N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from Liver Metastases of Ductal Adenocarcinoma of the Pancreas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. Antibodies and Reagents
2.3. Immunofluorescence Microscopy
2.4. Immunoblot Analysis
2.5. Immunohistochemistry
2.6. Statistics
3. Results
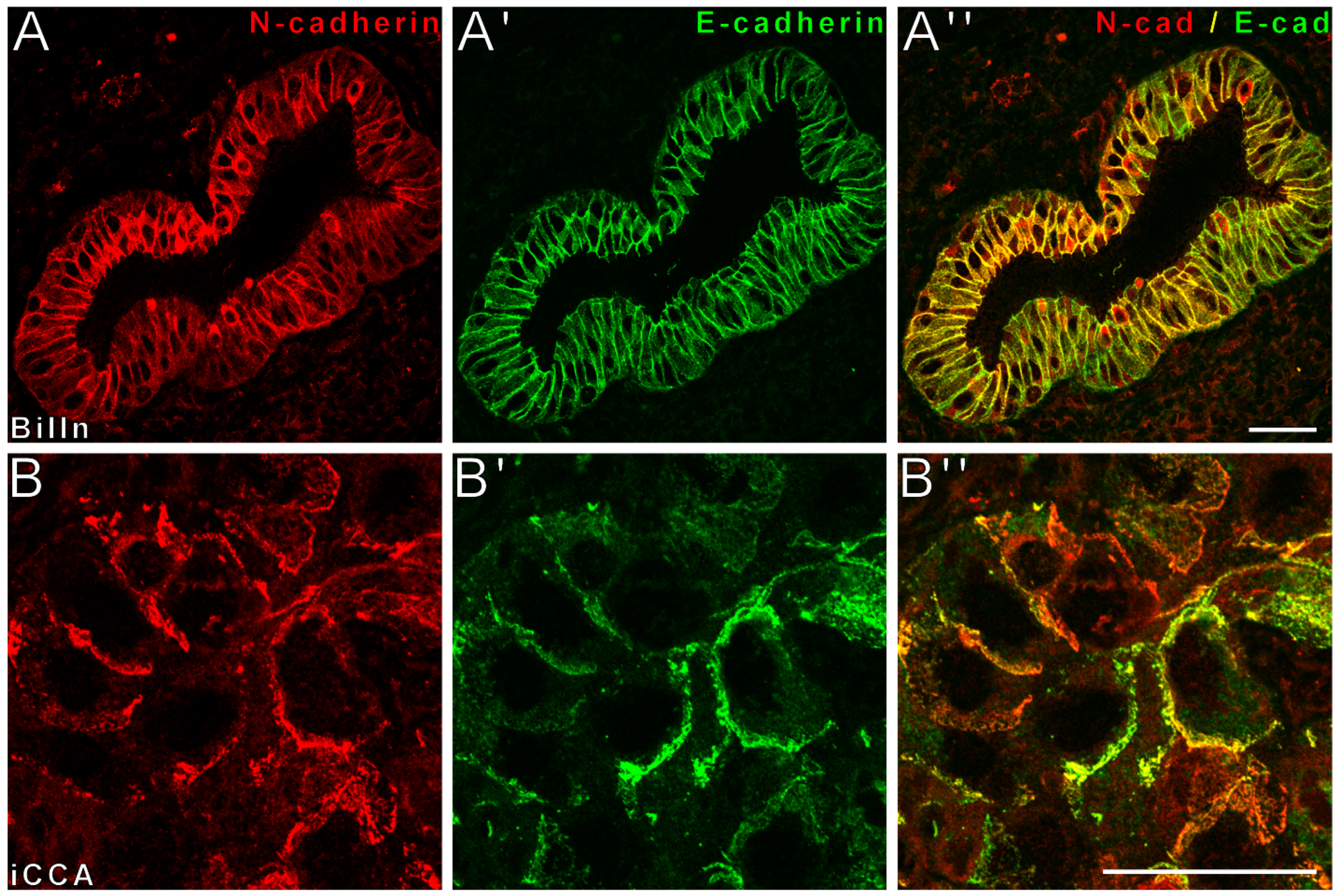
3.1. Colocalization of E- and N-Cadherin Characterizes Epithelia of the Physiological Biliary Tract
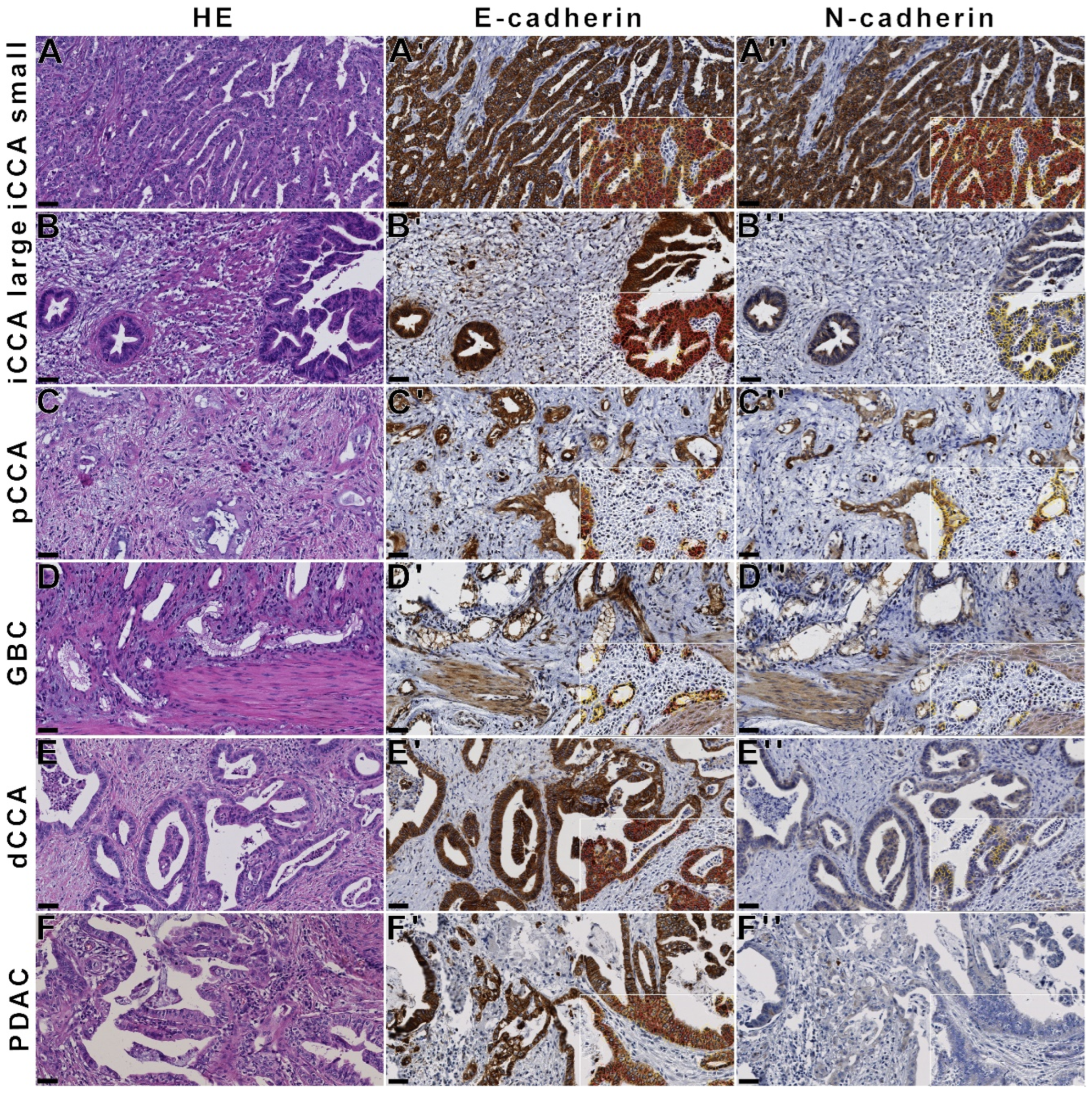
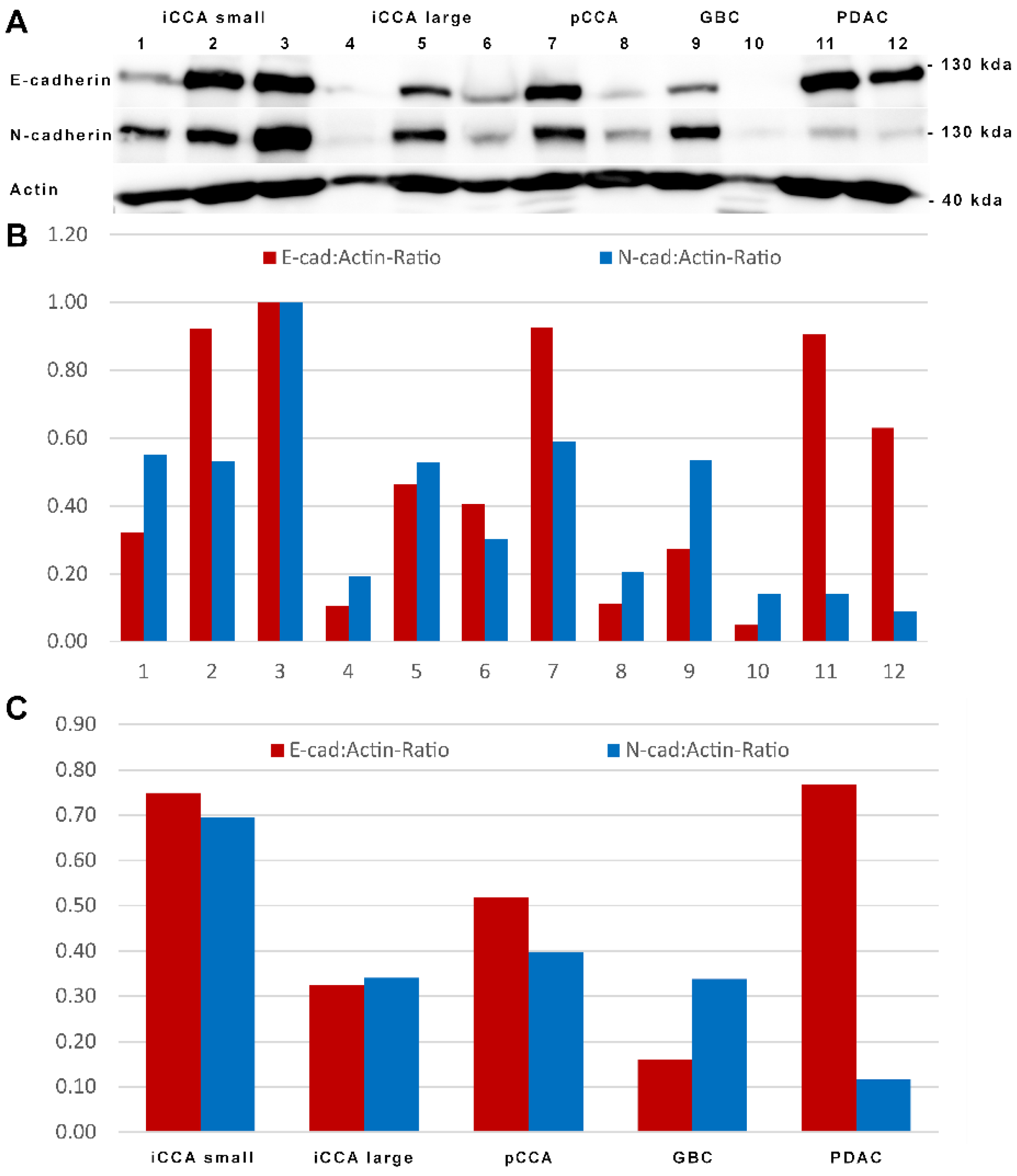
3.2. N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from PDAC
3.3. Expression of E- and N-Cadherin in BTC Is Retained in Respective Metastases
3.4. Significance of E- and N-Cadherin for the Prognosis of Patients with Carcinomas of the Pancreatobiliary System
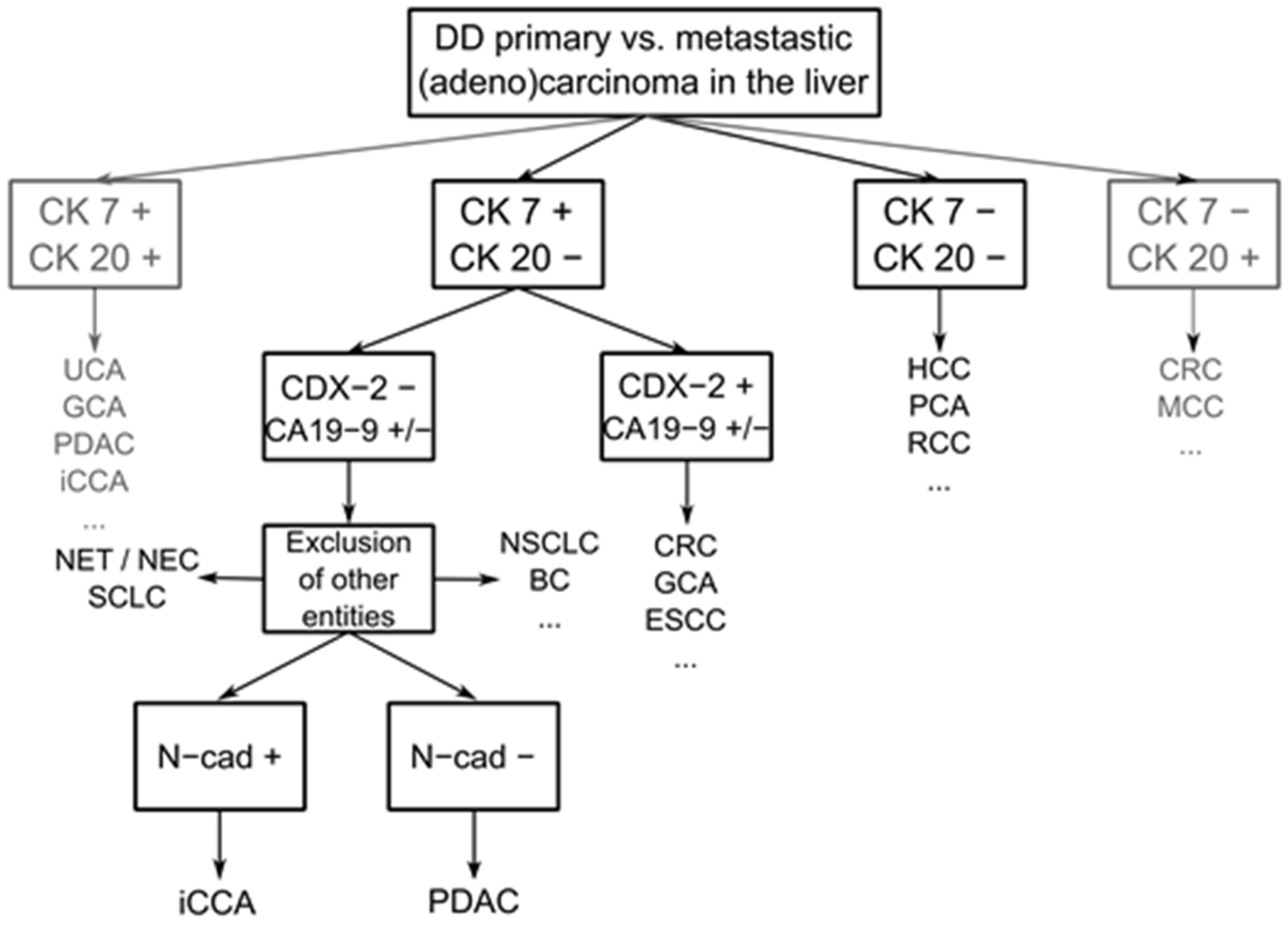
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Löhr, J.M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmuck, R.B.; de Carvalho-Fischer, C.V.; Neumann, C.; Pratschke, J.; Bahra, M. Distal bile duct carcinomas and pancreatic ductal adenocarcinomas: Postulating a common tumor entity. Cancer Med. 2016, 5, 88–99. [Google Scholar] [CrossRef]
- Lok, T.; Chen, L.; Lin, F.; Wang, H. Immunohistochemical distinction between intrahepatic cholangiocarcinoma and pancreatic ductal adenocarcinoma. Hum. Pathol. 2014, 45, 394–400. [Google Scholar] [CrossRef] [Green Version]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Digestive System Tumours: WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2019; Volume 1. [Google Scholar]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Zaccari, P.; Cardinale, V.; Severi, C.; Pedica, F.; Carpino, G.; Gaudio, E.; Doglioni, C.; Petrone, M.C.; Alvaro, D.; Arcidiacono, P.G.; et al. Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World J. Gastroenterol. 2019, 25, 4343–4359. [Google Scholar] [CrossRef]
