Efficacy of Immune Checkpoint Inhibitors against Advanced or Metastatic Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Definition of Endpoints for Treatment Efficacy
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
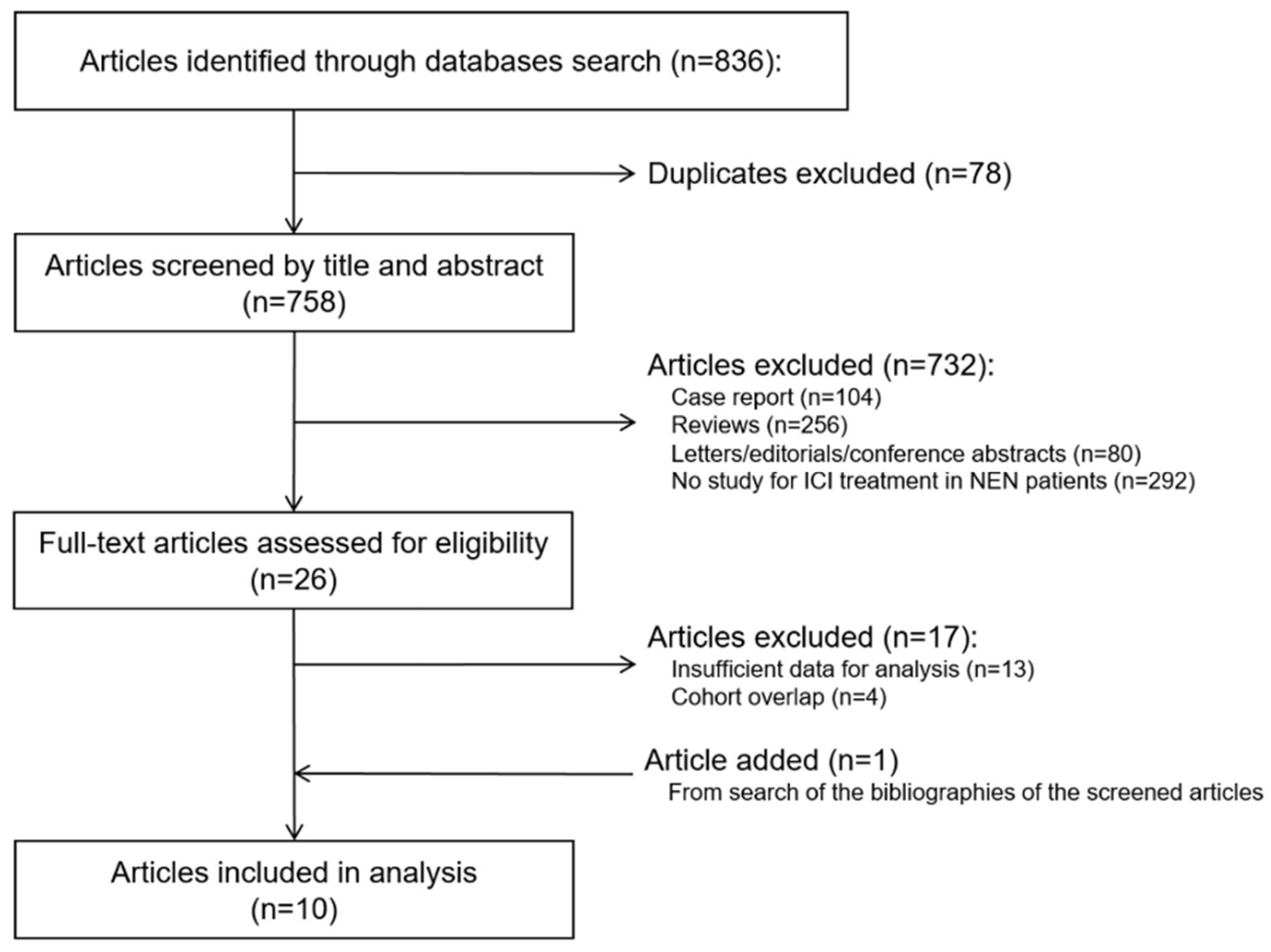
3.1. Study Search and Characteristics
3.2. Meta-Analysis
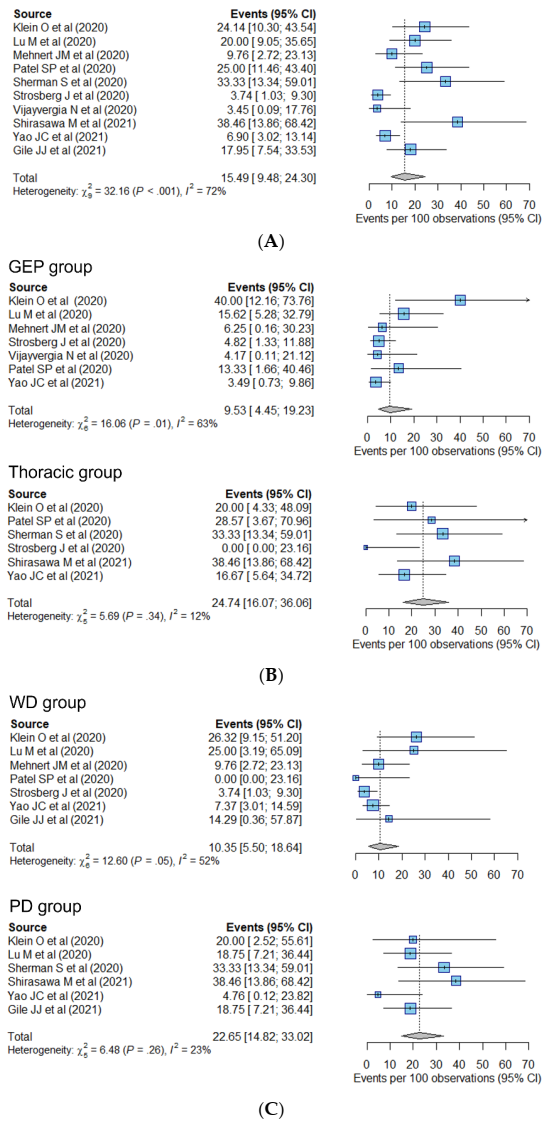
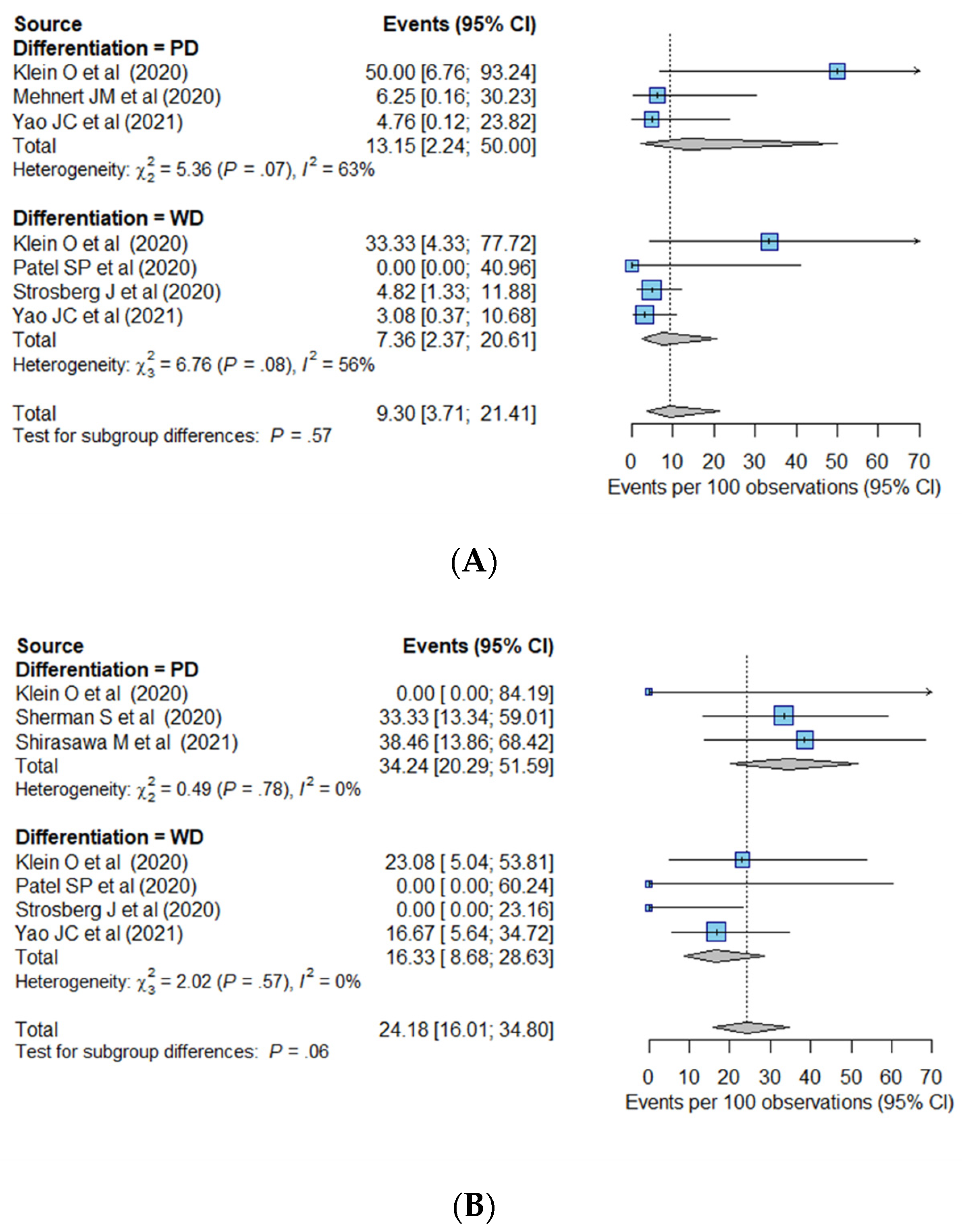
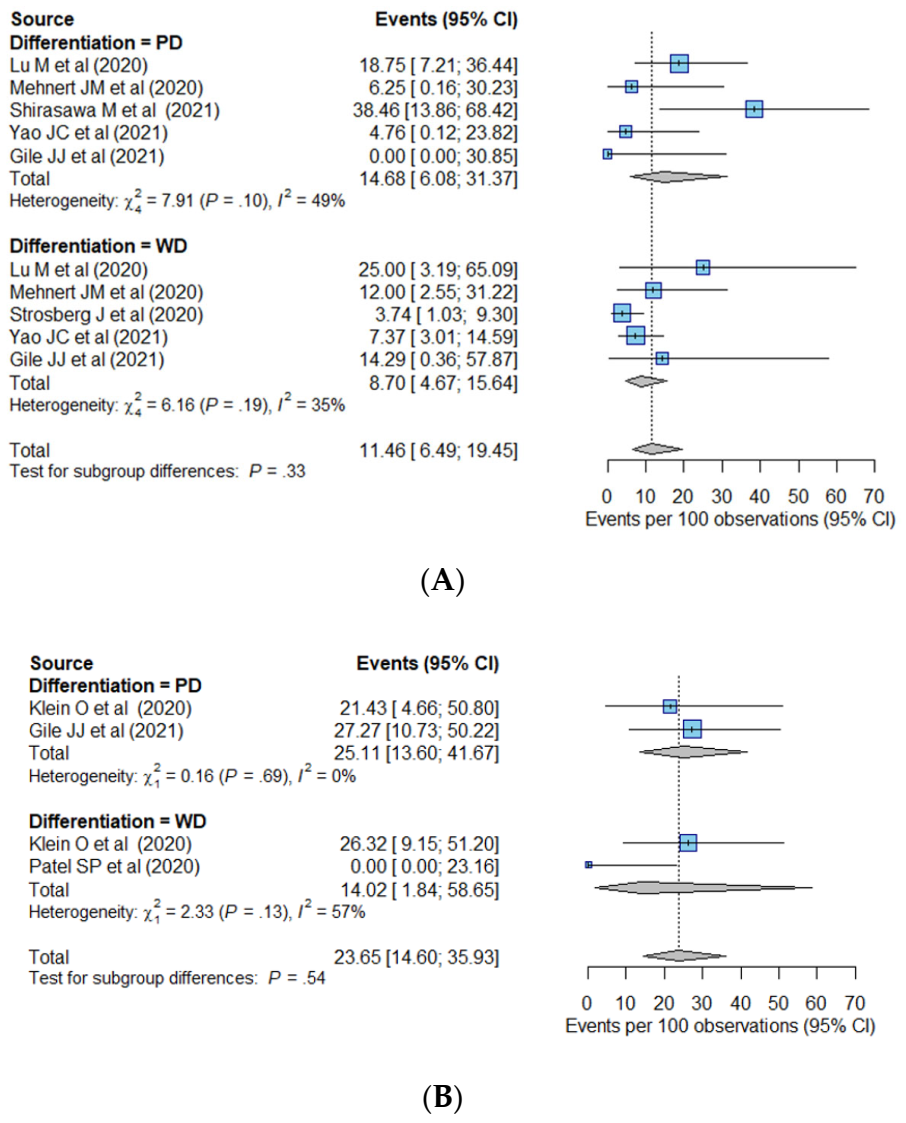
3.2.1. Response-Related Endpoint
3.2.2. Survival-Related Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, I.; Manuzzi, L.; Lamberti, G.; Ricci, A.D.; Tober, N.; Campana, D. Landscape and future perspectives of immunotherapy in neuroendocrine neoplasia. Cancers 2020, 12, 832. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2014, 121, 589–597. [Google Scholar] [CrossRef]
- Chang, J.S.; Chen, L.-T.; Shan, Y.-S.; Chu, P.-Y.; Tsai, C.-R.; Tsai, H.-J. An updated analysis of the epidemiologic trends of neuroendocrine tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef]
- Chauhan, A.; Kohn, E.; Del Rivero, J. Neuroendocrine Tumors-Less Well Known, Often Misunderstood, and Rapidly Growing in Incidence. JAMA Oncol. 2020, 6, 21–22. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Neuroendocrine and Adrenal Tumors (Version 4. 2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 17 December 2021).
- Yoo, C.; Oh, C.R.; Kim, S.T.; Bae, W.K.; Choi, H.J.; Oh, D.Y.; Lee, M.A.; Ryoo, B.Y. Systemic Treatment of Advanced Gastroenteropancreatic Neuroendocrine Tumors in Korea: Literature Review and Expert Opinion. Cancer Res. Treat. 2021, 53, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Klimstra, D.; Klöppel, G.; La Rosa, S.; Rindi, G. WHO Classification of Tumours: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 16–19. [Google Scholar]
