Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy
Abstract
:Simple Summary
Abstract
1. Introduction
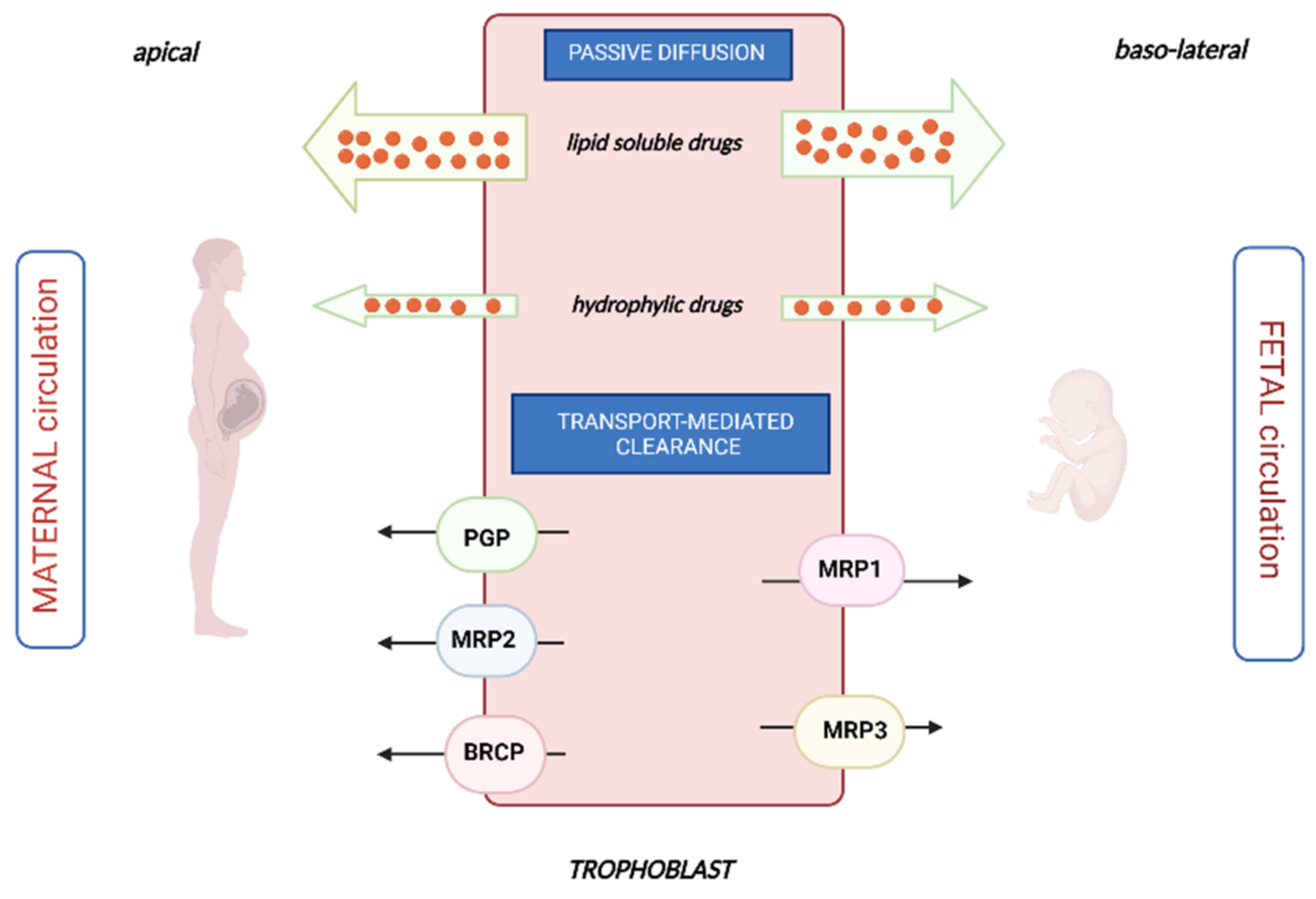
2. Transplacental Passage of Chemotherapy
3. Anticancer Agents during Pregnancy
3.1. Platinum Compounds
3.1.1. Cisplatin
3.1.2. Carboplatin
3.1.3. Oxaliplatin
3.2. Alkylating Agents
3.2.1. Cyclophosphamide
3.2.2. Ifosfamide
3.2.3. Dacarbazine
3.2.4. Busulfan
3.2.5. Procarbazine and Mechlorethamine (Chlormethine)
3.2.6. Chlorambucil
3.3. Antimetabolites
3.3.1. Cytarabine
3.3.2. Methotrexate
3.3.3. Gemcitabine
3.3.4. 5-Fluoruracil (5-FU)
3.3.5. Capecitabine
3.3.6. 6-Mercaptopurine
3.4. Antitumor Antibiotics
3.4.1. Anthracyclines: Daunorubicin, Doxorubicin, Epirubicin, Idarubicin
3.4.2. Other: Actinomycin-D, Bleomycin
3.5. Topoisomerase Inhibitor
3.5.1. Topoisomerase I Inhibitors: Irinotecan
3.5.2. Topoisomerase II Inhibitors: Etoposide
3.6. Antimitotic Agents
3.6.1. Plant Alkaloids: Vincristine and Vinblastine
3.6.2. Taxanes: Paclitaxel and Docetaxel
3.7. Targeted Agents
3.7.1. Rituximab
3.7.2. Trastuzumab
3.7.3. Lapatinib
3.7.4. Bevacizumab
3.7.5. Imatinib, Nilotinib and Dasatinib
3.7.6. Gefitinib and Erlotinib
3.7.7. Vemurafenib
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Haan, J.; Verheecke, M.; Van Calsteren, K.; Van Calster, B.; Shmakov, R.G.; Mhallem Gziri, M.; Halaska, M.J.; Fruscio, R.; Lok, C.A.R.; Boere, I.A.; et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: A 20-year international cohort study of 1170 patients. Lancet Oncol. 2018, 19, 337–346. [Google Scholar] [CrossRef]
- Eibye, S.; Kjær, S.K.; Mellemkjær, L. Incidence of pregnancy-associated cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013, 122, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, E.-S.; Hemida, R.A.-H.; Gamal, A.; El-Zafarany, M.; Toson, E.; El-Bayoumi, M.A. Cancer during pregnancy: Perinatal outcome after in utero exposure to chemotherapy. Arch. Gynecol. Obstet. 2012, 286, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Samlowski, W.E.; Grossman, D.; Bruggers, C.S.; Harris, R.M.; Zone, J.J.; Noyes, R.D.; Bowen, G.M.; Leachman, S.A. Metastatic melanoma in pregnancy: Risk of transplacental metastases in the infant. J. Clin. Oncol. 2003, 21, 2179–2186. [Google Scholar] [CrossRef]
- Alfasi, A.; Ben-Aharon, I. Breast cancer during pregnancy—Current paradigms, paths to explore. Cancers 2019, 11, 1669. [Google Scholar] [CrossRef] [Green Version]
- Poggio, F.; Tagliamento, M.; Pirrone, C.; Soldato, D.; Conte, B.; Molinelli, C.; Cosso, M.; Fregatti, P.; Del Mastro, L.; Lambertini, M. Update on the management of breast cancer during pregnancy. Cancers 2020, 12, 3616. [Google Scholar] [CrossRef]
- Parazzini, F.; Franchi, M.; Tavani, A.; Negri, E.; Peccatori, F.A. Frequency of pregnancy related cancer: A population based linkage study in Lombardy, Italy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2017, 27, 613–619. [Google Scholar] [CrossRef]
- Pentheroudakis, G.; Pavlidis, N. Cancer and pregnancy: Poena magna, not anymore. Eur. J. Cancer 2006, 42, 126–140. [Google Scholar] [CrossRef]
- Cardonick, E.; Iacobucci, A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004, 5, 283–291. [Google Scholar] [CrossRef]
- Adam, M.P. The all-or-none phenomenon revisited. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 664–669. [Google Scholar] [CrossRef]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Ferrara, P.; Attinà, G. Neonatal pharmacology and clinical implications. Drugs Context 2019, 8, 212608. [Google Scholar] [CrossRef] [Green Version]
- Brewer, M.; Kueck, A.; Runowicz, C.D. Chemotherapy in Pregnancy. Clin. Obstet. Gynecol. 2011, 54, 602–618. [Google Scholar] [CrossRef]
- Van Calsteren, K.; Amant, F. Chemotherapy during Pregnancy: Pharmacokinetics and Impact on foetal neurological development. Verh. K. Acad. Geneeskd. Belg. 2011, 73, 105–121. [Google Scholar]
- Kluetz, P.G.; Edelman, M.J. Successful treatment of small cell lung cancer during pregnancy. Lung Cancer 2008, 61, 129–130. [Google Scholar] [CrossRef]
- Geijteman, E.C.; Wensveen, C.W.; Duvekot, J.J.; van Zuylen, L. A child with severe hearing loss associated with maternal cisplatin treatment during pregnancy. Obstet. Gynecol. 2014, 124 (Pt 2) (Suppl S1), 454–456. [Google Scholar] [CrossRef]
- Elit, L.; Bocking, A.; Kenyon, C.; Natale, R. An endodermal sinus tumor diagnosed in pregnancy: Case report and review of the literature. Gynecol. Oncol. 1999, 72, 123–127. [Google Scholar] [CrossRef]
- Tabata, T.; Nishiura, K.; Tanida, K.; Kondo, E.; Okugawa, T.; Sagawa, N. Carboplatin chemotherapy in a pregnant patient with undifferentiated ovarian carcinoma: Case report and review of the literature. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2008, 18, 181–184. [Google Scholar] [CrossRef]