- Chu, P.G.; Schwarz, R.E.; Lau, S.K.; Yen, Y.; Weiss, L.M. Immunohistochemical Staining in the Diagnosis of Pancreatobiliary and Ampulla of Vater Adenocarcinoma: Application of CDX2, CK17, MUC1, and MUC2. Am. J. Surg. Pathol. 2005, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Rudini, N.; Dejana, E. Adherens junctions. Curr. Biol. 2008, 18, R1080–R1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasioukhin, V. Adherens junctions and cancer. Subcell. Biochem. 2012, 60, 379–414. [Google Scholar] [CrossRef] [PubMed]
- Brasch, J.; Harrison, O.J.; Honig, B.; Shapiro, L. Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol. 2012, 22, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Nelson, W.J. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 2008, 36, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, O. Evolution of the cadherin-catenin complex. Subcell. Biochem. 2012, 60, 9–35. [Google Scholar] [CrossRef]
- Stemmler, M.P. Cadherins in development and cancer. Mol. Biosyst. 2008, 4, 835–850. [Google Scholar] [CrossRef]
- Kourtidis, A.; Lu, R.; Pence, L.J.; Anastasiadis, P.Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017, 358, 78–85. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Pagano, R.E.; Takeichi, M. Adhesion of phospholipid vesicles to Chinese hamster fibroblasts. Role of cell surface proteins. J. Cell Biol. 1977, 74, 531–546. [Google Scholar] [CrossRef] [Green Version]
- Volk, T.; Geiger, B. A 135-kd membrane protein of intercellular adherens junctions. EMBO J. 1984, 3, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development 1988, 102, 639–655. [Google Scholar] [CrossRef]
- Riethmacher, D.; Brinkmann, V.; Birchmeier, C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc. Natl. Acad. Sci. USA 1995, 92, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Radice, G.L.; Rayburn, H.; Matsunami, H.; Knudsen, K.A.; Takeichi, M.; Hynes, R.O. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997, 181, 64–78. [Google Scholar] [CrossRef]
