Long-Term Exposure to Decabromodiphenyl Ether Promotes the Proliferation and Tumourigenesis of Papillary Thyroid Carcinoma by Inhibiting TRß
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cell Proliferation Assays
2.3. Cell Cycle and Cell Apoptosis Determination by Flow Cytometry
2.4. Migration and Invasion Assays
2.5. Quantitative Real-Time PCR (qRT–PCR)
2.6. Subcellular Protein Extraction and Western Blot Analysis
2.7. Immunofluorescence and Confocal Imaging
2.8. Transient Transfection and Dual-Luciferase Reporter Assay
2.9. RNA Sequencing and Bioinformatics Analysis
2.10. Tumour Xenografts
2.11. Histology and Immunohistochemistry
2.12. Statistical Analysis
3. Results
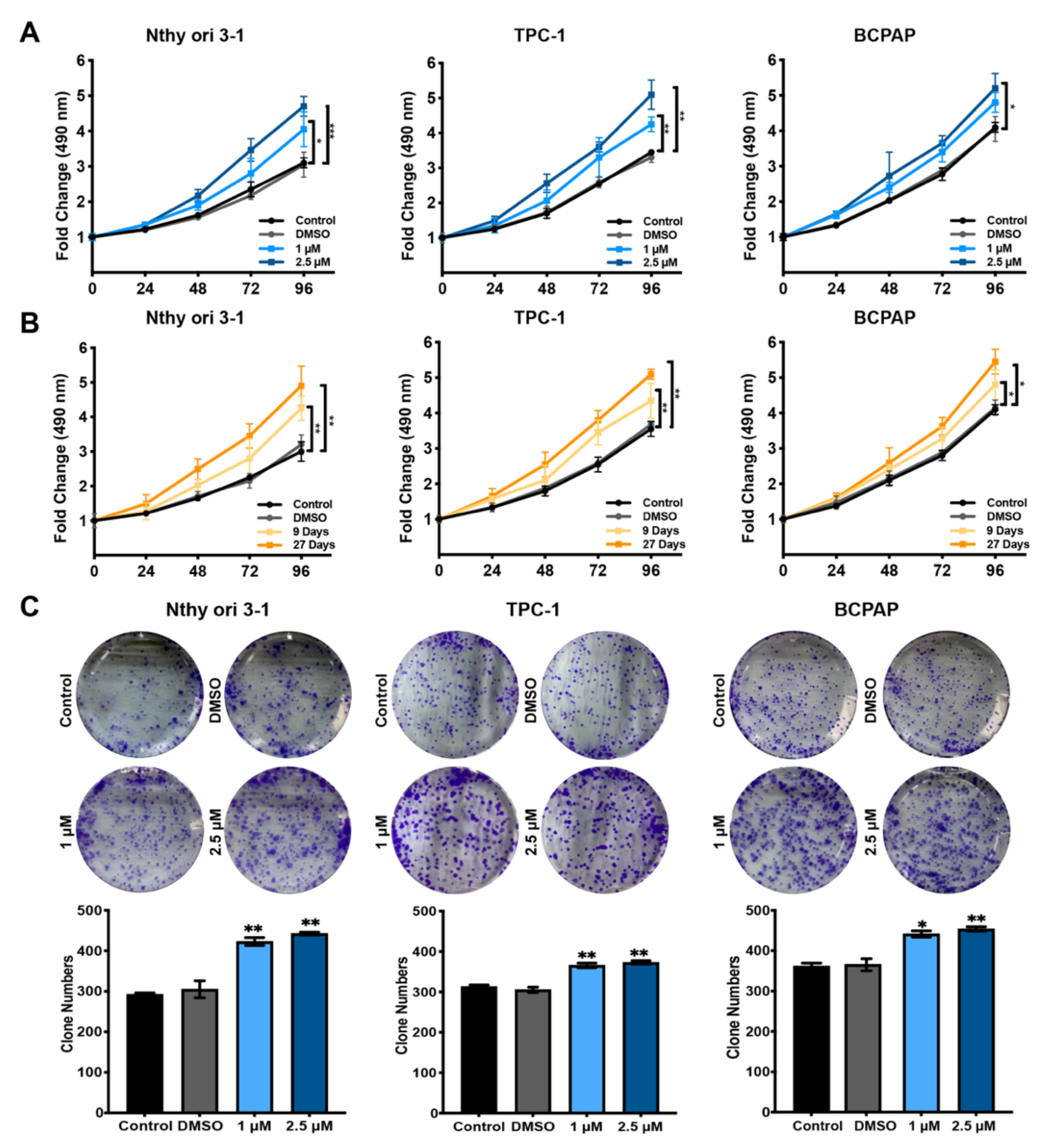
3.1. Hormesis Effect of BDE209 on Nthy-ori 3-1 and PTC Cell Proliferation
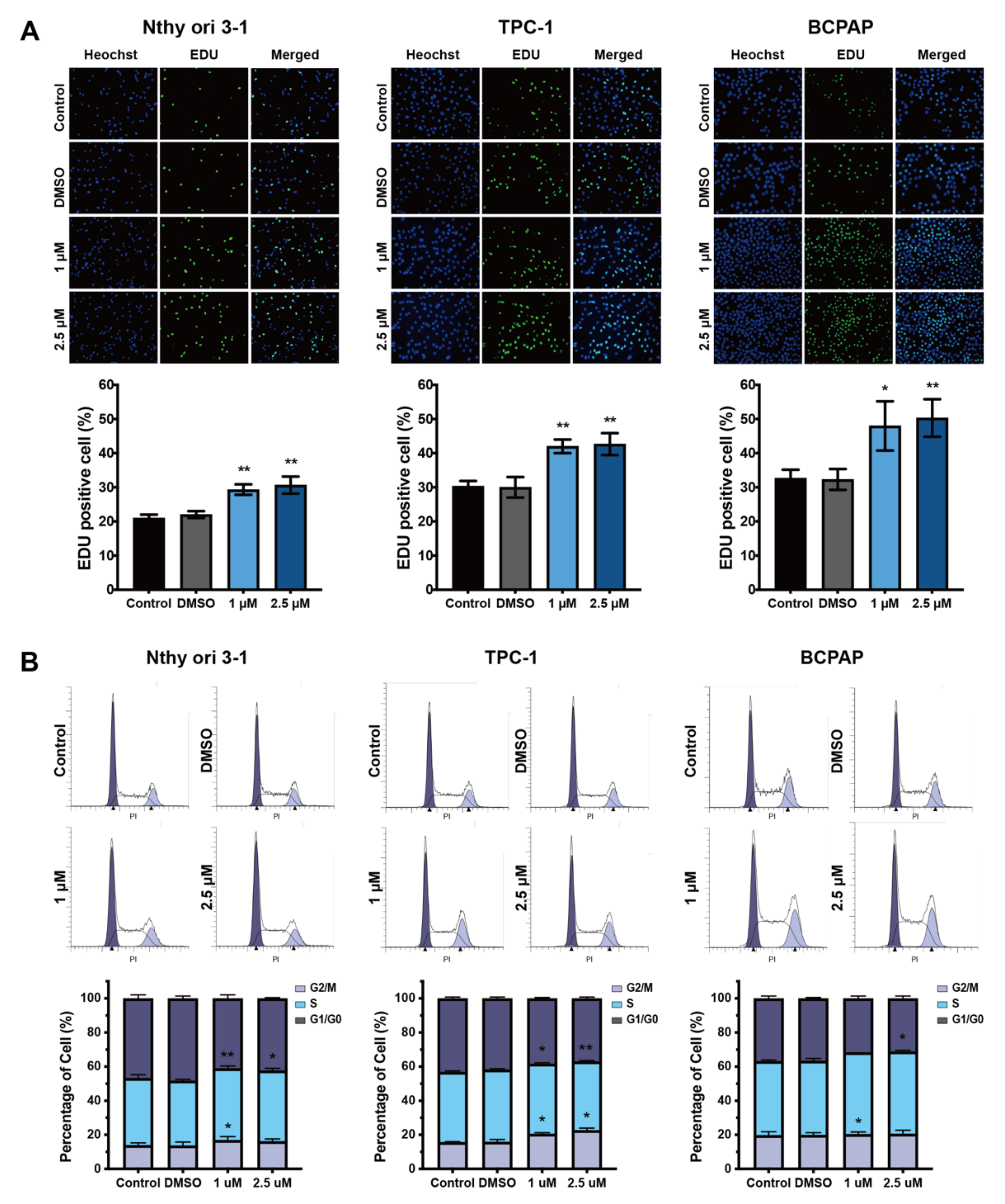
3.2. Cell Cycle Analysis of Nthy-ori 3-1 and PTC Cells after Exposure to BDE209
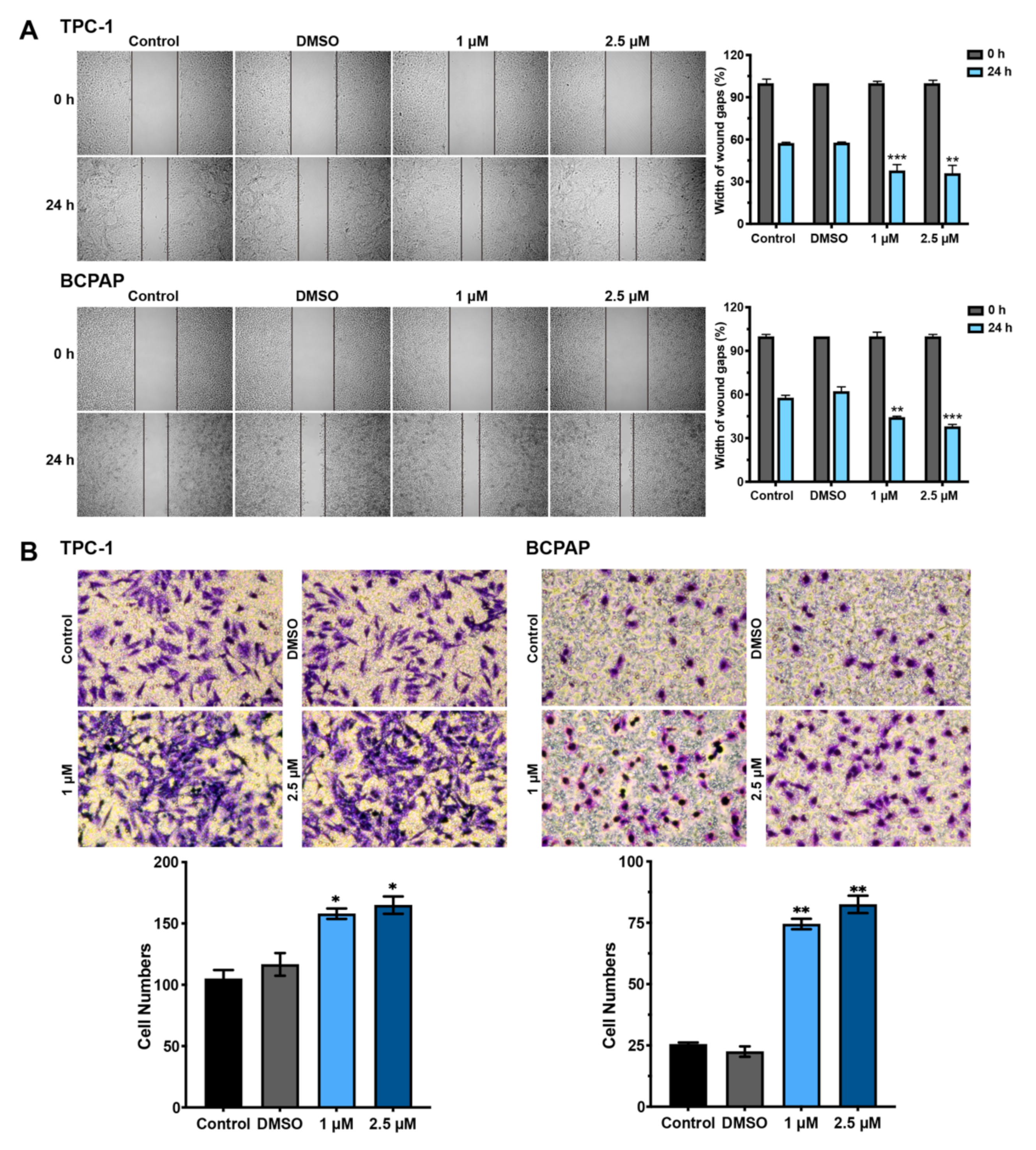
3.3. Hormesis Effect of BDE209 on Nthy-ori 3-1 and PTC Cell Invasion and Migration
3.4. BDE209 Promotes Tumourigenesis by Inhibiting TRß Expression and Function
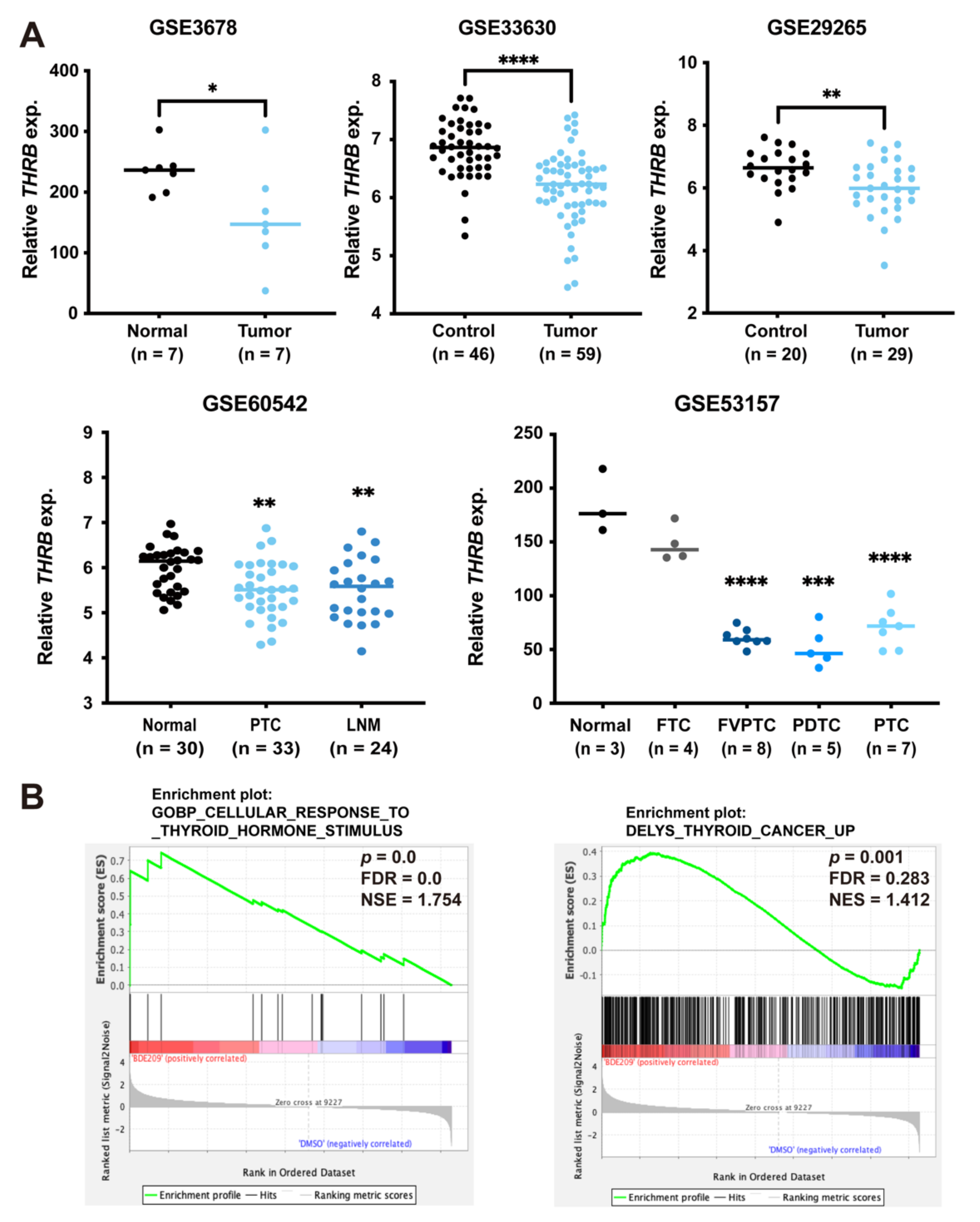
3.5. Inverse Association between Expression of TRß and Thyroid Cancer
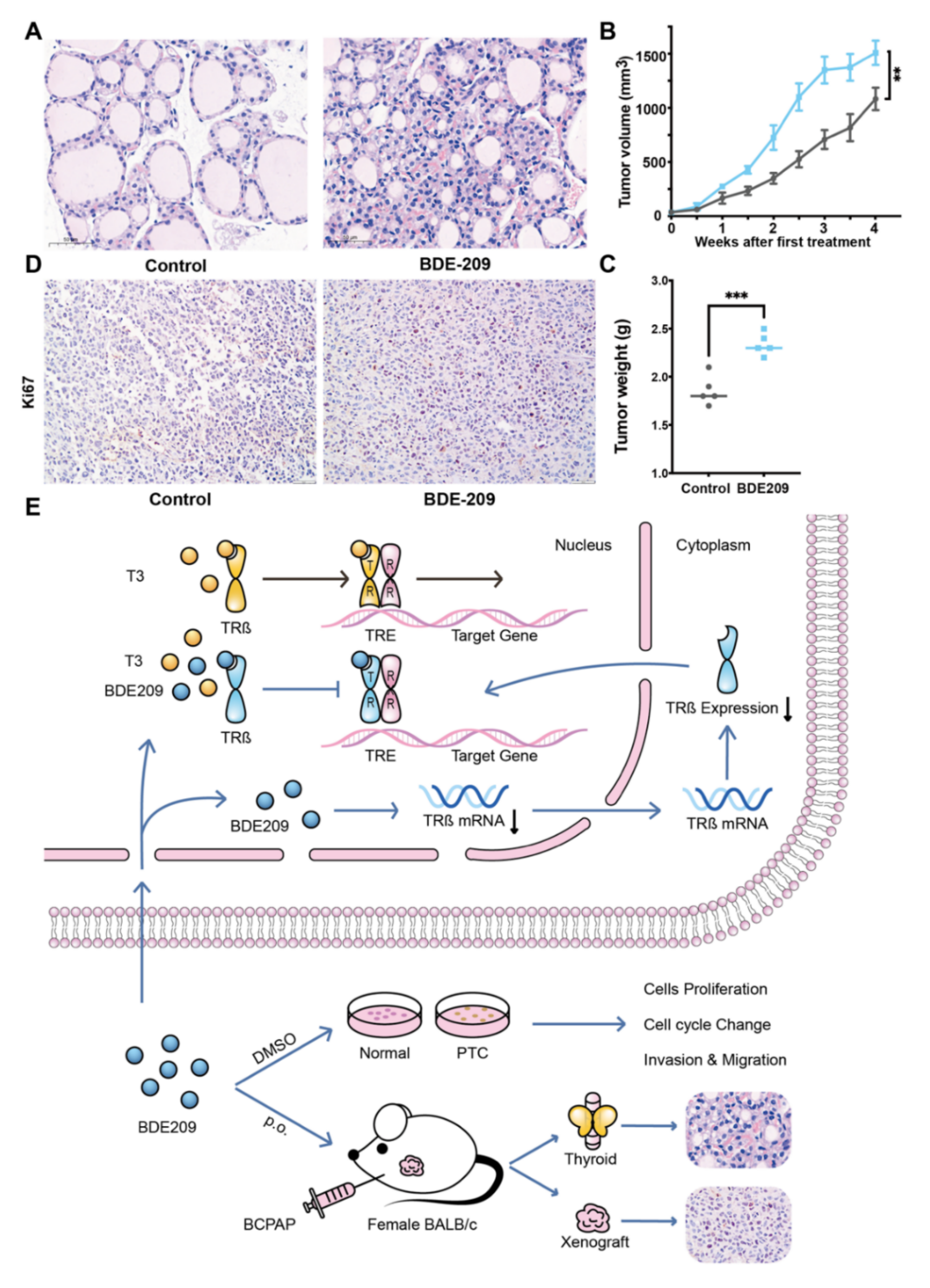
