Simple Summary
Poorly differentiated thyroid cancer is a rare subtype of thyroid cancer. The course of this disease can vary substantially. Treatment options consist of surgery and radioactive iodine therapy, if possible, and in tyrosine kinase inhibitors for patients where this is not possible. The aim of this study was to identify risk factors for the development of disease that does not respond to radioactive iodine therapy and for premature death, in order to better identify patients in need of more extensive tumor staging and treatment. We identified primary tumor size and infiltration of the tissue surrounding the thyroid gland as risk factors for the development of disease that does not respond to radioactive iodine therapy and tumor volume as a risk factor for early death.
Abstract
Background: The clinical phenotype of poorly differentiated thyroid cancer (PDTC) can vary substantially. We aim to evaluate risk factors for radioiodine refractory (RAI-R) disease and reduced overall survival (OS). Methods: We retrospectively screened our institutional database for PDTC patients. For the assessment of RAI-R disease, we included patients who underwent dual imaging with 18F-FDG-PET and 124I-PET/131I scintigraphy that met the internal standard of care. We tested primary size, extrathyroidal extension (ETE), and age >55 years as risk factors for RAI-R disease at initial diagnosis and during the disease course using uni- and multivariate analyses. We tested metabolic tumor volume (MTV), total lesion glycolysis (TLG) on 18F-FDG-PET, and the progression of stimulated thyroglobulin within 4–6 months of initial radioiodine therapy as prognostic markers for OS. Results: Size of primary >40 mm and ETE were significant predictors of RAI-R disease in the course of disease in univariate (81% vs. 27%, p = 0.001; 89% vs. 33%, p < 0.001) and multivariate analyses. Primary tumor size was an excellent predictor of RAI-R disease (AUC = 0.90). TLG/MTV > upper quartile and early thyroglobulin progression were significantly associated with shorter median OS (29.0 months vs. 56.9 months, p < 0.05; 57.8 months vs. not reached p < 0.005, respectively). Discussion: PDTC patients, especially those with additional risk factors, should be assessed for RAI-R disease at initial diagnosis and in the course of disease, allowing for early implementation of multimodal treatment. Primary tumor size >40 mm, ETE, and age >55 are significant risk factors for RAI-R disease. High MTV/TLG is a significant risk factor for premature death and can help identify patients requiring intervention.
1. Introduction
Poorly differentiated thyroid carcinoma (PDTC) is a rare subtype of thyroid carcinoma representing about 2–3% of thyroid cancers (TC) in the United States [1]. PDTCs show an intermediate level of differentiation when compared to anaplastic thyroid carcinoma on one side and papillary or follicular thyroid carcinoma on the other side of the spectrum [1]. Five-year survival rates range from approximately 65 to 85% and can vary substantially [2,3,4]. PDTC lesions are at an increased risk of being radioiodine refractory (RAI-R) at initial diagnosis or becoming so in the course of disease [5,6]. Subsequently, the benefit of radioactive iodine therapy (RAIT) in these patients is variable and the routine treatment with RAIT is therefore largely controversial. However, patients with PDTC should not be automatically precluded from undergoing RAIT, as RAI− avid disease is described in about 25% of patients [7]. It is therefore crucial to reliably identify patients likely to benefit from RAIT as well as patients with RAI-R TC or are at a high risk for progression to RAI-R TC. Given the toxicity profile of current RAI-R TC medications, such as hypertension, skin reactions and proteinuria [8,9] it is of additional importance to then reliably stratify patients into those at increased risk for cancer-related death and in need for therapeutic intervention vs. those that can be managed with active surveillance [10].
As metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are derived from 18F-FDG PET and have shown to be inversely correlated with progression-free survival and overall survival (OS) in different tumor entities, among which is RAI-R TC [11]. We hypothesize that it can aide risk stratification in patients with PDTC.
The aims of this study were to determine predictive factors for RAI-R TC in PDTC at initial diagnosis and in the course of disease, as well as to establish parameters that identify patients at a high risk for early mortality.
2. Methods
We retrospectively screened our institutional database for all patients treated at our hospital from 2007 until March 2020 meeting the following inclusion criteria:
Histopathologically confirmed poorly differentiated thyroid cancer (PDTC) in accordance with the Turin proposal [2]. Patients with an earlier initial diagnosis were considered if a re-evaluation of the original histopathology had been performed after the publication of the Turin proposal.
For the assessment of RAI-R TC: Dual imaging, defined as 18F-FDG PET and 124I-PET or 131I whole-body scan (WBS) meeting the internal standard of care for PDTC.
Patients with 18F-FDG PET but without iodine imaging were included for the analysis of MTV and TLG as prognostic factors for OS.
2.1. RAI Avidity
Based on initial dual imaging, patients were classified as RAI−avid (RAI+), RAI-Refractory (RAI−), and disease-free (DF). RAI-Refractory disease was defined as the absence of RAI-uptake in at least one lesion or radiological disease progression within one year after RAIT. For the assessment of RAI-R in the course of disease, structural persistence after the administration of cumulatively 22.0 GBq 131I was added as a criterion. We tested primary tumor size >40 mm, any extrathyroidal extension, and age > 55 years as putative risk factors for RAI-R disease at initial diagnosis and in the course of disease based on the evidence for their prognostic role in TC [12].