- Peralta Soler, A.; Knudsen, K.A.; Jaurand, M.C.; Johnson, K.R.; Wheelock, M.J.; Klein-Szanto, A.J.; Salazar, H. The differential expression of N-cadherin and E-cadherin distinguishes pleural mesotheliomas from lung adenocarcinomas. Hum. Pathol. 1995, 26, 1363–1369. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Value of E-cadherin and N-cadherin immunostaining in the diagnosis of mesothelioma. Hum. Pathol. 2003, 34, 749–755. [Google Scholar] [CrossRef]
- Moll, R.; Mitze, M.; Frixen, U.H.; Birchmeier, W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am. J. Pathol. 1993, 143, 1731–1742. [Google Scholar]
- Oda, T.; Kanai, Y.; Oyama, T.; Yoshiura, K.; Shimoyama, Y.; Birchmeier, W.; Sugimura, T.; Hirohashi, S. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc. Natl. Acad. Sci. USA 1994, 91, 1858–1862. [Google Scholar] [CrossRef] [Green Version]
- Straub, B.K.; Rickelt, S.; Zimbelmann, R.; Grund, C.; Kuhn, C.; Iken, M.; Ott, M.; Schirmacher, P.; Franke, W.W. E-N-cadherin heterodimers define novel adherens junctions connecting endoderm-derived cells. J. Cell Biol. 2011, 195, 873–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, J.E.; Morgan, T.K.; Grompe, M.; Sheppard, B.C.; Troxell, M.L.; Corless, C.L.; Streeter, P.R. The novel monoclonal antibody HPC2 and N-cadherin distinguish pancreatic ductal adenocarcinoma from cholangiocarcinoma. Hum. Pathol. 2012, 43, 1583–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akita, M.; Sawada, R.; Komatsu, M.; Suleman, N.; Itoh, T.; Ajiki, T.; Heaton, N.; Fukumoto, T.; Zen, Y. An immunostaining panel of C-reactive protein, N-cadherin, and S100 calcium binding protein P is useful for intrahepatic cholangiocarcinoma subtyping. Hum. Pathol. 