- Chan, D.; Freixinos, V.R.; Doherty, M.; Wasson, K.; Iscoe, N.; Raskin, W.; Hallet, J.; Myrehaug, S.; Law, C.; Thawer, A. Avelumab in Unresectable/Metastatic, Progressive, Grade 2–3 Neuroendocrine Neoplasms (NEN): Combined Results From NET-001 and NET-002 Trials. Pancreas 2021, 50, 450. [Google Scholar]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.; Ohrling, K. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, M.D.; Ott, P.A.; Zugazagoitia, J.; Ready, N.E.; Hann, C.L.; De Braud, F.G.; Antonia, S.J.; Ascierto, P.A.; Moreno, V.; Atmaca, A. Nivolumab (nivo) ± ipilimumab (ipi) in advanced small-cell lung cancer (SCLC): First report of a randomized expansion cohort from CheckMate 032. J. Clin. Oncol. 2017, 32, 8503. [Google Scholar] [CrossRef]
- Chung, H.C.; Lopez-Martin, J.A.; Kao, S.C.-H.; Miller, W.H.; Ros, W.; Gao, B.; Marabelle, A.; Gottfried, M.; Zer, A.; Delord, J.-P. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J. Clin. Oncol. 2018, 36, 8506. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Daud, A.; Friedlander, P.; Kluger, H.; Kohrt, H.; Kudchadkar, R.; Lipson, E.; Lundgren, L.; Margolin, K. 22LBA Activity of PD-1 blockade with pembrolizumab as first systemic therapy in patients with advanced Merkel cell carcinoma. Eur. J. Cancer 2015, 3, S720–S721. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred reporting items for a systematic review and meta-analysis of individual participant data: The PRISMA-IPD statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Decis. Mak. 2007, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ulbright, T.; Amin, M.; Balzer, B.; Berney, D.; Epstein, J.; Guo, C.; Idrees, M.; Looijenga, L.; Paner, G.; Rajpert-De Meyts, E. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Gilks, C. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Female Genital Organs; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Villaruz, L.C.; Socinski, M.A. The clinical viewpoint: Definitions, limitations of RECIST, practical considerations of measurement. Clin. Cancer Res. 2013, 15, 2629–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barat, M.; Nguyen, T.T.L.; Hollande, C.; Coty, J.B.; Hoeffel, C.; Terris, B.; Dohan, A.; Mallet, V.; Pol, S.; Soyer, P. LI-RADS v2018 major criteria: Do hepatocellular carcinomas in non-alcoholic steatohepatitis differ from those in virus-induced chronic liver disease on MRI? Eur. J. Radiol. 