3.6. BDE209 Promotes the Proliferation of Thyroid Follicular Cells and the BCPAP Cells in BALB/c Female Mouse Xenograft Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.S.; Dong, F.; Telatar, M.; Lorch, J.H.; Alexander, E.K.; Marqusee, E.; Cho, N.L.; Nehs, M.A.; Doherty, G.M.; Afkhami, M.; et al. Papillary Thyroid Carcinoma with High-Grade Features Versus Poorly Differentiated Thyroid Carcinoma: An Analysis of Clinicopathologic and Molecular Features and Outcome. Thyroid. Off. J. Am. Thyroid. Assoc. 2021, 31, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Kakudo, K.; Bychkov, A.; Bai, Y.; Li, Y.; Liu, Z.; Jung, C.K. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol. Int. 2018, 68, 641–664. [Google Scholar] [CrossRef] [Green Version]
- Watutantrige-Fernando, S.; Vianello, F.; Barollo, S.; Bertazza, L.; Galuppini, F.; Cavedon, E.; Censi, S.; Benna, C.; Ide, E.C.; Parisi, A.; et al. The Hobnail Variant of Papillary Thyroid Carcinoma: Clinical/Molecular Characteristics of a Large Monocentric Series and Comparison with Conventional Histotypes. Thyroid. Off. J. Am. Thyroid. Assoc. 2018, 28, 96–103. [Google Scholar] [CrossRef]
- Lin, Y.; Qin, S.; Li, Z.; Yang, H.; Fu, W.; Li, S.; Chen, W.; Gao, Z.; Miao, W.; Xu, H.; et al. Apatinib vs Placebo in Patients With Locally Advanced or Metastatic, Radioactive Iodine-Refractory Differentiated Thyroid Cancer: The REALITY Randomized Clinical Trial. JAMA Oncol. 2022, 8, 242–250. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Giordano, T.J. Genomic Hallmarks of Thyroid Neoplasia. Annu. Rev. Pathol. 2018, 13, 141–162. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Li, Y.; Zhou, Z.; Wu, C.; Liu, Z.; Hao, L.; Fan, S.; Jiang, F.; Xie, Y.; et al. Low dose of Bisphenol A enhance the susceptibility of thyroid carcinoma stimulated by DHPN and iodine excess in F344 rats. Oncotarget 2017, 8, 69874–69887. [Google Scholar] [CrossRef] [Green Version]
- Sui, S.; Ng, J.; Gao, Y.; Peng, C.; He, C.; Wang, G.; Liu, Z. Pollution characteristics and chronic health risk assessment of metals and metalloids in ambient PM(2.5) in Licheng District, Jinan, China. Environ. Geochem. Health 2020, 42, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Gao, Y.; Yuan, T.; He, C.; Peng, C.; Wang, Y.; Liu, Z. Pollution characteristics and health risk assessment of PM(2.5)-bound arsenic: A 7-year observation in the urban area of Jinan, China. Environ. Geochem. Health 2022, 1–12. [Google Scholar] [CrossRef]
- Goodman, J.E. Neurodevelopmental effects of decabromodiphenyl ether (BDE-209) and implications for the reference dose. Regul. Toxicol. Pharmacol. 2009, 54, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Yu, J.; Chen, L.; Wang, C.; Zhou, Y.; Hu, Y.; Shi, R.; Zhang, Y.; Cui, C.; Gao, Y.; et al. Polybrominated diphenyl ethers (PBDEs) and thyroid hormones in cord blood. Environ. Pollut. 2017, 229, 489–495. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.; Wester, P.G.; Klamer, H.J.; Lewis, W.E.; Boon, J.P. Do flame retardants threaten ocean life? Nature 1998, 394, 28–29. [Google Scholar] [CrossRef]