2021, 109, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, J.F.; Kandel, C.; Cazals-Hatem, D.; Bou-Hanna, C.; Gournay, J.; Jarry, A.; Laboisse, C.L. N-cadherin serves as diagnostic biomarker in intrahepatic and perihilar cholangiocarcinomas. Mod. Pathol. 2009, 22, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.S.; Bartsch, F.; Wagner, D.C.; Schindeldecker, M.; Heuft, L.-K.; Roth, W.; Lang, H.; Straub, B.K. Clinicopathological Significance of Syndecan-1 in Cholangiocarcinoma: A Study Based on Immunohistochemistry and Public Sequencing Data. J. Clin. Med. 2021, 10, 2745. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [Green Version]
- McCarty, K.S., Jr.; Szabo, E.; Flowers, J.L.; Cox, E.B.; Leight, G.S.; Miller, L.; Konrath, J.; Soper, J.T.; Budwit, D.A.; Creasman, W.T.; et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986, 46, 4244s–4248s. [Google Scholar]
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Farshidfar, F.; Zheng, S.; Gingras, M.C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef] [PubMed]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Győrffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, W. Does McNemar’s test compare the sensitivities and specificities of two diagnostic tests? Stat. Methods Med. Res. 2017, 26, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.H.; Yuan, R.H.; Chen, Y.L.; Yang, W.C.; Hsu, H.C.; Jeng, Y.M. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Mod. Pathol. 2011, 24, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Lei, H.J.; Chen, M.H.; Ho, H.L.; Chiu, L.Y.; Li, C.P.; Wang, Y.C. C-Reactive Protein (CRP) is a Promising Diagnostic Immunohistochemical Marker for Intrahepatic Cholangiocarcinoma and Is Associated with Better Prognosis. Am. J. Surg. Pathol. 2017, 41, 1630–1641. [Google Scholar] [CrossRef]