- Sood, A.K.; Shahin, M.S.; Sorosky, J.I. Paclitaxel and platinum chemotherapy for ovarian carcinoma during pregnancy. Gynecol. Oncol. 2001, 83, 599–600. [Google Scholar] [CrossRef]
- Romano, A.; Capozza, M.A.; Mastrangelo, S.; Maurizi, P.; Triarico, S.; Rolesi, R.; Attinà, G.; Fetoni, A.R.; Ruggiero, A. Assessment and management of platinum-related ototoxicity in children treated for cancer. Cancers 2020, 12, 1266. [Google Scholar] [CrossRef]
- Jeppesen, J.B.; Østerlind, K. Successful twin pregnancy outcome after in utero exposure to FOLFOX for metastatic colon cancer: A case report and review of the literature. Clin. Color. Cancer 2011, 10, 348–352. [Google Scholar] [CrossRef]
- Gensheimer, M.; Jones, C.A.; Graves, C.R.; Merchant, N.B.; Lockhart, A.C. Administration of oxaliplatin to a pregnant woman with rectal cancer. Cancer Chemother. Pharmacol. 2009, 63, 371–373. [Google Scholar] [CrossRef]
- Dogan, N.U.; Tastekin, D.; Kerimoglu, O.S.; Pekin, A.; Celik, C. Rectal cancer in pregnancy: A case report and review of the literature. J. Gastrointest. Cancer 2013, 44, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Robson, D.E.; Lewin, J.; Cheng, A.W.; O’Rourke, N.A.; Cavallucci, D.J. Synchronous colorectal liver metastases in pregnancy and post-partum. ANZ J. Surg. 2017, 87, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Zemlickis, D.; Lishner, M.; Erlich, R.; Koren, G. Teratogenicity and carcinogenicity in a twin exposed in utero to cyclophosphamide. Teratog. Carcinog. Mutagen. 1993, 13, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, K.; Heyns, L.; De Smet, F.; Van Eycken, L.; Gziri, M.M.; Van Gemert, W.; Halaska, M.; Vergote, I.; Ottevanger, N.; Amant, F. Cancer during pregnancy: An analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Safi, N.; Anazodo, A.; Dickinson, J.E.; Lui, K.; Wang, A.Y.; Li, Z.; Sullivan, E.A. In utero exposure to breast cancer treatment: A population-based perinatal outcome study. Br. J. Cancer 2019, 121, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Merimsky, O.; Le Chevalier, T.; Missenard, G.; Lepechoux, C.; Cojean-Zelek, I.; Mesurolle, B.; Le Cesne, A. Management of cancer in pregnancy: A case of Ewing’s sarcoma of the pelvis in the third trimester. Ann. Oncol. 1999, 10, 345–350. [Google Scholar] [CrossRef]
- Nakajima, W.; Ishida, A.; Takahashi, M.; Hirayama, M.; Washino, N.; Ogawa, M.; Takahashi, S.; Okada, K. Good outcome for infant of mother treated with chemotherapy for Ewing sarcoma at 25 to 30 weeks’ gestation. J. Pediatr. Hematol. 2004, 26, 308–311. [Google Scholar] [CrossRef]
- Avilès, A.; Nambo, M.-J.; Neri, N. Treatment of early stages Hodgkin lymphoma during pregnancy. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018006. [Google Scholar] [CrossRef] [Green Version]
- Avilés, A.; Neri, N. Hematological malignancies and pregnancy: A final report of 84 children who received chemotherapy in utero. Clin. Lymphoma 2001, 2, 173–177. [Google Scholar] [CrossRef]
- Cardonick, E.; Usmani, A.; Ghaffar, S. Perinatal outcomes of a pregnancy complicated by cancer, including neo-natal follow-up after in utero exposure to chemotherapy: Results of an international registry. Am. J. Clin. Oncol. 2010, 33, 221–228. [Google Scholar] [CrossRef]
- Diamond, I.; Anderson, M.M.; McCreadie, S.R. Transplacental transmission of busulfan (myleran) in a mother with leukemia. Production of fetal mafromation and cyomegaly. Pediatrics 1960, 25, 85–90. [Google Scholar] [CrossRef]
- Earll, J.M.; May, R.L. Busulfan therapy of myelocytic leukemia during pregnancy. Am. J. Obstet. Gynecol. 1965, 92, 580–581. [Google Scholar] [CrossRef]
- Lishner, M.; Zemlickis, D.; Degendorfer, P.; Panzarella, T.; Sutcliffe, S.; Koren, G. Maternal and foetal outcome following Hodgkin’s disease in pregnancy. Br. J. Cancer 1992, 65, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, D.T.; Parreño, M.G.H.; Batten, J.; Chamberlain, M.C. Management of malignant gliomas during pregnancy: A case series. Cancer 2008, 113, 3349–3354. [Google Scholar] [CrossRef]
- Ali, R.; Ozkalemkas, F.; Kimya, Y.; Koksal, N.; Ozkocaman, V.; Yorulmaz, H.; Eroglu, A.; Ozcelik, T.; Tunali, A. Pregnancy in chronic lymphocytic leukemia: Experience with fetal exposure to chlorambucil. Leuk. Res. 2009, 33, 567–569. [Google Scholar] [CrossRef]
- Ebert, U.; Löffler, H.; Kirch, W. Cytotoxic therapy and pregnancy. Pharmacol. Ther. 1997, 74, 207–220. [Google Scholar] [CrossRef]
- Niedermeier, D.M.; Frei-Lahr, D.A.; Hall, P.D. Treatment of acute myeloid leukemia during the second and third trimesters of pregnancy. Pharmacotherapy 2005, 25, 1134–1140. [Google Scholar] [CrossRef]