2021, 138, 109651. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovi, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials Control Clin Trials. Control. Clin. Trials 1986, 177. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P. Bias in meta-analysis detected by a simple, graphical test. Experts’ views are still needed. BMJ 1998, 316, 469. [Google Scholar]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-analysis of individual participant data: Rationale, conduct, and reporting. BMJ 2010, 340. [Google Scholar] [CrossRef] [Green Version]
- Gile, J.J.; Liu, A.J.; McGarrah, P.W.; Eiring, R.A.; Hobday, T.J.; Starr, J.S.; Sonbol, M.B.; Halfdanarson, T.R. Efficacy of Checkpoint Inhibitors in Neuroendocrine Neoplasms: Mayo Clinic Experience. Pancreas 2021, 50, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: A subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z. Efficacy, safety, and biomarkers of Toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: A Multiple-Center phase Ib trial. Clin. Cancer Res. 2020, 26, 2337–2345. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, S.; Rotem, O.; Shochat, T.; Zer, A.; Moore, A.; Dudnik, E. Efficacy of immune check-point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung Cancer 2020, 143, 40–46. [Google Scholar] [CrossRef]
- Shirasawa, M.; Yoshida, T.; Takayanagi, D.; Shiraishi, K.; Yagishita, S.; Sekine, K.; Kanda, S.; Matsumoto, Y.; Masuda, K.; Shinno, Y. Activity and Immune Correlates of Programmed Death-1 Blockade Therapy in Patients with Advanced Large Cell Neuroendocrine Carcinoma. Clin. Lung Cancer 2021, 22, 282–291.e6. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Sabari, J.K.; Julian, R.A.; Ni, A.; Halpenny, D.; Hellmann, M.D.; Drilon, A.E.; Li, B.T.; Poirier, J.T.; Rudin, C.M.; Rekhtman, N. Outcomes of advanced pulmonary large cell neuroendocrine carcinoma stratified by RB1 loss, SLFN11 expression, and tumor mutational burden. J. Clin. Oncol. 2018, 36, e20568. [Google Scholar] [CrossRef]