2.2. Response Evaluation
Treatment response was assessed one year after initial treatment and classified as either an excellent response, a biochemical incomplete response, a structural incomplete response, or an indeterminate response in adherence to current ATA guidelines [13]. Time to progression to RAI-R TC was defined as time from initial RAIT until at least one of the abovementioned criteria was met.
2.3. Predictive Factors for Overall Survival
Early thyroglobulin (Tg) progression, FDG- avid disease, MTV, TLG, and TNM stage were tested as prognostic factors for overall survival. Early Tg progression was defined as any increase in stimulated Tg at 4–6 months after initial treatment. FDG-avid disease was defined as the presence of any focal FDG-uptake unambiguously identified as neoplastic using both PET and CT information. AJCC TNM stage was stratified in accordance with the 7th edition.
MTV and TLG were obtained from 18F-FDG-PET using a research software prototype (MIWBAS, version 1.0, Siemens Medical Solutions USA, Inc., Knoxville, TN, USA). A local threshold of 50% of SUVmax was used. OS was defined as time from initial RAIT to death/last follow-up when analyzing early Tg progression and the time from FDG-PET/CT to death/last follow-up when analyzing MTV and TLG.
2.4. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Mac, version 26 (IBM Corp., Armonk, NY, USA). Interval data are given in the format mean ± standard deviation. Fisher’s exact test was used to determine statistical significance for differences among groups and logistic regression was used for multivariate analysis for the patients where tumor size and ETE were known. A p-value < 0.05 was considered statistically significant. A log-rank test using Kaplan-Meier curves was used to test assumed predictive factors for OS. Receiver operating characteristic (ROC) analysis was performed to assess the predictive value of primary tumor size for radioiodine refractoriness using area under the curve (AUC) as a metric. Youden’s J statistics were employed to identify the optimal cutoff value for primary tumor size and their association with radioiodine refractoriness.
3. Results
3.1. Imaging and Treatment Protocol
Initial RAIT was carried out on clinical indication in 47/51 patients after the oral administration of 3.9 ± 2.5 Gbq 131I. Prior dosimetry using 124I PET was employed to assess RAI avidity before initial and follow-up RAIT, if the likelihood for insufficient RAI uptake was deemed significant. Based on prior publications [14,15,16], in the presence of iodine-avid lesions on pretherapeutic 124I PET imaging, dosimetry-based RAIT activities aiming at tumor absorbed doses >85 Gy, while not exceeding a blood dose of 2.0 Gy, were administered. In the remainder, empirical activities were used.
On average, 124I PET was acquired 1 and 5 days after the oral administration of 26.2 ± 3.5 MBq 124I. 18F-FDG PET was performed 61.7 ± 9.1 min after the intravenous administration of 282.4 ± 65.2 MBq 18F-FDG. Mean interval between thyroidectomy and 18F-FDG PET was 4 months.
3.2. Study Cohort
Fifty-one patients (23 male, 28 female) were eligible for the assessment of RAI-R TC at initial diagnosis and in the course of disease. The mean patient age was 58.5 ± 17.3 years, and 30 (59%) patients were older than 55 years. In 2 patients the primary could not be evaluated (Tx) and the histopathological diagnosis was derived from metastatic tissue and in another 3, information about size of the primary tumor could not be retrieved. Thirty-one patients had a primary tumor >40 mm, and any extrathyroidal extension tumor was present in 28.
For the analysis of MTV and TLG as risk factors for OS, 4 patients had to be excluded, because the images were inaccessible, and two because of concurrent malignancies. An additional 11 patients that were referred to us for imaging only were included. These patients were not included in the assessment of RAI-R TC at initial diagnosis, as they were referred to our imaging center under the suspicion of RAI-R TC. Mean patient age of this cohort was 58.5 ± 18.0 years. Patient characteristics are provided in Table 1 and Table 2.

Table 1.
Patient characteristics for the assessment of radioiodine refractory (RAI-R) poorly differentiated thyroid carcinoma (PDTC) at initial disease and the prognostic factor of early thyroglobulin (Tg) progression.

Table 2.
Patient characteristics for the assessment of positron-emission tomography (PET) parameters as prognostic factors for overall survival.