- Powell, H.R.; Ekert, H. Methotrexate-induced congenital malformations. Med. J. Aust. 1971, 2, 1076–1077. [Google Scholar] [CrossRef]
- Kozlowski, R.D.; Steinbrunner, J.V.; MacKenzie, A.H.; Clough, J.D.; Wilke, W.S.; Segal, A.M. Outcome of first-trimester exposure to low-dose methotrexate in eight patients with rheumatic disease. Am. J. Med. 1990, 88, 589–592. [Google Scholar] [CrossRef]
- Buckley, L.M.; Bullaboy, C.A.; Leichtman, L.; Marquez, M. Multiple congenital anomalies associated with weekly low-dose methotrexate treatment of the mother. Arthritis Rheum. 1997, 40, 971–973. [Google Scholar] [CrossRef]
- Mantilla-Rivas, E.; Brennan, A.; Goldrich, A.; Bryant, J.R.; Oh, A.K.; Rogers, G.F. Extremity findings of methotrexate embryopathy. HAND 2020, 15, NP14–NP21. [Google Scholar] [CrossRef]
- Wiesweg, M.; Aydin, S.; Koeninger, A.; Stein, A.; Schara, U.; van Roye, C.; Hense, J.; Welt, A.; Schuler, M. Administration of gemcitabine for metastatic adenocarcinoma during pregnancy: A case report and review of the literature. AJP Rep. 2014, 4, 17–22. [Google Scholar]
- Castellanos, M.I.; Childress, K.J.; Ramirez, M.; Venkatramani, R. Fetal exposure to capecitabine and temozolomide during the first trimester: A case report. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101881. [Google Scholar] [CrossRef]
- Rogers, J.E.; Dasari, A.; Eng, C. The treatment of colorectal cancer during pregnancy: Cytotoxic chemotherapy and targeted therapy challenges. Oncologist 2016, 21, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Pellino, G.; Simillis, C.; Kontovounisios, C.; Baird, D.L.; Nikolaou, S.; Warren, O.; Tekkis, P.P.; Rasheed, S. Colorectal cancer diagnosed during pregnancy: Systematic review and treatment pathways. Eur. J. Gastroenterol. Hepatol. 2017, 29, 743–753. [Google Scholar] [CrossRef]
- Karp, G.I.; Von Oeyen, P.; Valone, F.; Khetarpal, V.K.; Israel, M.; Mayer, R.J.; Frigoletto, F.D.; Garnick, M.B. Doxorubicin in pregnancy: Possible transplacental passage. Cancer Treat. Rep. 1983, 67, 773–777. [Google Scholar]
- Gziri, M.M.; Hui, W.; Amant, F.; Van Calsteren, K.; Ottevanger, N.; Kapusta, L.; Mertens, L. Myocardial function in children after fetal chemotherapy exposure. A tissue Doppler and myocardial deformation imaging study. Eur. J. Pediatr. 2013, 172, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Avilés, A.; Neri, N.; Nambo, M.J. Long-term evaluation of cardiac function in children who received anthracyclines during pregnancy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006, 17, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Leyder, M.; Laubach, M.; Breugelmans, M.; Keymolen, K.; De Greve, J.; Foulon, W. Specific congenital malformations after exposure to cyclophosphamide, epirubicin and 5-fluorouracil during the first trimester of pregnancy. Gynecol. Obstet. Investig. 2011, 71, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Peccatori, F.A.; Scarfone, G.; Acaia, B.; Rossi, P.; Cascio, R.; Goldhirsch, A. Anthracyclines for gestational breast cancer: Course and outcome of pregnancy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 1511–1512. [Google Scholar] [CrossRef]
- Achtari, C.; Hohlfeld, P. Cardiotoxic transplacental effect of idarubicin administered during the second trimester of pregnancy. Am. J. Obstet. Gynecol. 2000, 183, 511–512. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimoya, K.; Ueda, S.; Wada, K.; Koyama, M.; Murata, Y. Idarubicin administered during pregnancy: Its effects on the fetus. Gynecol. Obstet. Investig. 2004, 58, 186–188. [Google Scholar] [CrossRef]
- Brito, V.B.; Nascimento, L.V.; Nunes, R.B.; Moura, D.J.; Lago, P.D.; Saffi, J. Exercise during pregnancy decreases doxorubicin-induced cardiotoxic effects on neonatal hearts. Toxicology 2016, 368–369, 46–57. [Google Scholar] [CrossRef]
- Taylor, J.; Amanze, A.; Di Federico, E.; Verschraegen, C. Irinotecan use during pregnancy. Obstet. Gynecol. 2009, 114 (Pt 2), 451–452. [Google Scholar] [CrossRef]
- Cirillo, M.; Musola, M.; Cassandrini, P.A.; Lunardi, G.; Venturini, M. Irinotecan during pregnancy in metastatic colon cancer. Tumori 2012, 98, 155e–157e. [Google Scholar] [CrossRef]
- Motegi, M.; Takakura, S.; Takano, H.; Tanaka, T.; Ochiai, K. Adjuvant chemotherapy in a pregnant woman with endodermal sinus tumor of the ovary. Obstet. Gynecol. 2007, 109, 537–540. [Google Scholar] [CrossRef]
- Maggen, C.; Dierickx, D.; Lugtenburg, P.; Laenen, A.; Cardonick, E.; Shmakov, R.G.; Bellido, M.; Cabrera-Garcia, A.; Gziri, M.M.; Halaska, M.J.; et al. Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: A multicentre, retrospective, cohort study. Lancet Haematol. 2019, 6, e551–e561. [Google Scholar] [CrossRef]
