Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. EV Isolation from AGS and SNU-16 Cell Lines
2.3. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.4. Nanoparticle Tracking Analysis (NTA)
2.5. Western Blot Analysis
2.6. EVs Labeling
2.7. EVs Internalization Assay
2.8. Cell Growth Assay
2.9. Migration and Invasion Assays
2.10. Tube Formation Assay
2.11. Matrigel Plug Assay
2.12. Immunohistochemistry (IHC)
2.13. Human Angiogenesis Antibody Array
2.14. Lentiviral Knockdown of ANG2
2.15. Statistical Analysis
3. Results
3.1. ANG2 Is Expressed in Human Gastric Cancer and Omental Metastasis Samples
3.2. Isolation and Characterization of Gastric Cancer-Derived EVs
3.3. Uptake of Gastric Cancer Derived EVs by Endothelial Cells
3.4. Gastric Cancer-Derived EVs Promote Proliferation, Migration, and Invasion of Endothelial Cells
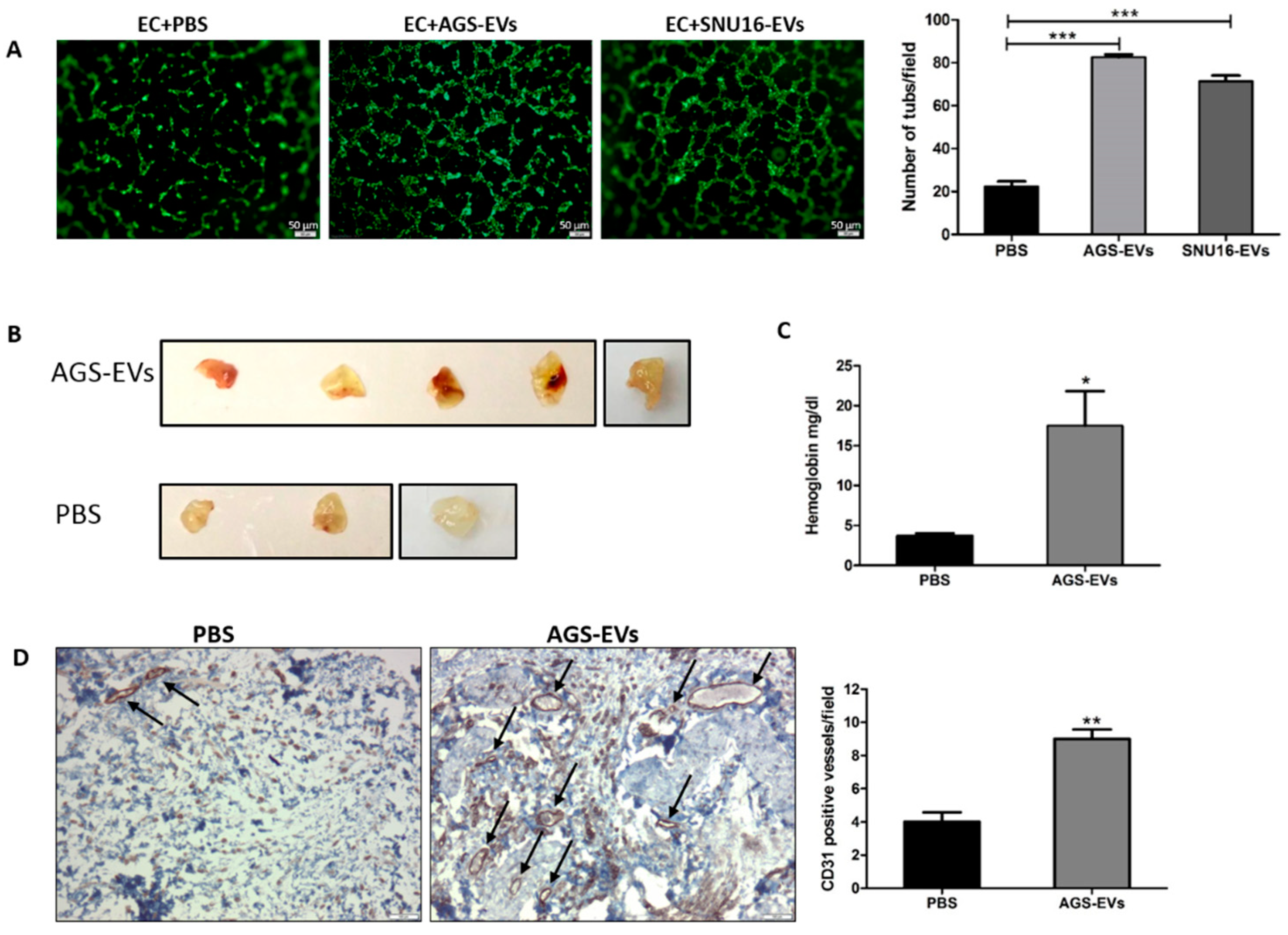
3.5. Gastric Cancer-Derived EVs Promote Tube Formation In Vitro and Blood Vessels In Vivo
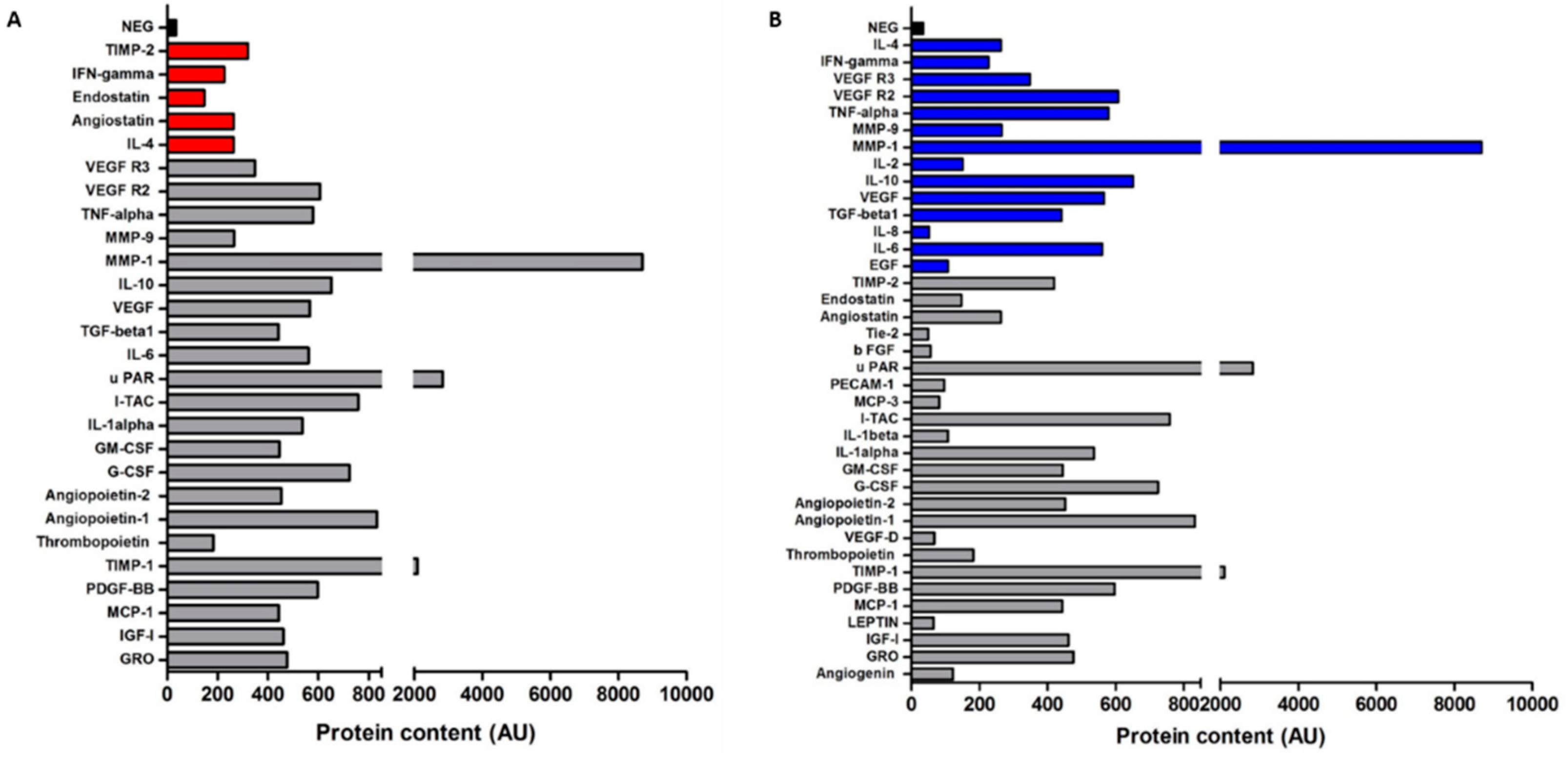
3.6. Gastric Cancer-Derived EVs Contain Various Angiogenic Proteins
3.7. Gastric Cancer-Derived EVs Increase the Expression of ANG2 in Endothelial Cells
3.8. ANG2 Derived from Gastric Cancer EVs Mediates Gastric Cancer Induced Angiogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.S.; Reid, T.D.; White, C.; Willicombe, A.; Blackshaw, G.; Clark, G.W.; Havard, T.J.; Escofet, X.; Crosby, T.D.; Roberts, S.A.; et al. Influence of a regional centralised upper gastrointestinal cancer service model on patient safety, quality of care and survival. Clin. Oncol. 2013, 25, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.D.; Rizzo, A.; Rojas Llimpe, F.L.; Di Fabio, F.; De Biase, D.; Rihawi, K. Novel HER2-Directed Treatments in Advanced Gastric Carcinoma: AnotHER Paradigm Shift? Cancers 2021, 13, 1664. [Google Scholar] [CrossRef] [PubMed]
- Rihawi, K.; Ricci, A.D.; Rizzo, A.; Brocchi, S.; Marasco, G.; Pastore, L.V.; Llimpe, F.L.R.; Golfieri, R.; Renzulli, M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021, 22, 3805. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Ricci, A.D.; Maggio, I.; Massucci, M.; Rojas Limpe, F.L.; Fabio, F.D.; Ardizzoni, A. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Future Oncol. 2020, 16, 4409–4418. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [Green Version]