- Levra, M.G.; Mazieres, J.; Valette, C.A.; Molinier, O.; Planchard, D.; Frappat, V.; Ferrer, L.; Toffart, A.C.; Moro-Sibilot, D. P1. 07-012 efficacy of immune checkpoint inhibitors in large cell neuroendocrine lung cancer: Results from a French retrospective cohort: Topic: Drug treatment alone and in combination with radiotherapy. J. Thorac. Oncol. 2017, 12, S702–S703. [Google Scholar] [CrossRef]
- Forde, P.M.; Hooker, C.M.; Boikos, S.A.; Petrini, I.; Giaccone, G.; Rudin, C.M.; Yang, S.C.; Illei, P.B.; Hann, C.L.; Ettinger, D.S. Systemic therapy, clinical outcomes, and overall survival in locally advanced or metastatic pulmonary carcinoid: A brief report. J. Thorac. Oncol. 2014, 9, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.R.; Wirth, L.J.; Nishino, M.; Chen, A.B.; Sholl, L.M.; Kulke, M.H.; McNamee, C.J.; Jänne, P.A.; Johnson, B.E. Chemotherapy for locally advanced and metastatic pulmonary carcinoid tumors. Lung Cancer 2014, 86, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Niho, S.; Kenmotsu, H.; Sekine, I.; Ishii, G.; Ishikawa, Y.; Noguchi, M.; Oshita, F.; Watanabe, S.-i.; Nakajima, R.; Tada, H. Combination chemotherapy with irinotecan and cisplatin for large-cell neuroendocrine carcinoma of the lung: A multicenter phase II study. J. Thorac. Oncol. 2013, 8, 980–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Treut, J.; Sault, M.; Lena, H.; Souquet, P.; Vergnenegre, A.; Le Caer, H.; Berard, H.; Boffa, S.; Monnet, I.; Damotte, D. Multicentre phase II study of cisplatin-etoposide chemotherapy for advanced large-cell neuroendocrine lung carcinoma: The GFPC 0302 study. Ann. Oncol. 2013, 24, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Niho, S.; Ishii, G.; Hishida, T.; Yoshida, J.; Nishimura, M.; Yoh, K.; Goto, K.; Ohmatsu, H.; Ohe, Y. Clinical features of unresectable high-grade lung neuroendocrine carcinoma diagnosed using biopsy specimens. Lung Cancer 2012, 75, 368–373. [Google Scholar] [CrossRef]
