 7, srep46181. [Google Scholar] [CrossRef]
- Acharya, M.M.; Green, K.N.; Allen, B.D.; Najafi, A.R.; Syage, A.; Minasyan, H.; Le, M.T.; Kawashita, T.; Giedzinski, E.; Parihar, V.K.; et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci. Rep. 2016, 6, 31545. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Jung, J.-S.; Kim, T.-H.; Lim, S.-J.; Oh, E.-S.; Kim, J.-Y.; Ji, K.-A.; Joe, E.-H.; Cho, K.-H.; Han, I.-O. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol. Dis. 2006, 21, 457–467. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The Subventricular Zone Is Able to Respond to a Demyelinating Lesion After Localized Radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Tappenbeck, N.; Schröder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560. [Google Scholar] [CrossRef]
- Gonzalvez-Garcia, M.; Martinez, C.M.; Villanueva, V.; Garcia-Hernandez, A.; Blanquer, M.; Meseguer-Olmo, L.; Onate Sancez, R.E.; Moraleda, J.; Rodriguez-Lozano, F. Preclinical Studies of the Biosafety and Efficacy of Human Bone Marrow Mesenchymal Stem Cells Pre-Seeded into beta-TCP Scaffolds after Transplantation. Materials 2018, 11, 1349. [Google Scholar] [CrossRef]
- Waterman, R.S.; Morgenweck, J.; Nossaman, B.D.; Scandurro, A.; Scandurro, S.; Betancount, A. Anti-inflammatory mesenchymal stem cells (MSC2) attenuate symptoms of painful diabetic peripheral neuropathy. Stem Cells Transl. Med. 2012, 1, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Vohrer, J.; Schmal, H.; Kasten, P.; Fellenberg, J.; Suedkamp, N.P.; Mehlhorn, A. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy 2008, 10, 784–795. [Google Scholar] [CrossRef]
- Lin, A.-L.; Powell, D.; Caban-Holt, A.; Jicha, G.; Robertson, W.; Gold, B.; Davis, R.; Abner, E.; Wilcock, D.; Schmitt, F.; et al. 1 H-MRS metabolites in adults with Down syndrome: Effects of dementia. Neuroimage Clin. 2016, 11, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.; Namboodiri, M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- da Costa Goncalves, F.; Grings, M.; Nunes, N.S.; Pinto, F.; Alves Garcez, T.; Visioli, F.; Leipnitz, G.; Paz, A. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol. Lett. 2017, 39, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Liao, H.; Wang, H.; Rong, X.; Li, E.; Xu, R.-H.; Peng, Y. Mesenchymal Stem Cells Attenuate Radiation-Induced Brain Injury by Inhibiting Microglia Pyroptosis. Biomed. Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.; Geissler, E.; Schlitt, H.; Baan, C.; Dahlker, M.; Hoogduijn, M. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef]
- Azevedo, R.I.; Minskaia, E.; Fernandes-Platzgummer, A.; Viera, A.; da Silva, C.; Cabral, J.; Lacerda, J. Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells 2020, 38, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Tasso, R.; Ilengo, C.; Quarto, R.; Cancedda, R.; Caspi, R.R.; Pennesi, G. Mesenchymal Stem Cells Induce Functionally Active T-Regulatory Lymphocytes in a Paracrine Fashion and Ameliorate Experimental Autoimmune Uveitis. Investig. Opthalmology Vis. Sci. 2012, 53, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilera, Y.; Mellado-Damas, N.; Olmedo-Moreno, L.; López, V.; Panadero-Morón, C.; Benito, M.; Guerrero-Cázares, H.; Márquez-Vega, C.; Martín-Montalvo, A.; Capilla-González, V. Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice. Cancers 2021, 13, 1169. https://doi.org/10.3390/cancers13051169
Aguilera Y, Mellado-Damas N, Olmedo-Moreno L, López V, Panadero-Morón C, Benito M, Guerrero-Cázares H, Márquez-Vega C, Martín-Montalvo A, Capilla-González V. Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice. Cancers. 2021; 13(5):1169. https://doi.org/10.3390/cancers13051169
Chicago/Turabian StyleAguilera, Yolanda, Nuria Mellado-Damas, Laura Olmedo-Moreno, Víctor López, Concepción Panadero-Morón, Marina Benito, Hugo Guerrero-Cázares, Catalina Márquez-Vega, Alejandro Martín-Montalvo, and Vivian Capilla-González. 2021. "Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice" Cancers 13, no. 5: 1169. https://doi.org/10.3390/cancers13051169
APA StyleAguilera, Y., Mellado-Damas, N., Olmedo-Moreno, L., López, V., Panadero-Morón, C., Benito, M., Guerrero-Cázares, H., Márquez-Vega, C., Martín-Montalvo, A., & Capilla-González, V. (2021). Preclinical Safety Evaluation of Intranasally Delivered Human Mesenchymal Stem Cells in Juvenile Mice. Cancers, 13(5), 1169. https://doi.org/10.3390/cancers13051169