- Haynes, B.A.; Yang, L.F.; Huyck, R.W.; Lehrer, E.J.; Turner, J.M.; Barabutis, N.; Correll, V.L.; Mathiesen, A.; McPheat, W.; Semmes, O.J.; et al. Endothelial-to-Mesenchymal Transition in Human Adipose Tissue Vasculature Alters the Particulate Secretome and Induces Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2168–2191. [Google Scholar] [CrossRef]
- Thurston, G.; Wang, Q.; Baffert, F.; Rudge, J.; Papadopoulos, N.; Jean-Guillaume, D.; Wiegand, S.; Yancopoulos, G.D.; McDonald, D.M. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development 2005, 132, 3317–3326. [Google Scholar] [CrossRef] [Green Version]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Kontos, C.D.; Annex, B.H.; Popel, A.S. Angiopoietin-Tie Signaling Pathway in Endothelial Cells: A Computational Model. iScience 2019, 20, 497–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, D.; Apte, M. Angiopoietin inhibitors: A review on targeting tumor angiogenesis. Eur. J. Pharmacol. 2021, 899, 174021. [Google Scholar] [CrossRef] [PubMed]
- Etoh, T.; Inoue, H.; Tanaka, S.; Barnard, G.F.; Kitano, S.; Mori, M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001, 61, 2145–2153. [Google Scholar] [PubMed]
- Sun, X.D.; Liu, X.E.; Wu, J.M.; Cai, X.J.; Mou, Y.P.; Li, J.D. Expression and significance of angiopoietin-2 in gastric cancer. World J. Gastroenterol. 2004, 10, 1382–1385. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Leong, A.; Kim, M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int. J. Mol. Sci. 2020, 21, 8689. [Google Scholar] [CrossRef]
- Li, R.; Song, X.; Guo, Y.; Song, P.; Duan, D.; Chen, Z.S. Natural Products: A Promising Therapeutics for Targeting Tumor Angiogenesis. Front. Oncol. 2021, 11, 772915. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Thery, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lam, E.W.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappello, F.; Fais, S. Extracellular vesicles in cancer pros and cons: The importance of the evidence-based medicine. Semin. Cancer Biol. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Gao, Y.; Lin, C.; An, Q.; Feng, Y.; Liu, Y.; Liu, D.; Luo, H.; Wang, D. The Biology and Function of Extracellular Vesicles in Cancer Development. Front. Cell Dev. Biol. 2021, 9, 777441. [Google Scholar] [CrossRef]
- Adem, B.; Vieira, P.F.; Melo, S.A. Decoding the Biology of Exosomes in Metastasis. Trends Cancer 2020, 6, 20–30. [Google Scholar] [CrossRef]