- Brackett, D.G.; Neyaz, A.; Arora, K.; Masia, R.; Mattia, A.; Zukerberg, L.; Misdraji, J.; Goyal, L.; Zhu, A.X.; Ferrone, C.R.; et al. Cholangiolar pattern and albumin in situ hybridisation enable a diagnosis of intrahepatic cholangiocarcinoma. J. Clin. Pathol. 2020, 73, 23–29. [Google Scholar] [CrossRef]
- Sigel, C.S.; Drill, E.; Zhou, Y.; Basturk, O.; Askan, G.; Pak, L.M.; Vakiani, E.; Wang, T.; Boerner, T.; Do, R.K.G.; et al. Intrahepatic Cholangiocarcinomas Have Histologically and Immunophenotypically Distinct Small and Large Duct Patterns. Am. J. Surg. Pathol. 2018, 42, 1334–1345. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, T.; Zhang, Q.; Fu, Q.; Hu, Y.; Xiang, M.; Peng, H.; Zheng, T.; Lu, L.; Shi, H. Upregulated N-cadherin expression is associated with poor prognosis in epithelial-derived solid tumours: A meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12903. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Lammens, T.; Swerts, K.; Derycke, L.; De Craemer, A.; De Brouwer, S.; De Preter, K.; Van Roy, N.; Vandesompele, J.; Speleman, F.; Philippé, J.; et al. N-cadherin in neuroblastoma disease: Expression and clinical significance. PLoS ONE 2012, 7, e31206. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, F.J.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z. E-cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta 2012, 1826, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, W.W.; Rickelt, S. Mesenchymal–epithelial transitions: Spontaneous and cumulative syntheses of epithelial marker molecules and their assemblies to novel cell junctions connecting human hematopoietic tumor cells to carcinomatoid tissue structures. Int. J. Cancer 2011, 129, 2588–2599. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F. Beyond E-cadherin: Roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 2014, 14, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, A.M.; Na, T.Y.; Gumbiner, B.M. E-cadherin in contact inhibition and cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef] [PubMed]