- La Rosa, S.; Uccella, S. Classification of neuroendocrine neoplasms: Lights and shadows. Rev. Endocr. Metab. Disord. 2020, 22, 527–538. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 2018, 36, 633. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Kim, S.T.; Ha, S.Y.; Lee, S.; Ahn, S.; Lee, J.; Park, S.H.; Park, J.O.; Lim, H.Y.; Kang, W.K.; Kim, K.-M. The impact of PD-L1 expression in patients with metastatic GEP-NETs. J. Cancer 2016, 7, 484. [Google Scholar] [CrossRef] [Green Version]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The tumor microenvironment in neuroendocrine tumors: Biology and therapeutic implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Maiorano, B.A.; Azzali, I.; Liverani, C.; Bocchini, M.; Fausti, V.; Di Menna, G.; Grassi, I.; Sansovini, M.; Riva, N.; et al. Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 476. [Google Scholar] [CrossRef]
- Scherer, R.W.; Saldanha, I.J. How should systematic reviewers handle conference abstracts? A view from the trenches. Syst. Rev. 2019, 8, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.A.; Raj, N.P.; Aggarwal, R.R.; Calabrese, S.; DeMore, A.; Dhawan, M.S.; Fattah, D.; Fong, L.; Grabowsky, J.; Hope, T.A. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part B (pembrolizumab + chemotherapy). J. Clin. Oncol. 2021, 39, 4148. [Google Scholar] [CrossRef]
- Fang, L.; Arvind, D.; Dowlati, A.; Mohamed, A. Role of immunotherapy in gastro-enteropancreatic neuroendocrine neoplasms (gep-nens): Current advances and future directions. J. Neuroendocr. 2021, 33, e12943. [Google Scholar] [CrossRef]
- Mulvey, C.; Raj, N.P.; Chan, J.A.; Aggarwal, R.R.; Cinar, P.; Hope, T.A.; Kolli, K.; Zhang, L.; Calabrese, S.; Grabowsky, J.A. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part A (pembrolizumab alone). J. Clin. Oncol. 2019, 37, 363. [Google Scholar] [CrossRef]