- Gulei, D.; Petrut, B.; Tigu, A.B.; Onaciu, A.; Fischer-Fodor, E.; Atanasov, A.G.; Ionescu, C.; Berindan-Neagoe, I. Exosomes at a glance—Common nominators for cancer hallmarks and novel diagnosis tools. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 564–577. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Liu, C.G.; Goh, R.; Yu, F.; Ma, Z.; Wang, L. Noncoding RNAs of Extracellular Vesicles in Tumor Angiogenesis: From Biological Functions to Clinical Significance. Cells 2022, 11, 947. [Google Scholar] [CrossRef]
- Wu, H.; Fu, M.; Liu, J.; Chong, W.; Fang, Z.; Du, F.; Liu, Y.; Shang, L.; Li, L. The role and application of small extracellular vesicles in gastric cancer. Mol. Cancer 2021, 20, 71. [Google Scholar] [CrossRef]
- Tang, D.; Liu, S.; Shen, H.; Deng, G.; Zeng, S. Extracellular Vesicles Promote the Formation of Pre-Metastasis Niche in Gastric Cancer. Front. Immunol. 2022, 13, 813015. [Google Scholar] [CrossRef] [PubMed]
- Kersy, O.; Loewenstein, S.; Lubezky, N.; Sher, O.; Simon, N.B.; Klausner, J.M.; Lahat, G. Omental Tissue-Mediated Tumorigenesis of Gastric Cancer Peritoneal Metastases. Front. Oncol. 2019, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Koifman, N.; Biran, I.; Aharon, A.; Brenner, B.; Talmon, Y. A direct-imaging cryo-EM study of shedding extracellular vesicles from leukemic monocytes. J. Struct. Biol. 2017, 198, 177–185. [Google Scholar] [CrossRef]
- Issman, L.; Brenner, B.; Talmon, Y.; Aharon, A. Cryogenic transmission electron microscopy nanostructural study of shed microparticles. PLoS ONE 2013, 8, e83680. [Google Scholar] [CrossRef] [Green Version]
- Bellare, J.R.; Davis, H.T.; Scriven, L.E.; Talmon, Y. Controlled environment vitrification system: An improved sample preparation technique. J. Electron Microsc. Tech. 1988, 10, 87–111. [Google Scholar] [CrossRef]
- Hazan-Halevy, I.; Rosenblum, D.; Weinstein, S.; Bairey, O.; Raanani, P.; Peer, D. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 2015, 364, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Paggetti, J.; Haderk, F.; Seiffert, M.; Janji, B.; Distler, U.; Ammerlaan, W.; Kim, Y.J.; Adam, J.; Lichter, P.; Solary, E.; et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 2015, 126, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Nizri, E.; Greenman-Maaravi, N.; Bar-David, S.; Ben-Yehuda, A.; Weiner, G.; Lahat, G.; Klausner, J. Analysis of histological and immunological parameters of metastatic lymph nodes from colon cancer patients reveals that T-helper 1 type immune response is associated with improved overall survival. Medicine 2016, 95, e5340. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Mao, S.; Zhu, H. Comparison of immunohistology using pan-CK and EMA in the diagnosis of lymph node metastasis of gastric cancer, particularly micrometastasis and isolated tumor cells. Oncol. Lett. 2013, 5, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Kang, B.; Kim, O.Y.; Choi, D.S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Schekman, R. Extracellular Vesicles and Cancer: Caveat Lector. Annu. Rev. Cancer Biol. 2018, 2, 395–411. [Google Scholar] [CrossRef]