- Ikonomou, M.G.; Teas, H.J.; Gerlach, R.; Higgs, D.; Addison, R.F. Residues of PBDEs in northeastern Pacific marine fish: Evidence for spatial and temporal trends. Environ. Toxicol. Chem. 2011, 30, 1261–1271. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, L.; Li, J.; Wu, Y. Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere 2018, 198, 522–536. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, M.; Wu, H.; Niu, Q.; Lu, X.; He, J.; Huang, F. Plasma polybrominated diphenyl ethers, urinary heavy metals and the risk of thyroid cancer: A case-control study in China. Environ. Pollut. 2021, 269, 116162. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Du, Y.; Li, R.; Zhou, T.; Wang, Y.; Chen, T.; Wang, D.; Shi, Z. Polybrominated diphenyl ethers in serum from residents living in a brominated flame retardant production area: Occurrence, influencing factors, and relationships with thyroid and liver function. Environ. Pollut. 2021, 270, 116046. [Google Scholar] [CrossRef]
- Sa, R.; Liang, R.; Qiu, X.; He, Z.; Liu, Z.; Chen, L. Targeting IGF2BP2 Promotes Differentiation of Radioiodine Refractory Papillary Thyroid Cancer via Destabilizing RUNX2 mRNA. Cancers 2022, 14, 1268. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Liu, Z.; Ma, J.; Lin, X.; Qin, Y.; Nishihara, E.; Miyauchi, A.; Kakudo, K. Hashimoto’s Thyroiditis with Increased IgG4-Positive Plasma Cells: Using Thyroid-Specific Diagnostic Criteria May Identify Early Phase IgG4 Thyroiditis. Thyroid 2020, 30, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, X.; Hu, J.; Liu, J.; Wang, X.; Jia, W.; Yu, Z.; Gao, L.; Dou, B.; Zhao, R.; et al. RUVBL1 promotes enzalutamide resistance of prostate tumors through the PLXNA1-CRAF-MAPK pathway. Oncogene 2022. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, W.; Zhang, J.; Liu, J.; Sun, F.; Liu, H.; Hu, J.; Wang, X.; Wang, X.; Su, P.; et al. KIF15-Mediated Stabilization of AR and AR-V7 Contributes to Enzalutamide Resistance in Prostate Cancer. Cancer Res. 2021, 81, 1026–1039. [Google Scholar] [CrossRef] [PubMed]
- Stubbings, W.A.; Harrad, S. Extent and mechanisms of brominated flame retardant emissions from waste soft furnishings and fabrics: A critical review. Environ. Int. 2014, 71, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Li, M.; Jin, J.; Wang, Y.; Bu, Y.; Xu, M.; Yang, X.; Liu, A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ. Toxicol. Chem. 2013, 32, 1242–1247. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human internal and external exposure to PBDEs--a review of levels and sources. Int. J. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef]