- Chelghoum, Y.; Vey, N.; Raffoux, E.; Huguet, F.; Pigneux, A.; Witz, B.; Pautas, C.; de Botton, S.; Guyotat, D.; Lioure, B.; et al. Acute leukemia during pregnancy: A report on 37 patients and a review of the literature. Cancer 2005, 104, 110–117. [Google Scholar] [CrossRef]
- Mir, O.; Berveiller, P.; Goffinet, F.; Treluyer, J.-M.; Serreau, P.R.; Goldwasser, F.; Rouzier, R. Taxanes for breast cancer during pregnancy: A systematic review. Ann. Oncol. 2010, 21, 425–426. [Google Scholar] [CrossRef]
- Lambertini, M.; Peccatori, F.; Azim, H.A. Targeted agents for cancer treatment during pregnancy. Cancer Treat. Rev. 2015, 41, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, E.F.; Murray, E.R.; Kelman, A.; Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011, 117, 1499–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrikopoulou, A.; Apostolidou, K.; Chatzinikolaou, S.; Bletsa, G.; Zografos, E.; Dimopoulos, M.-A.; Zagouri, F. Trastuzumab administration during pregnancy: Un update. BMC Cancer 2021, 21, 463. [Google Scholar]
- Kaygusuz, I.; Eser, A.; Gümüş, I.I.; Köşüş, A.; Yenidünya, S.; Namuslu, M.; Kafali, H. Effect of anti-vascular endothelial growth factor antibody during early fetal development in rats. J. Matern. Neonatal Med. 2014, 27, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Georgalas, I.; Giavaras, G.; Anastasiou, E.; Ntana, Z.; Petrou, C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010, 88, e136. [Google Scholar] [CrossRef]
- Polizzi, S.; Mahajan, V. Intravitreal anti-VEGF injections in pregnancy: Case series and review of literature. J. Ocul. Pharmacol. Ther. 2015, 31, 605–610. [Google Scholar] [CrossRef]
- Dolai, T.K.; Bhargava, R.; Mahapatra, M.; Mishra, P.; Seth, T.; Pati, H.P.; Saxena, R. Is imatinib safe during pregnancy? Leuk. Res. 2009, 33, 572–573. [Google Scholar] [CrossRef]
- Pye, S.M.; Cortes, J.; Ault, P.; Hatfield, A.; Kantarjian, H.; Pilot, R.; Rosti, G.; Apperley, J.F. The effects of imatinib on pregnancy outcome. Blood 2008, 111, 5505–5508. [Google Scholar] [CrossRef] [Green Version]
- Abruzzese, E.; Mauro, M.; Apperley, J.; Chelysheva, E. Tyrosine kinase inhibitors and pregnancy in chronic myeloid leukemia: Opinion, evidence, and recommendations. Ther. Adv. Hematol. 2020, 11. [Google Scholar] [CrossRef]
- Gil, S.; Goetgheluck, J.; Paci, A.; Broutin, S.; Friard, S.; Couderc, L.; Ayoubi, J.; Picone, O.; Tcherakian, C. Efficacy and safety of gefitinib during pregnancy: Case report and literature review. Lung Cancer 2014, 85, 481–484. [Google Scholar] [CrossRef]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Carter, A.M. Animal models of human placentation—A Review. Placenta 2007, 28 (Suppl. A), S41–S47. [Google Scholar] [CrossRef]
- Enders, A.C.; Carter, A.M. What can comparative studies of placental structure tell us?—A review. Placenta 2004, 25 (Suppl. A), S3–S9. [Google Scholar] [CrossRef]
- Maltepe, E.; Bakardjiev, A.I.; Fisher, S.J. The placenta: Transcriptional, epigenetic, and physiological integration during development. J. Clin. Investig. 2010, 120, 1016–1025. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213 (Suppl. S4), S115–S122. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Benoit, L.; Mir, O.; Vialard, F.; Berveiller, P. Cancer during pregnancy: A review of preclinical and clinical transplacental transfer of anticancer agents. Cancers 2021, 13, 1238. [Google Scholar] [CrossRef]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X. Contributions of drug transporters to blood-placental barrier. Adv. Exp. Med. Biol. 2019, 1141, 505–548. [Google Scholar]
- Van Calsteren, K.; Verbesselt, R.; Beijnen, J.; Devlieger, R.; De Catte, L.; Chai, D.; Van Bree, R.; Heyns, L.; de Hoon, J.; Amant, F. Transplacental transfer of anthracyclines, vinblastine, and 4-hydroxy-cyclophosphamide in a baboon model. Gynecol. Oncol. 2010, 119, 594–600. [Google Scholar] [CrossRef]
- Bell, D.J.; Kerr, D.J. Pharmacokinetic considerations in the use of anticancer drugs during pregnancy: Challenges and new developments. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1341–1344. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, E.; Al-Harmi, J.; Nandakumaran, M.; Al-Shammari, M. Transport kinetics of cisplatin in the perfused human placental lobule in vitro. J. Matern. Fetal Neonatal Med. 2008, 21, 726–731. [Google Scholar] [CrossRef]
- Pascual, M.J.; Macias, R.I.; Garcia-Del-Pozo, J.; Serrano, M.A.; Marin, J.J. Enhanced efficiency of the placental barrier to cisplatin through binding to glycocholic acid. Anticancer Res. 2001, 21, 2703–2707. [Google Scholar]