- Kuriyama, N.; Yoshioka, Y.; Kikuchi, S.; Azuma, N.; Ochiya, T. Extracellular Vesicles Are Key Regulators of Tumor Neovasculature. Front. Cell Dev. Biol. 2020, 8, 611039. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Ge, S.; Ning, T.; Bai, M.; Li, J.; Li, S.; Sun, W.; Deng, T.; Zhang, L.; et al. Exosome-Derived miR-130a Activates Angiogenesis in Gastric Cancer by Targeting C-MYB in Vascular Endothelial Cells. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 2466–2475. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Vera, Y.M.; Marchat, L.A.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Astudillo-De La Vega, H.; Echavarria-Zepeda, R.; Lopez-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2019, 43, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Li, J.; Yang, H.; Zhang, H.; Zhou, Z.; Deng, T.; Zhu, K.; Ning, T.; Fan, Q.; Ying, G.; et al. miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1772–1783. [Google Scholar] [CrossRef]
- Du, J.; Liang, Y.; Li, J.; Zhao, J.M.; Wang, Z.N.; Lin, X.Y. Gastric Cancer Cell-Derived Exosomal microRNA-23a Promotes Angiogenesis by Targeting PTEN. Front. Oncol. 2020, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, S.; Du, K.; Zheng, N.; Liu, Y.; Chen, H.; Xie, G.; Ma, Y.; Zhou, Y.; Zheng, Y.; et al. Gastric cancer-secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2021, 112, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Huang, J.; Yu, K.; Chen, X.; He, Y.; Qi, D.; Wu, Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer 2020, 20, 996. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Delle Monache, S.; Di Francesco, M.; Sanita, P.; D’Ascenzo, S.; Gravina, G.L.; Festuccia, C.; Dolo, V. From glioblastoma to endothelial cells through extracellular vesicles: Messages for angiogenesis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 12743–12753. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Ekstrom, E.J.; Bergenfelz, C.; von Bulow, V.; Serifler, F.; Carlemalm, E.; Jonsson, G.; Andersson, T.; Leandersson, K. WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 2014, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.K.; Zhang, H.; Liu, P.; Tsao, S.W.; Lung, M.L.; Mak, N.K.; Ngok-Shun Wong, R.; Ying-Kit Yue, P. Proteomic analysis of exosomes from nasopharyngeal carcinoma cell identifies intercellular transfer of angiogenic proteins. Int. J. Cancer 2015, 137, 1830–1841. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Huang, Q.; Chen, Y.; Wang, Q.; Sang, R.; Wang, L.; Xie, Y.; Chen, W. Tumor-Derived Extracellular Vesicles Regulate Cancer Progression in the Tumor Microenvironment. Front. Mol. Biosci. 2021, 8, 796385. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Hacker, U.T.; Escalona-Espinosa, L.; Consalvo, N.; Goede, V.; Schiffmann, L.; Scherer, S.J.; Hedge, P.; Van Cutsem, E.; Coutelle, O.; Buning, H. Evaluation of Angiopoietin-2 as a biomarker in gastric cancer: Results from the randomised phase III AVAGAST trial. Br. J. Cancer 2016, 114, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.Y.; Wei, J.X.; Lv, L.H.; Han, Q.F.; Yang, W.B.; Li, G.L.; Wang, P.X.; Wu, S.B.; Duan, J.X.; Zhuo, W.F.; et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun. Signal. CCS 2020, 18, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanishi, Y.; Hu, B.; Jarzynka, M.J.; Guo, P.; Elishaev, E.; Bar-Joseph, I.; Cheng, S.Y. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007, 67, 4254–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, A.J.; Wikstrom, P.; Granfors, T.; Egevad, L.; Stattin, P.; Bergh, A. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. Prostate 2005, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalfon, T.; Loewenstein, S.; Gerstenhaber, F.; Leibou, S.; Geller, H.; Sher, O.; Nizri, E.; Lahat, G. Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2. Cancers 2022, 14, 2953. https://doi.org/10.3390/cancers14122953
Kalfon T, Loewenstein S, Gerstenhaber F, Leibou S, Geller H, Sher O, Nizri E, Lahat G. Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2. Cancers. 2022; 14(12):2953. https://doi.org/10.3390/cancers14122953
Chicago/Turabian StyleKalfon, Talya, Shelly Loewenstein, Fabian Gerstenhaber, Stav Leibou, Hen Geller, Osnat Sher, Eran Nizri, and Guy Lahat. 2022. "Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2" Cancers 14, no. 12: 2953. https://doi.org/10.3390/cancers14122953
APA StyleKalfon, T., Loewenstein, S., Gerstenhaber, F., Leibou, S., Geller, H., Sher, O., Nizri, E., & Lahat, G. (2022). Gastric Cancer-Derived Extracellular Vesicles (EVs) Promote Angiogenesis via Angiopoietin-2. Cancers, 14(12), 2953. https://doi.org/10.3390/cancers14122953