- Kulke, M.H.; Wu, B.; Ryan, D.P.; Enzinger, P.C.; Zhu, A.X.; Clark, J.W.; Earle, C.C.; Michelini, A.; Fuchs, C.S. A phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Dig. Dis. Sci. 2006, 51, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, P.W.; Leventakos, K.; Hobday, T.J.; Molina, J.R.; Finnes, H.D.; Westin, G.F.; Halfdanarson, T.R. Efficacy of second-line chemotherapy in extrapulmonary neuroendocrine carcinoma. Pancreas 2020, 49, 529–533. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Design | Sample Size * | Primary Site | Grade/ Differentiation | Prior Systemic Therapy | Details of Prior Systemic Therapy | Disease State | ICI Regimen | Follow-Up † (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Klein (2020) | Phase 2 Clinical Trial | 29 | Lung, thymus, GI, prostate, unknown | WD or PD (Low [n = 3], intermediate [n = 13], high [n = 13]) | 89.7% | Chemotherapy 86% (platinum/etoposide or temozolomide/capecitabine) | Advanced or metastatic | Ipilimumab + nivolumab | Up to 26 |
| Lu (2020) | Phase 1b Clinical Trial | 40 | Pancreas, GI | WD (n = 8), PD (n = 32) | 100% | PRRT 21% | Metastatic, Ki-67 ≥ 10%, PD on prior therapy | Toripalimab | Up to 24 |
| Mehnert (2020) | Phase 1b Clinical Trial | 41 | Pancreas, lung, GI, others | WD | 70.7% | Everolimus 7% | Advanced or metastatic, PD on prior therapy | Pembrolizumab | Up to 24 |
| Patel (2020) | Phase 2 Clinical Trial | 32 | Lung, thymus, GI, cervix, prostate, unknown | WD or PD (low [n = 4], intermediate [n = 10], high [n = 18]) | 100% | Sunitinib 7% | PD on prior therapy and no available standard therapy | Ipilimumab + nivolumab | Up to 15 |
| Sherman (2020) | Retrospective | 18 | Lung | Large cell NEC | NA | Pembrolizumab 3% | Advanced | Monotherapy (nivolumab, pembrolizumab, atezolizumab, or durvalumab) or Combination (nivolumab + Ipilimumab) | 6.2 |