- Lanowska, M.; Köhler, C.; Oppelt, P.; Schmittel, A.; Gottschalk, E.; Hasenbein, K.; Schneider, A.; Marnitz, S. Addressing concerns about cisplatin application during pregnancy. J. Perinat. Med. 2011, 39, 279–285. [Google Scholar] [CrossRef]
- Amant, F.; Halaska, M.J.; Fumagalli, M.; Steffensen, K.D.; Lok, C.; Van Calsteren, K.; Han, S.N.; Mir, O.; Fruscio, R.; Uzan, C.; et al. Gynecologic cancers in pregnancy: Guidelines of a second international consensus meeting. Int. J. Gynecol. Cancer 2014, 24, 394–403. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.; Hasenburg, A.; Johansson, A.L.V.; Lambertini, M.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, A.; Trombatore, G.; Triarico, S.; Arena, R.; Ferrara, P.; Scalzone, M.; Pierri, F.; Riccardi, R. Platinum compounds in children with cancer: Toxicity and clinical management. Anticancer Drugs 2013, 24, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Sergentanis, T.N.; Chrysikos, D.; Bartsch, R. Platinum derivatives during pregnancy in cervical cancer: A systematic review and meta-analysis. Obstet. Gynecol. 2013, 121, 337–343. [Google Scholar] [CrossRef]
- Mir, O.; Berveiller, P.; Ropert, S.; Goffinet, F.; Goldwasser, F. Use of platinum derivatives during pregnancy. Cancer 2008, 113, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G. Cisplatin-induced nephrotoxicity in children: What is the best protective strategy? J. Oncol. Pharm. Pract. 2021, 27, 180–186. [Google Scholar] [CrossRef]
- Van Calsteren, K.; Verbesselt, R.; Van Bree, R.; Heyns, L.; De Bruijn, E.; De Hoon, J.; Amant, F. Substantial variation in transplacental transfer of chemotherapeutic agents in a mouse model. Reprod. Sci. 2011, 18, 57–63. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, vi160–vi170. [Google Scholar] [CrossRef]
- Makoshi, Z.; Perrott, C.; Al-Khatani, K.; Al-Mohaisen, F. Chemotherapeutic treatment of colorectal cancer in pregnancy: Case report. J. Med. Case Rep. 2015, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- D’Incalci, M.; Sessa, C.; Colombo, N.; De Palo, G.; Semprini, A.E.; Pardi, G. Transplacental passage of cyclophosphamide. Cancer Treat. Rep. 1982, 66, 1681–1682. [Google Scholar]
- Rengasamy, P. Congenital malformations attributed to prenatal exposure to cyclophosphamide. Anti-Cancer Agents Med. Chem. 2017, 17, 1211–1227. [Google Scholar] [CrossRef]
- Avilés, A.; Díaz-Maqueo, J.C.; Talavera, A.; Guzmán, R.; García, E.L. Growth and development of children of mothers treated with chemotherapy during pregnancy: Current status of 43 children. Am. J. Hematol. 1991, 36, 243–248. [Google Scholar] [CrossRef]
- Passera, S.; Contarino, V.; Scarfone, G.; Scola, E.; Fontana, C.; Peccatori, F.; Cinnante, C.; Counsell, S.; Ossola, M.; Pisoni, S.; et al. Effects of in-utero exposure to chemotherapy on fetal brain growth. Int. J. Gynecol. Cancer 2019, 29, 1195–1202. [Google Scholar] [CrossRef]
- Helal, M. Prenatal effects of transplacental exposure to ifosfamide in rats. Biotech. Histochem. 2016, 91, 357–368. [Google Scholar] [CrossRef]
- Kantrowitz-Gordon, I.; Hays, K.; Kayode, O.; Kumar, A.R.; Kaplan, H.G.; Reid, J.M.; Safgren, S.L.; Ames, M.M.; Easterling, T.R.; Hebert, M.F. Pharmacokinetics of dacarbazine (DTIC) in pregnancy. Cancer Chemother. Pharmacol. 2018, 81, 455–460. [Google Scholar] [CrossRef]
- Basler, A.; Theiss, I.; Röhrborn, G. Cytogenetic effects of busulfan in vivo on bone marrow cells and oocytes of adult mice and liver cells of transplacentally exposed embryos. Environ. Mutagen. 1979, 1, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Otsuji, M.; Takahara, M.; Naruse, T.; Guan, D.; Harada, M.; Zhe, P.; Takagi, M.; Ogino, T. Developmental abnormalities in rat embryos leading to tibial ray deficiencies induced by busulfan. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Brada, M.J.; van den Bent, M.; Tonn, J.-C.; Pentheroudakis, G.; on behalf of the ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii93–iii101. [Google Scholar] [CrossRef] [PubMed]
- Malek, F.A.; Möritz, K.U.; Fanghänel, J. Effects of prenatal procarbazine administration on intrauterine development in rats. Ann. Anat. Anat. Anz. 2003, 185, 117–119. [Google Scholar] [CrossRef]
- Weingartner, J.; Proff, P.; Fanghanel, J.; Kundt, G.; Gedrange, T.; Kubein-Meesenburg, D.; Gredes, T. Different bone sesitivity to malformations induced by procarbazine in fetal rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 17–25. [Google Scholar]
- Padmanabhan, R.; Samad, P. Chlorambucil-induced postclosure exencephaly and axial skeletal abnormalities in rat fetuses. Reprod. Toxicol. 1999, 13, 189–201. [Google Scholar] [CrossRef]