3.3. Initial RAI Avidity
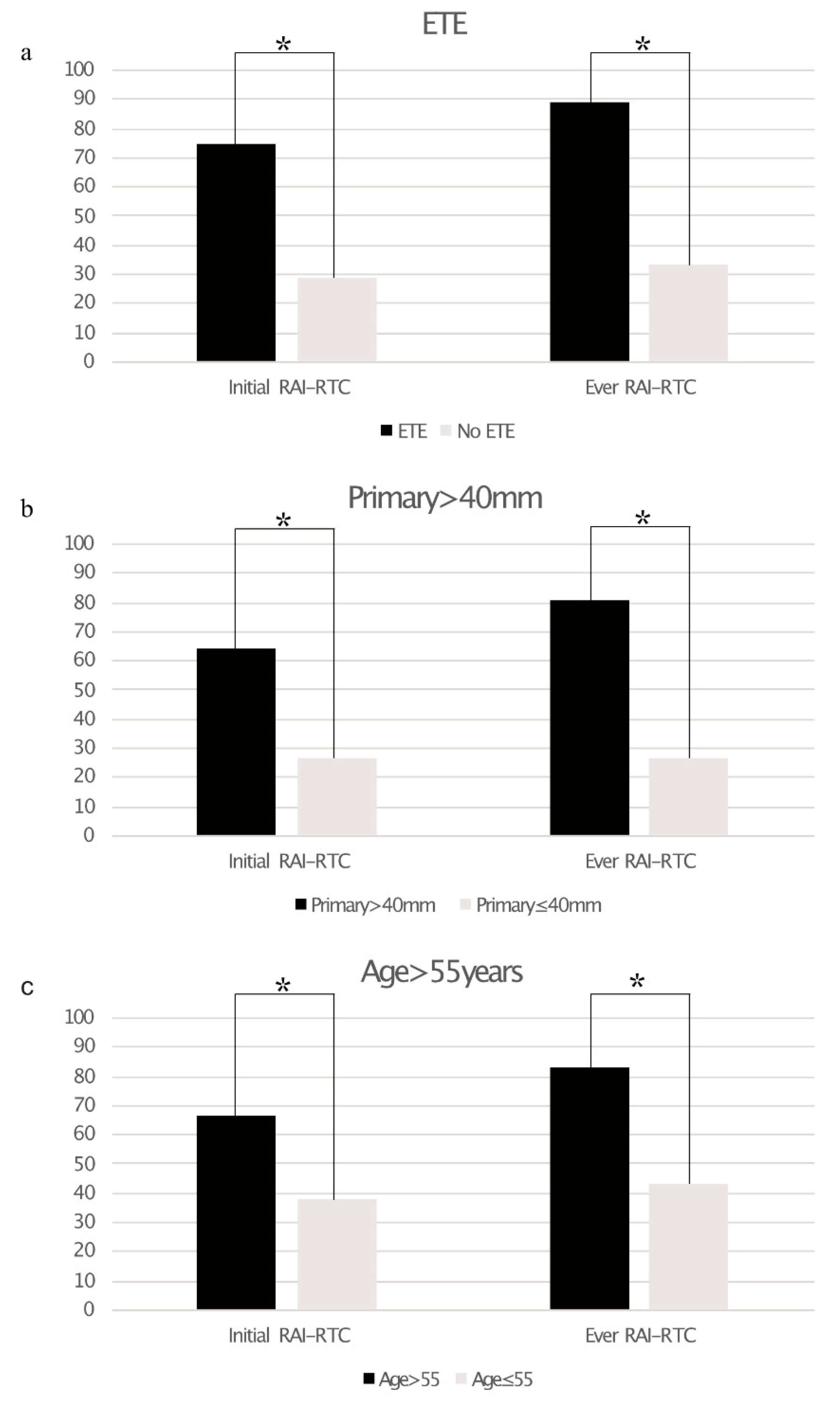
A total of 19/51 (37%) of patients showed any neoplastic RAI uptake as assessed by 124I-PET alone (n = 4), 131I-WBS alone (n = 19) or both (n = 28). Post-therapeutic 131I-WBS detected additional lesions vs. 124I-PET in three patients. Using the aforementioned criteria, 16 patients were DF, 7 patients RAI+, and 28 patients RAI−. Of the latter 25 showed tumor lesions without iodine uptake at initial imaging and three patients developed radiological disease progression within 12 months of RAIT (mean: 8.0 ± 2.0 months). Patients with a primary tumor size >40 mm (relative risk 64.5% vs. 26.7%; p < 0.05), extrathyroidal tumor extension (75.0% vs. 28.6%; p < 0.005), and age >55 years (66.7% vs. 38.1%; p < 0.05) were significantly more likely to show RAI-R TC at initial diagnosis than patients without these risk factors. All of these risk factors were significantly associated with a higher risk for RAI-R TC in the course of disease (primary size >40 mm: 80.6% vs. 26.7%; p < 0.005, extrathyroidal extension: 89.3% vs. 33.3%; p < 0.001; age > 55 years: 83.3% vs. 42.9%, p < 0.005). Figure 1a–c and Table 3 gives an overview of the impact of the assessed risk factors on RAI-R TC.
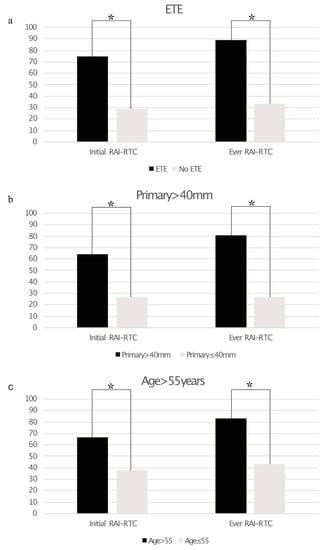
Figure 1.
Bar graphs showing the prevalence of radioiodine refractory thyroid cancer (RAI-R TC) based on extrathyroidal extension ((a), ETE), size of primary tumor >40 mm (b), and age >55 years (c). Statistically significant differences are marked with an asterisk *.

Table 3.
Risk factors for radioiodine refractory disease at initial diagnosis and in the course of disease.