| Shirasawa (2020) | Retrospective | 13 | Lung | Large cell NEC | NA | NA | Advanced or metastatic | Nivolumab or pembrolizumab | NA |
| Strosberg (2020) | Phase 2 Clinical Trial | 107 | Lung, pancreas, GI, liver, ovary, unknown | WD | 97.2% | Chemotherapy 65.9% | Mostly metastatic (106/107), PD on prior therapy | Pembrolizumab | 24.2 |
| Vijayvergia (2020) | Phase 2 Clinical Trial | 29 | Thymus, pancreas, GI, kidney | Grade 3 WD or PD | 100% | Everolimus 31.7% | Advanced or metastatic, PD on prior therapy | Pembrolizumab | Up to 36 |
| Yao (2021) | Phase 2 Clinical Trial | 116 | Lung, thymus, pancreas, GI, gallbladder, unknown | WD (n = 95), PD (n = 21) | 100% | Somatostatin analogues 29.3% | Metastatic, PD on prior therapy | Spartalizumab | 13.4 |
| Gile (2021) | Retrospective | 39 | Pancreas, GI, head and neck, bladder, uterus, gallbladder, unknown | WD (n = 7), PD (n = 32) | NA | Sunitinib 9.8% | Metastatic (81%) | Monotherapy (pembrolizumab, Nivolumab, or atezolizumab) or Combination (nivolumab + ipilimumab) | NEC, 10.7; NET, 79.8 |
| Variables | Pooled ORR (95% CI) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Overall (n = 426) | 15.5% (9.5–24.3%) | - | - | ||
| Primary site | 0.13 | 0.02 | |||
| GEP (n = 266) | 9.5% (4.5–19.2%) | Reference | Reference | ||
| Thoracic (n = 97) | 24.7% (16.1–36.1%) * | 2.33 (0.75–7.22) | 0.13 | 2.59 (1.18–5.72) | |
| Differentiation | 0.08 | 0.03 | |||
| WD (n = 291) | 10.4% (5.5–18.6%) | Reference | Reference | ||
| PD (n = 126) | 22.7% (14.8–33.0%) * | 2.72 (0.88–8.40) | 0.08 | 2.60 (1.11–6.10) | |
| Drug regimen | 0.002 | 0.001 | |||
| Monotherapy (n = 363) | 10.1% (5.0–19.3%) | Reference | Reference | ||
| Combination (n = 83) | 25.3% (17.1–35.7%) * | 5.12 (1.97–13.61) | 0.002 | 4.45 (1.92–10.33) | |
| Survival Data | Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Overall survival | Primary site | 0.06 | 0.37 | ||
| Thoracic (n = 66) | Reference | Reference | |||
| GEP (n = 102) | 2.27 (0.95–5.44) | 1.53 (0.61–3.85) | |||