- Artlich, A.; Möller, J.; Tschakaloff, A.; Schwinger, E.; Kruse, K.; Gortner, L. Teratogenic effects in a case of maternal treatment for acute myelocytic leukaemia—Neonatal and infantile course. Eur. J. Pediatr. 1994, 153, 488–491. [Google Scholar]
- National Toxicology Program. NTP monograph: Developmental effects and pregnancy outcomes associated with cancer chemotherapy use during pregnancy. NTP Monogr. 2013, 2, i-214. [Google Scholar]
- Martín, M.C.; Barbero, P.; Groisman, B.; Aguirre, M.; Koren, G. Methotrexate embryopathy after exposure to low weekly doses in early pregnancy. Reprod. Toxicol. 2014, 43, 26–29. [Google Scholar] [CrossRef]
- Esumi, Y.; Mitsugi, K.; Seki, H.; Takao, A.; Kawai, M. Placental transfer, lacteal transfer and plasma protein binding of gemcitabine. Xenobiotica Fate Foreign Compd. Biol. Syst. 1994, 24, 957–964. [Google Scholar] [CrossRef]
- Gurumurthy, M.; Koh, P.; Singh, R.H.; Bhide, A.; Satodia, P.; Hocking, M.; Anbarasu, A.; Wood, L. Metastatic non-small-cell lung cancer and the use of gemcitabine during pregnancy. J. Perinatol. 2009, 29, 63–65. [Google Scholar] [CrossRef] [Green Version]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv22–iv40. [Google Scholar] [CrossRef]
- Germann, N.; Goffinet, F.; Goldwasser, F. Anthracyclines during pregnancy: Embryo–fetal outcome in 160 patients. Ann. Oncol. 2004, 15, 146–150. [Google Scholar] [CrossRef]
- Willemse, P.H.; van der Sijde, R.; Sleijfer, D.T. Combination chemotherapy and radiation for stage iv breast cancer during pregnancy. Gynecol. Oncol. 1990, 36, 281–284. [Google Scholar] [CrossRef]
- Barni, S.; Ardizzoia, A.; Zanetta, G.; Stracchi, E.; Lissoni, P.; Tancini, G. Weekly doxorubicin chemotherapy for breast cancer in pregnancy. A case report. Tumori J. 1992, 78, 349–350. [Google Scholar] [CrossRef]
- Soininen, S.K.; Repo, J.K.; Karttunen, V.; Auriola, S.; Vähäkangas, K.H.; Ruponen, M. Human placental cell and tissue uptake of doxorubicin and its liposomal formulations. Toxicol. Lett. 2015, 239, 108–114. [Google Scholar] [CrossRef]
- Eliesen, G.A.M.; van Hove, H.; Meijer, M.H.; Broek, P.H.H.V.D.; Pertijs, J.; Roeleveld, N.; van Drongelen, J.; Russel, F.G.M.; Greupink, R. Toxicity of anticancer drugs in human placental tissue explants and trophoblast cell lines. Arch. Toxicol. 2021, 95, 557–571. [Google Scholar] [CrossRef]
- Van Calsteren, K.; Hartmann, D.; Van Aerschot, L.; Verbesselt, R.; Van Bree, R.; D’Hooge, R.; Amant, F. Vinblastine and doxorubicin administration to pregnant mice affects brain development and behaviour in the offspring. Neurotoxicology 2009, 30, 647–657. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Osborne, E.M.; Chihara, D.; Lai, P.; Zhou, S.; Ramirez, M.M.; Oki, Y.; Hagemeister, F.B.; Rodriguez, A.M.; Samaniego, F.; et al. Maternal and fetal outcomes after therapy for Hodgkin or non-Hodgkin lymphoma diagnosed during pregnancy. JAMA Oncol. 2016, 2, 1065–1069. [Google Scholar] [CrossRef] [Green Version]
- Padberg, S.; Mick, I.M.; Frenzel, C.; Greil, R.; Hilberath, J.; Schaefer, C. Transient congenital dilated cardiomyopathy after maternal R-CHOP chemotherapy during pregnancy. Reprod. Toxicol. 2017, 71, 146–149. [Google Scholar] [CrossRef]
- Framarino-dei-Malatesta, M.; Perrone, G.; Giancotti, A.; Ventriglia, F.; Derme, M.; Iannini, I.; Tibaldi, V.; Galoppi, P.; Sammartino, P.; Cascialli, G.; et al. Epirubicin: A new entry in the list of fetal cardiotoxic drugs? Intrauterine death of one fetus in a twin pregnancy. Case report and review of literature. BMC Cancer 2015, 15, 951. [Google Scholar] [CrossRef] [Green Version]
- Dilek, I.; Topcu, N.; Demir, C.; Bay, A.; Uzun, K.; Gul, A.; Oner, A.F.; Ugras, S. Hematological malignancy and pregnancy: A single-institution experience of 21 cases. Int. J. Lab. Hematol. 2006, 28, 170–176. [Google Scholar] [CrossRef]
- Parodi, E.; Alluto, A.; Moggio, G.; Liberale, V.; Frigerio, M.; Sismondi, P. Transient ventricular hypocinesia after in utero anthracyclines exposure: A case-report and review of the literature. J. Matern. Neonatal Med. 2012, 25, 189–192. [Google Scholar] [CrossRef]
- Baumgärtner, A.K.; Oberhoffer, R.; Jacobs, V.R.; Ostermayer, E.; Menzel, H.; Voigt, M.; Schneider, K.T.M.; Pildner von Steinburg, S. Reversible foetal cerebral ventriculomegaly and cardiomyopathy under chemotherapy for maternal AML. Onkologie 2009, 32, 40–43. [Google Scholar] [CrossRef]
- Tuchmann-Duplessis, H.; Hiss, D.; Mottot, G.; Rosner, I. Embryotoxic and teratogenic effect of actinomycin D in the Syrian hamster. Toxicology 1973, 1, 131–133. [Google Scholar] [CrossRef]