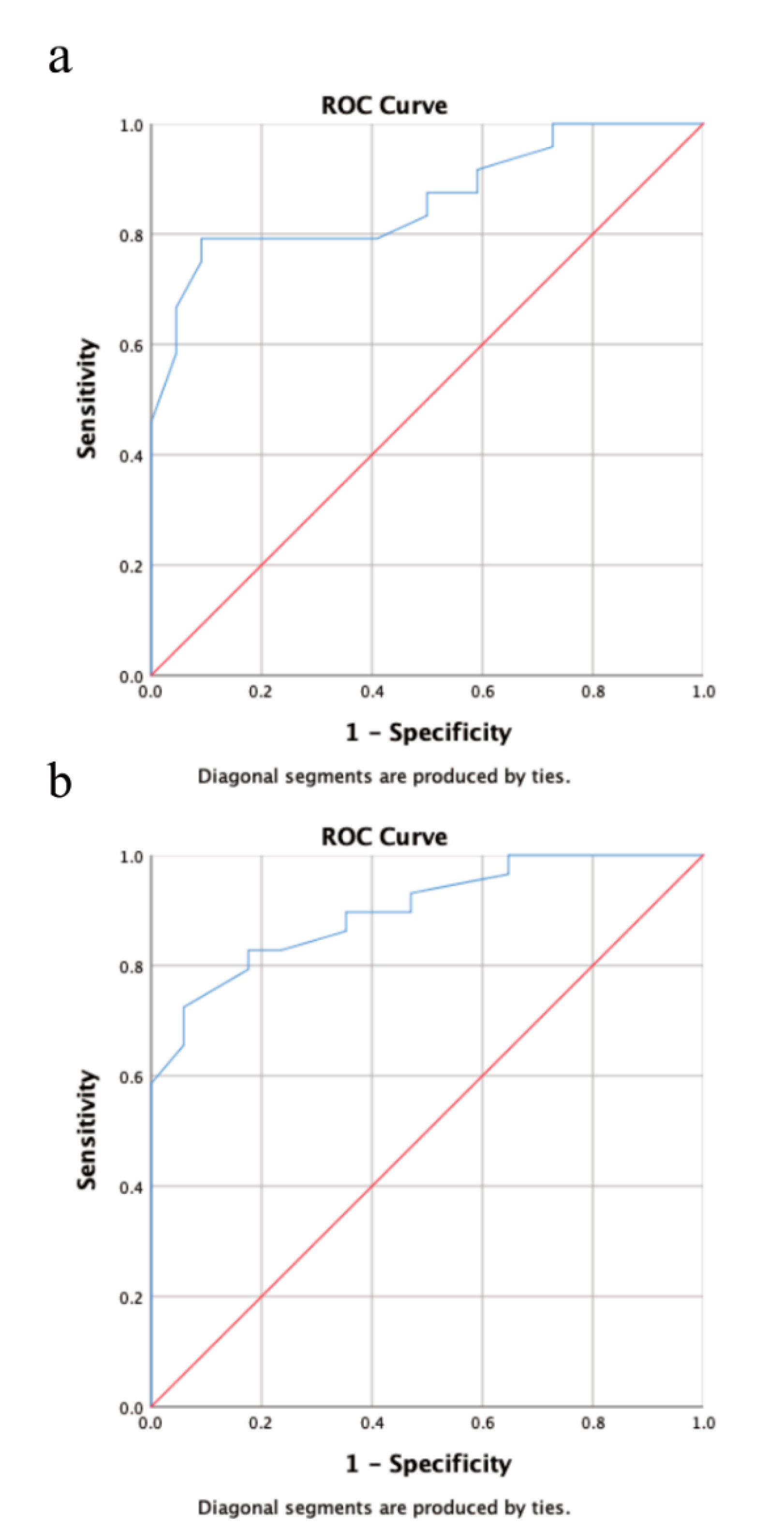
Multivariate analysis revealed a statistically significant impact of extrathyroidal extension on RAI-R TC at initial diagnosis (p = 0.016; hazard ratio (95% confidence interval): 5.2 (1.3–19.8)), while primary tumor size >40 mm was barely not statistically significant (p = 0.08). Both factors were statistically significant for the onset of RAI-R TC in the course of disease (primary tumor size >40 mm: p = 0.007; hazard ratio (95% confidence interval): 11.6 (1.9–69.7); extrathyroidal extension: p = 0.003; 14.8 (2.5–87.0)). The results of the multivariate analysis are provided in Table 4. The AUC for the predictive value of primary tumor size was 0.86 for RAI-R TC at initial diagnosis and 0.9 for RAI-R TC in the course of disease. Youden’s J statistics identified a primary tumor size of 52.5 mm as the optimal cutoff value for the risk of RAI-R TC in the course of the disease (specificity: 94% sensitivity: 72.4%). The results of the ROC analysis are provided in Figure 2.

Table 4.
(a) Multivariate model of risk factors for RAI-R TC at initial diagnosis. ETE = extrathyroidal extension; CI = confidential interval. (b) Multivariate model of risk factors for RAI-R TC during the course of disease. ETE = extrathyroidal extension; CI = confidential interval.