- Bi, X.; Thomas, G.O.; Jones, K.C.; Qu, W.; Sheng, G.; Martin, F.L.; Fu, J. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 2007, 41, 5647–5653. [Google Scholar] [CrossRef]
- Gu, H.; Sui, S.; Cui, X.; Han, B.; Zhang, C.; Qi, M.; Li, C.; Liu, Z. Thyroid carcinoma producing β-human chorionic gonadotropin shows different clinical behavior. Pathol. Int. 2018, 68, 207–213. [Google Scholar] [CrossRef]
- Wang, L.; Zou, W.; Zhong, Y.; An, J.; Zhang, X.; Wu, M.; Yu, Z. The hormesis effect of BDE-47 in HepG2 cells and the potential molecular mechanism. Toxicol. Lett. 2012, 209, 193–201. [Google Scholar] [CrossRef]
- Kuriyama, S.N.; Talsness, C.E.; Grote, K.; Chahoud, I. Developmental exposure to low dose PBDE 99: Effects on male fertility and neurobehavior in rat offspring. Environ. Health Perspect. 2005, 113, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Talsness, C.E.; Kuriyama, S.N.; Sterner-Kock, A.; Schnitker, P.; Grande, S.W.; Shakibaei, M.; Andrade, A.; Grote, K.; Chahoud, I. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ. Health Perspect. 2008, 116, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, X.L.; Li, Z.; Li, C.Q.; Zheng, Y.Y.; Xu, L.D.; Chen, J.; Lin, R.; Song, J.; Yu, C.H.; Yue, M.; et al. Probe-based endomicroscopy for in vivo detection of gastric intestinal metaplasia and neoplasia: A