- Yamauchi, H.; Katayama, K.-I.; Ueno, M.; Kanemitsu, H.; Nam, C.; Mikami, T.; Saito, A.; Ishida, Y.; Uetsuka, K.; Doi, K.; et al. Etoposide induces TRP53-dependent apoptosis and TRP53-Independent cell cycle arrest in trophoblasts of the developing mouse placenta. Biol. Reprod. 2009, 80, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-Y.; Nava-Ocampo, A.A.; Kim, T.-J.; Shim, J.-U.; Park, C.-T. Pregnancy outcome after prenatal exposure to bleomycin, etoposide and cisplatin for malignant ovarian germ cell tumors: Report of 2 cases. Reprod. Toxicol. 2005, 19, 557–561. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Hao, Z.; Li, L.; Lu, J.; Kang, H.; Lu, Y.; You, Y.; Li, L.; Chen, Q.; et al. Requirement for etoposide in the treatment of pregnancy related hemophagocytic lymphohistiocytosis: A multicenter retrospective study. Orphanet J. Rare Dis. 2019, 14, 50. [Google Scholar] [CrossRef]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G. Temozolomide and oral etoposide in children with recurrent malignant brain tumors. Drugs Context 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Triarico, S.; Romano, A.; Attinà, G.; Capozza, M.A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Vincristine-induced peripheral neuropathy (VIPN) in pediatric tumors: Mechanisms, risk factors, strategies of prevention and treatment. Int. J. Mol. Sci. 2021, 22, 4112. [Google Scholar] [CrossRef]
- Jones, R.T.; Weinerman, B.H. MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) given during pregnancy. Obstet. Gynecol. 1979, 54, 477. [Google Scholar] [PubMed]
- Mulvihill, J.J.; McKeen, E.A.; Rosner, F.; Zarrabi, M.H. Pregnancy outcome in cancer patients. Experience in a large cooperative group. Cancer 1987, 60, 1143–1150. [Google Scholar] [CrossRef]
- Zagouri, F.; Sergentanis, T.N.; Chrysikos, D.; Filipits, M.; Bartsch, R. Taxanes for ovarian cancer during pregnancy: A systematic review. Oncology 2012, 83, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Cardonick, E.; Bhat, A.; Gilmandyar, D.; Somer, R. Maternal and fetal outcomes of taxane chemotherapy in breast and ovarian cancer during pregnancy: Case series and review of the literature. Ann. Oncol. 2012, 23, 3016–3023. [Google Scholar] [CrossRef]
- Zagouri, F.; Korakiti, A.-M.; Zakopoulou, R.; Kyriazoglou, A.; Zografos, E.; Haidopoulos, D.; Apostolidou, K.; Papatheodoridi, M.A.; Dimopoulos, M.A. Taxanes during pregnancy in cervical cancer: A systematic review and pooled analysis. Cancer Treat. Rev. 2019, 79, 101885. [Google Scholar] [CrossRef]
- Berveiller, P.; Mir, O. Taxanes during pregnancy: Probably safe, but still to be optimized. Oncology 2012, 83, 239–240. [Google Scholar] [CrossRef]
- Cardonick, E.; Broadrup, R.; Xu, P.; Doan, M.T.; Jiang, H.; Snyder, N.W. Preliminary results of identification and quantification of paclitaxel and its metabolites in human meconium from newborns with gestational chemotherapeutic exposure. PLoS ONE 2019, 14, e0211821. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.A.; Watson, W.J.; Leslie, K.K. Targeted treatment using monoclonal antibodies and tyrosine-kinase inhibitors in pregnancy. Lancet Oncol. 2007, 8, 738–743. [Google Scholar] [CrossRef]
- Burotto, M.; Gormaz, J.G.; Samtani, S.; Valls, N.; Silva, R.; Rojas, C.; Portiño, S.; de la Jara, C. Viable pregnancy in a patient with metastatic melanoma treated with double checkpoint immunotherapy. Semin. Oncol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- Rey, J.; Coso, D.; Roger, V.; Bouayed, N.; Belmecheri, N.; Ivanov, V.; Gastaut, J.A.; Bouabdallah, R. Rituximab combined with chemotherapy for lymphoma during pregnancy. Leuk. Res. 2009, 33, e8–e9. [Google Scholar] [CrossRef]
- Decker, M.; Rothermundt, C.; Holländer, G.; Tichelli, A.; Rochlitz, C. Rituximab plus CHOP for treatment of diffuse large B-cell lymphoma during second trimester of pregnancy. Lancet Oncol. 2006, 7, 693–694. [Google Scholar] [CrossRef]
- Das, G.; Damotte, V.; Gelfand, J.M.; Bevan, C.; Cree, B.A.C.; Do, L.; Green, A.J.; Hauser, S.L.; Bove, R. Rituximab before and during pregnancy: A systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e453. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, B.; Tiemann, M.; Salwender, H.; Verpoort, K.; Wenger, M.K.; Schmitz, N. The effects of rituximab treatment during pregnancy on a neonate. Haematologica 2006, 91, 1426–1427. [Google Scholar]
- Sekar, R.; Stone, P. Trastuzumab use for metastatic breast cancer in pregnancy. Obstet. Gynecol. 2007, 110 (Pt 2), 507–510. [Google Scholar] [CrossRef]
- Kelly, H.; Graham, M.; Humes, E.; Dorflinger, L.J.; Boggess, K.A.; O’Neil, B.H.; Harris, J.; Spector, N.L.; Dees, E.C. Delivery of a healthy baby after first-trimester maternal exposure to lapatinib. Clin. Breast Cancer 2006, 7, 339–341. [Google Scholar] [CrossRef]