| Differentiation | <0.001 | <0.001 | |||
| WD (n = 243) | Reference | Reference | |||
| PD (n = 57) | 4.76 (2.40–9.44) * | 4.20 (2.04–8.66) | |||
| Drug regimen | 0.16 | NA | |||
| Monotherapy (n = 306) | Reference | Reference | |||
| Combination (n = 61) | 1.37 (0.88–2.13) | NA | |||
| Progression free survival | Primary site | 0.02 | 0.27 | ||
| Thoracic (n = 86) | Reference | Reference | |||
| GEP (n = 127) | 1.59 (1.07–2.35) | 1.27 (0.83–1.95) | |||
| Differentiation | <0.001 | <0.001 | |||
| WD (n = 276) | Reference | Reference | |||
| PD (n = 65) | 3.00 (1.88–4.79) * | 2.64 (1.59–4.37) | |||
| Drug regimen | 0.51 | NA | |||
| Monotherapy (n = 306) | Reference | Reference | |||
| Combination (n = 61) | 0.78 (0.38–1.61) | NA | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.-J.; Park, H.-J.; Kim, K.-W.; Suh, C.-H.; Yoo, C.; Chae, Y.-K.; Tirumani, S.H.; Ramaiya, N.H. Efficacy of Immune Checkpoint Inhibitors against Advanced or Metastatic Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 794. https://doi.org/10.3390/cancers14030794
Park E-J, Park H-J, Kim K-W, Suh C-H, Yoo C, Chae Y-K, Tirumani SH, Ramaiya NH. Efficacy of Immune Checkpoint Inhibitors against Advanced or Metastatic Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(3):794. https://doi.org/10.3390/cancers14030794
Chicago/Turabian StylePark, Eun-Joo, Hyo-Jung Park, Kyung-Won Kim, Chong-Hyun Suh, Changhoon Yoo, Young-Kwang Chae, Sree Harsha Tirumani, and Nikhil H. Ramaiya. 2022. "Efficacy of Immune Checkpoint Inhibitors against Advanced or Metastatic Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis" Cancers 14, no. 3: 794. https://doi.org/10.3390/cancers14030794
APA StylePark, E.-J., Park, H.-J., Kim, K.-W., Suh, C.-H., Yoo, C., Chae, Y.-K., Tirumani, S. H., & Ramaiya, N. H. (2022). Efficacy of Immune Checkpoint Inhibitors against Advanced or Metastatic Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Cancers, 14(3), 794. https://doi.org/10.3390/cancers14030794