multicenter randomized controlled trial. Endoscopy 2017, 49, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Hecht, S.S.; Felton, J.S.; Conney, A.H.; Loeb, L.A. Environmental and chemical carcinogenesis. Semin. Cancer Biol. 2004, 14, 473–486. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.A. A perspective on the potential health risks of PBDEs. Chemosphere 2002, 46, 745–755. [Google Scholar] [CrossRef]
- Meerts, I.A.; van Zanden, J.J.; Luijks, E.A.; van Leeuwen-Bol, I.; Marsh, G.; Jakobsson, E.; Bergman, A.; Brouwer, A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000, 56, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.M.; Guo, L.H.; Gao, Y.; Zhang, B.T.; Wan, B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol. Appl. Pharmacol. 2013, 268, 256–263. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [Green Version]
- Ibhazehiebo, K.; Iwasaki, T.; Kimura-Kuroda, J.; Miyazaki, W.; Shimokawa, N.; Koibuchi, N. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ. Health Perspect. 2011, 119, 168–175. [Google Scholar] [CrossRef]
- Gore, A.C. Endocrine-Disrupting Chemicals. JAMA Intern. Med. 2016, 176, 1705–1706. [Google Scholar] [CrossRef]
- Thomas, G.A.; Williams, E.D. Thyroid stimulating hormone (TSH)-associated follicular hypertrophy and hyperplasia as a mechanism of thyroid carcinogenesis in mice and rats. IARC Sci. Publ. 1999, 147, 45–59. [Google Scholar]