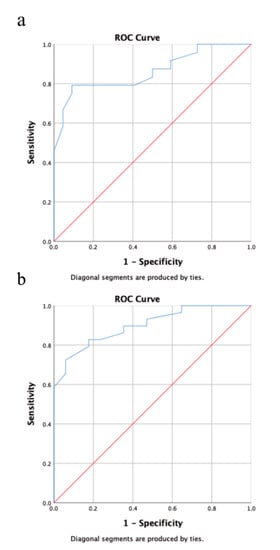
Figure 2.
Receiver operating characteristic (ROC) analysis showing the predictive value for primary tumor size for RAI-R TC at initial diagnosis (a) and during the course of disease. (b) Using area under curve (AUC) as a metric primary tumor size was an excellent predictor for RAI-R TC at initial diagnosis (AUC = 0.86) and during the course of disease (AUC = 0.90).
3.4. Therapy Response
A total of 14/16 (88%) of initial DF patients showed excellent responses and 2/16 (13%) showed biochemical incomplete responses according to ATA guidelines. A total of 5/7 (%) RAI+ patients showed excellent responses, 1/7 (14%) each showed biochemical incomplete responses and structural incomplete responses. Progression to RAI-R TC occurred in all patients with biochemical (n = 3) or structural (n = 1) incomplete responses after a mean interval of 29 ± 8 months, and only in 2/21 (10%; p = 0.002) of excellent responses after a mean interval of 183.5 ± 5 months. Treatment response could not be evaluated in 4/28 (14%) RAI− patients, of these 2 were lost to follow-up and 2 died within less than a year after initial treatment. All of these presented with iodine-negative lesions at initial imaging. 2 (7%) initially RAI− patients showed excellent responses (following resection of lymph node metastases) and 2 showed biochemical incomplete responses. Twenty-one (75%) of initially RAI− patients showed structural incomplete response.
3.5. Survival Analysis
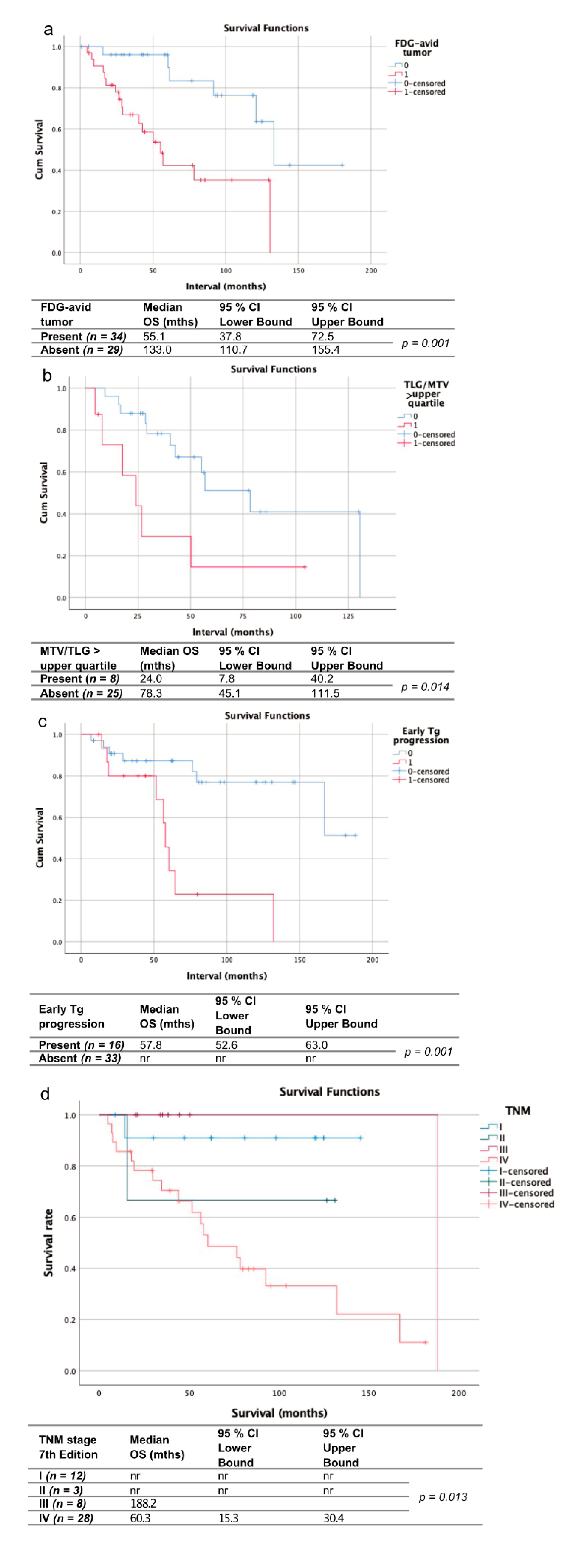
Mean follow-up time after 18F-FDG PET was 57.0 ± 43.9 months. Twenty-one patients of this cohort died during follow-up after a median time (range) of 49.7 ± 49.8 months after initial diagnosis. Neoplastic FDG uptake was present in 31 patients. Mean MTV (TLG) in these patients was 58.1 (1159.3) mL. Any measurable MTV or TLG was significantly associated with shorter OS (median OS: 50.2 vs. 133.0 months; p < 0.005). Values above the upper quartile for MTV (>49.0 mL) and TLG (>229.0 mL) of our cohort were significantly associated with shorter OS (median OS: 29.0 vs. 56.9 months; p < 0.05). Early Tg progression was assessable in 43 patients, and present in 15 (35%), who were significantly associated with shorter OS (57.8 months vs. not reached; p < 0.005). As expected, RAI− patients were overrepresented in the cohort of patients with early Tg progression (93% RAI− vs. 7% RAI+ patients).