- Techasen, A.; Loilome, W.; Namwat, N.; Khuntikeo, N.; Puapairoj, A.; Jearanaikoon, P.; Saya, H.; Yongvanit, P. Loss of E-cadherin promotes migration and invasion of cholangiocarcinoma cells and serves as a potential marker of metastasis. Tumour Biol. 2014, 35, 8645–8652. [Google Scholar] [CrossRef]
- Jiang, F.; Fang, D.B.; Lin, J.; Chen, Q.; Zhu, L.X.; Yu, H.Z. Correlation of LARP1 and E-cadherin expression with prognosis of intrahepatic cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 3559–3566. [Google Scholar]
- Mao, X.; Chen, D.; Wu, J.; Li, J.; Zhou, H.; Wu, Y.; Duan, X. Differential expression of fascin, E-cadherin and vimentin: Proteins associated with survival of cholangiocarcinoma patients. Am. J. Med. Sci. 2013, 346, 261–268. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.M.; Liu, C.; Xiao, B.L.; Lu, J.X.; Zou, S.Q. Correlation of aPKC-iota and E-cadherin expression with invasion and prognosis of cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 70–75. [Google Scholar]
- Ashida, K.; Terada, T.; Kitamura, Y.; Kaibara, N. Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: An immunohistochemical study. Hepatology 1998, 27, 974–982. [Google Scholar] [CrossRef]
- Németh, Z.; Szász, A.M.; Somorácz, A.; Tátrai, P.; Németh, J.; Gyorffy, H.; Szíjártó, A.; Kupcsulik, P.; Kiss, A.; Schaff, Z. Zonula occludens-1, occludin, and E-cadherin protein expression in biliary tract cancers. Pathol. Oncol. Res. 2009, 15, 533–539. [Google Scholar] [CrossRef]