- Crofton, K.M. Thyroid disrupting chemicals: Mechanisms and mixtures. Int. J. Androl. 2008, 31, 209–223. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cui, X.; Zhao, Q.; Sun, F.; Zhao, R.; Feng, T.; Sui, S.; Han, B.; Liu, Z. Long-Term Exposure to Decabromodiphenyl Ether Promotes the Proliferation and Tumourigenesis of Papillary Thyroid Carcinoma by Inhibiting TRß. Cancers 2022, 14, 2772. https://doi.org/10.3390/cancers14112772
Wang X, Cui X, Zhao Q, Sun F, Zhao R, Feng T, Sui S, Han B, Liu Z. Long-Term Exposure to Decabromodiphenyl Ether Promotes the Proliferation and Tumourigenesis of Papillary Thyroid Carcinoma by Inhibiting TRß. Cancers. 2022; 14(11):2772. https://doi.org/10.3390/cancers14112772
Chicago/Turabian StyleWang, Xinpei, Xiujie Cui, Qian Zhao, Feifei Sun, Ru Zhao, Tingting Feng, Shaofeng Sui, Bo Han, and Zhiyan Liu. 2022. "Long-Term Exposure to Decabromodiphenyl Ether Promotes the Proliferation and Tumourigenesis of Papillary Thyroid Carcinoma by Inhibiting TRß" Cancers 14, no. 11: 2772. https://doi.org/10.3390/cancers14112772
APA StyleWang, X., Cui, X., Zhao, Q., Sun, F., Zhao, R., Feng, T., Sui, S., Han, B., & Liu, Z. (2022). Long-Term Exposure to Decabromodiphenyl Ether Promotes the Proliferation and Tumourigenesis of Papillary Thyroid Carcinoma by Inhibiting TRß. Cancers, 14(11), 2772. https://doi.org/10.3390/cancers14112772