OS differed significantly depending on AJCC TNM (7th edition) stage (p = 0.013) with the shortest median OS being observed in patients with AJCC TNM stage IV (60.3 months vs. 188.2 months in grade III; median OS not reached in stages I and II).
Mean follow-up time after initial diagnosis was 61.1 ± 46.8 months. Sixteen patients of this cohort died during follow-up time, after a median time (range) of 39.0 ± 39.8 (3–160) months (14 RAI−, 1 RAI+). Figure 3 gives an overview of all the assessed parameters.
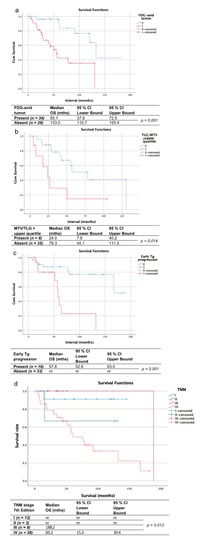
Figure 3.
Kaplan-Meier curves and charts showing the overall survival of patients with vs. without 18F-FDG-avid tumor at initial FDG-PET (a), with metabolic tumor volume/total lesion glycolysis (MTV/TLG) in the upper quartile (b) vs. the remaining patients with FDG-avid tumor, patients with early Tg progression vs. those without (c) and stratified by the AJCC/TNM stage according to the 7th edition (d). nr = not reached
4. Discussion
This study shows a high prevalence of RAI-R disease in patients with PDTC, even at initial diagnosis. 39% of patients without iodine non-avid lesions on initial imaging eventually progress to RAI-R disease, 33% of these progressing within one year. Risk factors for RAI-R PDTC at initial diagnosis or in the course of disease are primary tumor size >40 mm, extrathyroidal extension, and age >55 years. Furthermore, biochemical or structural incomplete response after initial treatment were significantly associated with late (≥12 months) occurrence of RAI-R disease.
The high prevalence of RAI-R disease in PDTC patients with these risk factors calls for the implementation of early cross-sectional imaging for treatment guidance and the evaluation of multimodal treatment. Still, neoplastic RAI uptake was present in 37% of patients and Tg response observed in a considerable fraction of patients. Therefore, PDTC patients should not be automatically precluded from undergoing RAIT.
To the authors’ knowledge, this is the first study to examine the association between RAI-R TC and the aforementioned histopathological or clinical risk factors in PDTC, although prior studies have shown these factors to be generally associated with a poor patient outcome [4,17]. Additionally, a prior study by de la Fouchardiére et al. has shown TERT-mutations to play a significant role in RAI-R PDTC and a higher recurrence rate for PDTC patients with incomplete responses; however, radioiodine-avidity of recurrent lesions was not reported [4].
Our study also shows the potential of TLG and MTV as metrics for poor patient outcome, which is in line with studies on different tumor entities, in which higher MTV and TLG are associated with shorter progression-free survival and overall survival [11,18,19,20,21,22,23,24,25]. Yet, to the authors’ knowledge, this is the first study to specifically analyze PDTC patients. A study by Manohar et al. [11] on RAI-R TC showed a statistically significant association of MTV and TLG > median with progression-free survival and a higher hazard ratio between log-MTV/log-TLG and death. This might be explained by the relatively long overall survival in patients with thyroid cancer. Therefore, depending on the follow-up time a significant association with MTV/TLG might only be observed at the extreme end of the spectrum, which can aide the stratification of patients with a grim vs. intermediate-to-excellent prognosis.
In our study cohort early progression of stimulated Tg after initial RAIT was also significantly associated with shorter OS. Of note, the fraction of RAI-R TC patients among patients without early Tg progression was 30%. A prior study by Wang et al. yielded similar results and shown a significant association between progression of stimulated Tg after the first RAIT on the one hand and the occurrence of RAI-R TC on the other hand [26]. Yet to our knowledge similar analyses have neither been performed in PDTC patients nor with regards to OS. Early Tg progression seems to be of particular value for the stratification of patients with intermediate vs. excellent prognosis. On the other hand, PDTCs can frequently be Tg-negative [27]. Additionally, our cohort patients without a radioiodine avid tumor at initial diagnosis did not undergo a second 124I-PET/CT or RAIT and early progression of stimulated Tg was thus not assessable. RAI− were also overrepresented among patients with early Tg progression, thus these differences might just reflect the different outcome between RAI− and RAI+ patients.