- Tian, X.; Cao, Z.; Ding, Q.; Li, Z.; Zhang, C. Prognostic value of multiple epithelial mesenchymal transition-associated proteins in intrahepatic cholangiocarcinoma. Oncol. Lett. 2019, 18, 2059–2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.S.; Kang, J.; Lee, J.; Yoo, T.K.; Kim, S.H.; Lee, A. Analysis of the molecular subtypes of preoperative core needle biopsy and surgical specimens in invasive breast cancer. J. Pathol. Transl. Med. 2020, 54, 87–94. [Google Scholar] [CrossRef] [PubMed]


| E-Cadherin | N-Cadherin | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Subcategory | Cases | Score | H-Score | High [%] † | Score | H-Score | High [%] † | Ki-67 |
| Lymph node metastases | iCCA small duct type | 18 | 2.88 ± 0.28 | 232.59 ± 39.18 | 100 | 1.93 ± 0.98 | 64.06 ± 63.07 | 64.29 | 23.02 ± 14.96 |
| iCCA large duct type | 15 | 2.98 ± 0.06 | 206.04 ± 33.28 | 100 | 1.12 ± 0.85 | 41.78 ± 45.50 | 33.33 | 23.01 ± 11.51 | |
| pCCA | 45 | 2.90 ± 0.32 | 226.83 ± 54.26 | 100 | 0.79 ± 0.63 | 36.78 ± 47.16 | 25.64 | 21.80 ± 17.08 | |
| GBC | 34 | 2.86 ± 0.49 | 241.85 ± 39.49 | 96.97 | 0.47 ± 0.66 | 13.07 ± 37.30 | 6.45 | 26.80 ± 22.63 | |
| dCCA | 13 | 3.00 ± 0 | 259.14 ± 15.95 | 100 | 0.73 ± 0.59 | 34.51 ± 47.32 | 8.33 | 24.79 ± 17.76 | |
| PDAC | 67 | 2.75 ± 0.53 | 178.56 ± 62.63 | 97.01 | 0.20 ± 0.44 | 2.09 ± 7.54 | 3.13 | n.a. | |
| Primary carcinoma | iCCA small duct type | 196 | 2.90 ± 0.29 | 162.35± 76.25 | 98.96 | 1.76 ± 0.92 | 38.97 ± 53.32 | 67.01 | 16.06 ± 16.06 |
| iCCA large duct type | 116 | 2.85 ± 0.32 | 149.46 ± 72.36 | 99.13 | 1.30 ± 0.93 | 22.64 ± 38.24 | 50.34 | 19.73 ± 15.91 | |
| pCCA | 328 | 2.94 ± 0.27 | 173.06 ± 62.25 | 99.05 | 0.63 ± 0.73 | 17.47 ± 33.88 | 17.57 | 17.81 ± 16.26 | |
| GBC | 228 | 2.78 ± 0.47 | 137.94 ± 76.6 | 97.29 | 0.45 ± 0.67 | 7.17 ± 20.40 | 12.67 | 27.91 ± 18.18 | |
| dCCA | 185 | 2.93 ± 0.19 | 155.78 ± 71.91 | 100 | 0.35 ± 0.59 | 5.67 ± 17.14 | 8.74 | 21.30 ± 17.75 | |
| PDAC | 131 | 2.89 ± 0.24 | 177.36 ± 50.45 | 100 | 0.34 ± 0.44 | 3.02 ± 5.60 | 3.85 | n.a. | |