Limitations of this study include the small sample size and its retrospective nature. Therefore, the study results need to be confirmed in prospective studies and larger collectives. Additionally, any ETE was classified as such, with no distinction being made between minimal and macroscopic ETE. As most patients were initially diagnosed before the 2017 revision of the AJCC/TNM classification, the degree of ETE was not assessable in multiple cases. However, a series of studies have indeed shown a negative prognostic impact of minimal ETE [28,29]. Another limitation is the potential misclassification of four patients who underwent 124I PET but not RAIT as RAI-R TC. As shown by Kist et al. [30], radioiodine-avid lesions can be observed on the WBS after RAIT in patients, in whom 124I PET did not reveal any neoplastic iodine-uptake. This was the case in 3/28 patients in our study cohort who underwent both imaging modalities (10.7%), therefore a large impact on the study results seems unlikely.
5. Conclusions
Our results confirm a high prevalence of RAI-R TC in PDTC patients with risk factors, such as primary tumor size >40 mm, or extrathyroidal extension, or age >55 years. Patients with these risk factors should be assessed for the presence of iodine negative lesions and evaluated for the need of multimodal treatment, but not automatically precluded from RAIT. Additionally, early Tg progression, MTV, and TLG appear to be promising metrics to stratify patient prognosis, and subsequently aide treatment planning.
Author Contributions
Conceptualization, D.K. and M.W.; Data curation, L.K. and M.W.; Formal analysis, R.S. and M.W.; Supervision, K.H., S.T., F.W., L.U., D.F.-S., R.G. and C.R.; Visualization, T.B., H.D. and M.W.; Writing—original draft, M.W.; Writing—review & editing, D.K., R.S., L.K., K.H., S.T., T.B., F.W., L.U., D.F.-S., R.G. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Institutional Review Board Statement
The retrospective analysis of available patient data was performed in accordance with the declaration of Helsinki and approved by the local ethics committee (ethics committee, University Duisburg-Essen, faculty of medicine; proposal 20-9379-BO).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy laws but are available from the corresponding author on reasonable request.
Conflicts of Interest
D.K., R.S., L.K., S.T., H.D., F.W., D.F.-S., R.G., C.R., and M.W. have nothing to disclose. L.U. is a Speaker/Advisory Board Member for Bayer Healthcare and received a Siemens Healthcare & Research grant (Siemens Healthcare) outside of the submitted work. K.H. reports personal fees from Bayer, personal fees and other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, outside the submitted work. T.B. received honoraria and/or expenses for invited speeches from Eisai, Lilly, Bayer Pharma and Liberum.
References
- Sanders, E.M., Jr.; Livolsi, V.A.; Brierley, J.; Shin, J.; Randolph, G.W. An evidence-based review of poorly differentiated thyroid cancer. World J. Surg. 2007, 31, 934–945. [Google Scholar] [PubMed]
- Volante, M.; Landolfi, S.; Chiusa, L.; Palestini, N.; Motta, M.; Codegone, A.; Torchio, B.; Papotti, M.G. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns. Cancer 2004, 100, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Kasai, N.; Sugano, H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer 1983, 52, 1849–1855. [Google Scholar] [CrossRef]
- de la Fouchardière, C.; Decaussin-Petrucci, M.; Berthiller, J.; Descotes, F.; Lopez, J.; Lifante, J.-C.; Peix, J.-L.; Giraudet, A.-L.; Delahaye, A.; Masson, S.; et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur. J. Cancer 2018, 92, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Bussolati, G.; Papotti, M. The story of poorly differentiated thyroid carcinoma: From Langhans’ description to the Turin proposal via Juan Rosai. Semin. Diagn. Pathol. 2016, 33, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Underwood, H.J.; Shaha, A.R.; Patel, K.N. Variable response to radioactive iodine treatment in poorly differentiated thyroid carcinoma. Gland. Surg. 2019, 8, 589–590. [Google Scholar] [CrossRef]
- Hiltzik, D.; Carlson, D.L.; Tuttle, R.M.; Chuai, S.; Ishill, N.; Shaha, A.; Shah, J.P.; Singh, B.; Ghossein, R.A. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: A clinicopathologic study of 58 patients. Cancer 2006, 106, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Shah, R.R.; Morganroth, J. Tyrosine Kinase Inhibitors: Their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013, 36, 413–426. [Google Scholar] [CrossRef]
- Shah, R.R.; Morganroth, J.; Shah, D.R. Cardiovascular safety of tyrosine kinase inhibitors: With a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013, 36, 295–316. [Google Scholar] [CrossRef]
- Kreissl, M.C.; Janssen, M.J.; Nagarajah, J. Current Treatment Strategies in Metastasized Differentiated Thyroid Cancer. J. Nucl. Med. 2019, 60, 9–15. [Google Scholar] [CrossRef]
- Manohar, P.M.; Beesley, L.J.; Bellile, E.L.; Worden, F.P.; Avram, A.M. Prognostic Value of FDG-PET/CT Metabolic Parameters in Metastatic Radioiodine-Refractory Differentiated Thyroid Cancer. Clin. Nucl. Med. 2018, 43, 641–647. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Haugen, B.R. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: What is new and what has changed? Cancer 2017, 123, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Jentzen, W.; Hoppenbrouwers, J.; van Leeuwen, P.; van der Velden, D.; van de Kolk, R.; Poeppel, T.D.; Nagarajah, J.; Brandau, W.; Bockisch, A.; Rosenbaum-Krumme, S. Assessment of lesion response in the initial radioiodine treatment of differentiated thyroid cancer using 124I PET imaging. J. Nucl. Med. 2014, 55, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Maxon, H.R., 3rd; Englaro, E.E.; Thomas, S.R.; Hertzberg, V.S.; Hinnefeld, J.D.; Chen, L.S.; Smith, H.; Cummings, D.; Aden, M.D. Radioiodine-131 therapy for well-differentiated thyroid cancer--a quantitative radiation dosimetric approach: Outcome and validation in 85 patients. J. Nucl. Med. 1992, 33, 1132–1136. [Google Scholar]
- Maxon, H.R.; Thomas, S.R.; Hertzberg, V.S.; Kereiakes, J.G.; Chen, I.-W.; Sperling, M.I.; Saenger, E.L. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N. Engl. J. Med. 1983, 309, 937–941. [Google Scholar] [CrossRef]
- Jung, T.S.; Kim, T.Y.; Kim, K.W.; Oh, Y.L.; Park, D.J.; Cho, B.Y.; Shong, Y.K.; Kim, W.B.; Park, Y.J.; Jung, J.H.; et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr. J. 2007, 54, 265–274. [Google Scholar] [CrossRef]
- Bazan, J.G.; Koong, A.C.; Kapp, D.S.; Quon, A.; Graves, E.E.; Loo, B.W.; Chang, D.T. Metabolic tumor volume predicts disease progression and survival in patients with squamous cell carcinoma of the anal canal. J. Nucl. Med. 2013, 54, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Paidpally, V.; Chirindel, A.; Chung, C.H.; Richmon, J.; Koch, W.; Quon, H.; Subramaniam, R.M. FDG volumetric parameters and survival outcomes after definitive chemoradiotherapy in patients with recurrent head and neck squamous cell carcinoma. Am. J. Roentgenol. 2014, 203, W139–W145. [Google Scholar] [CrossRef][Green Version]
- Weber, M.; Kersting, D.; Umutlu, L.; Schäfers, M.; Rischpler, C.; Fendler, W.P.; Buvat, I.; Herrmann, K.; Seifert, R. Just another “Clever Hans”? Neural networks and FDG PET-CT to predict the outcome of patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Soydal, Ç.; Yüksel, C.B.; Küçük, N.Ö.; Ökten, I.; Özkan, E.; Erdoğan, B.D. Prognostic Value of Metabolic Tumor Volume Measured by 18F-FDG PET/CT in Esophageal Cancer Patients. Mol. Imaging Radionucl. Ther. 2014, 23, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Choi, J.Y.; Shim, Y.M.; Kim, K.; Lee, S.J.; Cho, Y.S.; Lee, J.Y.; Lee, K.-H.; Kim, B.-T. Prognostic value of metabolic tumor volume measured by 18f-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann. Surg. Oncol. 2009, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- La, T.H.; Filion, E.J.; Turnbull, B.B.; Chu, J.N.; Lee, P.; Nguyen, K.; Maxim, P.; Quon, A.; Graves, E.E.; Loo, B.W.; et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int. J. Radiat. Oncol. 2009, 74, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.J.; Jeung, H.-C.; Park, H.S.; Park, J.S.; Rha, S.Y.; Choi, H.J.; Lee, J.-H.; Jeon, T.J. Significance of Metabolic Tumor Volume and Total Lesion Glycolysis Measured Using (18)F-FDG PET/CT in Locally Advanced and Metastatic Gallbladder Carcinoma. Yonsei Med. J. 2019, 60, 604–610. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Baba, S.; Endo, M.; Setsu, N.; Iida, K.; Fukushi, J.-I.; Kawaguchi, K.; Okada, S.; Bekki, H.; Isoda, T.; et al. Metabolic Tumor Volume by (18)F-FDG PET/CT Can Predict the Clinical Outcome of Primary Malignant Spine/Spinal Tumors. BioMed Res. Int. 2017, 2017, 8132676. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Li, H.; Li, X.; Lin, Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PLoS ONE 2017, 12, e0179664. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Grewal, R.K.; Larson, S.M. Radioactive iodine therapy in poorly differentiated thyroid cancer. Nat. Clin. Pract. Oncol. 2007, 4, 665–668. [Google Scholar] [CrossRef]
- Moon, H.J.; Kim, E.-K.; Chung, W.Y.; Yoon, J.H.; Kwak, J.Y. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: Is it a real prognostic factor? Ann. Surg. Oncol. 2011, 18, 1916–1923. [Google Scholar] [CrossRef]
- Radowsky, J.S.; Howard, R.S.; Burch, H.B.; Stojadinovic, A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014, 24, 241–244. [Google Scholar] [CrossRef]
- Kist, J.W.; De Keizer, B.; Van Der Vlies, M.; Brouwers, A.H.; Huysmans, D.A.; Van Der Zant, F.M.; Hermsen, R.; Stokkel, M.P.; Hoekstra, O.S.; Vogel, W.V.; et al. 124I PET/CT to Predict the Outcome of Blind 131I Treatment in Patients with Biochemical Recurrence of Differentiated Thyroid Cancer: Results of a Multicenter Diagnostic Cohort Study (THYROPET). J. Nucl. Med. 2015, 57, 701–707. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).