| Premalignant lesion | BilIN | 167 | 2.99 ± 0.06 | 240.74 ± 34.73 | 100 | 0.88 ± 0.65 | 34.22 ± 42.22 | 23.87 | 22.33 ± 17.07 |
| PanIn | 107 | 2.98 ± 0.24 | 218.49 ± 42.16 | 100 | 0.51 ± 0.79 | 17.58 ± 38.88 | 14.56 | n.a. | |
| Non-neoplastic tissue | Small bile ducts | 59 | 2.80 ± 0.34 | 165.88 ± 71.12 | 100 | 2.29 ± 0.59 | 88.32 ± 56.56 | 97.96 | 3.00 ± 5.48 |
| Large bile ducts | 42 | 2.97 ± 0.16 | 239.47 ± 36.2 | 100 | 1.03 ± 0.72 | 24.02 ± 29.17 | 34.15 | 5.49 ± 7.89 | |
| Perihilar bile ducts | 65 | 3.00 ± 0 | 218.40 ± 50.39 | 100 | 0.98 ± 0.79 | 38.70 ± 41.70 | 33.33 | 5.82 ± 6.92 | |
| Gallbladder epithelium | 148 | 3.00 ± 0.05 | 179.03 ± 54.39 | 100 | 0.47 ± 0.66 | 19.72 ± 40.23 | 12.84 | 8.64 ± 7.73 | |
| Pancreatic ducts | 92 | 2.85 ± 0.34 | 188.45 ± 42.85 | 100 | 0.59 ± 0.70 | 50.58 ± 70.39 | 8.14 | n.a. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, T.S.; Goeppert, B.; Hausen, A.; Witzel, H.R.; Bartsch, F.; Schindeldecker, M.; Gröger, L.-K.; Ridder, D.A.; Cahyadi, O.; Esposito, I.; et al. N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from Liver Metastases of Ductal Adenocarcinoma of the Pancreas. Cancers 2022, 14, 3091. https://doi.org/10.3390/cancers14133091
Gerber TS, Goeppert B, Hausen A, Witzel HR, Bartsch F, Schindeldecker M, Gröger L-K, Ridder DA, Cahyadi O, Esposito I, et al. N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from Liver Metastases of Ductal Adenocarcinoma of the Pancreas. Cancers. 2022; 14(13):3091. https://doi.org/10.3390/cancers14133091
Chicago/Turabian StyleGerber, Tiemo S., Benjamin Goeppert, Anne Hausen, Hagen R. Witzel, Fabian Bartsch, Mario Schindeldecker, Lisa-Katharina Gröger, Dirk A. Ridder, Oscar Cahyadi, Irene Esposito, and et al. 2022. "N-Cadherin Distinguishes Intrahepatic Cholangiocarcinoma from Liver Metastases of Ductal Adenocarcinoma of the Pancreas" Cancers 14, no. 13: 3091. https://doi.org/10.3390/cancers14133091