- Cross, S.N.; Ratner, E.; Rutherford, T.J.; Schwartz, P.E.; Norwitz, E.R. Bevacizumab-mediated interference with VEGF signaling is sufficient to induce a preeclampsia-like syndrome in nonpregnant women. Rev. Obstet. Gynecol. 2012, 5, 2–8. [Google Scholar]
- Yadav, U.; Solanki, S.L.; Yadav, R. Chronic myeloid leukemia with pregnancy: Successful management of pregnancy and delivery with hydroxyurea and imatinib continued till delivery. J. Cancer Res. Ther. 2013, 9, 484–486. [Google Scholar] [CrossRef]
- Conchon, M.; Sanabani, S.S.; Bendit, I.; Santos, F.M.; Serpa, M.; Dorliac-Llacer, P.E. Two successful pregnancies in a woman with chronic myeloid leukemia exposed to nilotinib during the first trimester of her second pregnancy: Case study. J. Hematol. Oncol. 2009, 2, 42. [Google Scholar] [CrossRef]
- Rivas, G.; Llinás, N.; Bonilla, C.; Rubiano, J.; Cuello, J.; Arango, N. Use of erlotinib throughout pregnancy: A case-report of a patient with metastatic lung adenocarcinoma. Lung Cancer 2012, 77, 469–472. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Puzanov, I.; Kim, K.B.; Ribas, A.; McArthur, G.A.; Sosman, J.A.; O’Dwyer, P.J.; Lee, R.J.; Grippo, J.F.; Nolop, K.; et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 2010, 363, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Pagan, M.; Jinks, H.; Sewell, M. Treatment of metastatic malignant melanoma during pregnancy with a BRAF Kinase inhibitor. Case Rep. Womens Health 2019, 24, e00142. [Google Scholar] [CrossRef] [PubMed]
- Maleka, A.; Enblad, G.; Sjörs, G.; Lindqvist, A.; Ullenhag, G.J. Treatment of metastatic malignant melanoma with vemurafenib during pregnancy. J. Clin. Oncol. 2013, 31, e192–e193. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T.; Lerma, V.; González-Valle, O.; González Herrada, C.; de Abajo, F.J. Vemurafenib-induced toxic epidermal necrolysis: Possible cross-reactivity with other sulfonamide compounds. Br. J. Dermatol. 2016, 174, 621–624. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.; van Thienen, J.V.; Casaer, M.; Hannivoort, R.A.; Van Calsteren, K.; van Tuyl, M.; van Gerwen, M.M.; Debeer, A.; Amant, F.; Painter, R.C. Severe adverse reaction to vemurafenib in a pregnant woman with metastatic melanoma. Case Rep. Oncol. 2018, 11, 119–124. [Google Scholar] [CrossRef] [PubMed]
| Antineoplastic Agents | Maternal Tumor | Pregnancy and Fetal Outcomes |
|---|---|---|
| Platinum compounds | ||
| Cisplatin | Cervical cancer, ovarian cancer, non-small cell lung cancer (NSCLC) | No congenital malformations (T2–T3) [14] Severe bilateral perceptive hearing loss (T2–T3) [15] Cerebral ventriculomegaly and cerebral atrophy (T2–T3) [16] |
| Carboplatin | Cervical cancer, ovarian cancer, NSCLC | No congenital malformations (T2–T3) [17,18] Spontaneous miscarriage of a fetus with gastroschisis (T2–T3) [19] |
| Oxaliplatin | Colorectal carcinoma | No congenital malformations (T2–T3) [20] Small for gestational age at birth (T2–T3) [21] Hypothyroidism (T2–T3) [22] Spontaneous miscarriage at 33 weeks of gestational age (T2–T3) [23] |
| Alkylating agents | ||
| Cyclophosphamide | Breast cancer, Hodgkin’s lymphoma | Teratogenic effects and multiple congenital anomalies (T1) [24,25] No congenital malformations (T2–T3) [20] Preterm birth (T2,T3) [26] |
| Ifosfamide | Ewing sarcoma, soft tissue sarcoma | Anhydramnios and intrauterine growth arrest (T2–T3) [27] No congenital malformations (T2–T3) [28] |
| Dacarbazine | Melanoma, Hodgkin’s lymphoma | No congenital malformations (T1–T2–T3) [29,30] Plagiocephaly, syndactyly (T2–T3) [31] |
| Busulfan | Chronic myeloid leukemia, myeloablative-conditioning | Teratogenic effects and multiple congenital malformations (T1–T2–T3) [32] Renal agenesis and liver calcifications (T2) [33] |
| Procarbazine Mechlorethamine | Anaplastic astrocytoma, oligodendroglioma, Hodgkin’s disease | Hydrocephaly and perinatal death (T1) [34] Syndactyly (T2) [34] No congenital malformations [35] |
| Chlorambucil | Chronic lymphocytic leukemia, Hodgkin’s lymphoma | No congenital malformations [36] |
| Antimetabolites | ||
| Cytarabine | Acute lymphocytic and non-lymphocytic leukemia, chronic myelogenous leukemia | Teratogenic effects (T1–T2–T3) [37] Intrauterine growth retardation (IUGR), intrauterine death [38] |
| Methotrexate | Leukemia, breast cancer, lung cancers, non-Hodgkin lymphoma, osteosarcoma, autoimmune diseases, dermatologic conditions, ectopic pregnancies, pregnancy termination | Spontaneous miscarriage and birth defects (T1) [39] Craniofacial, cardiac, pulmonary, gastrointestinal, genitourinary and musculoskeletal anomalies (T1–T2–T3) [40,41,42] |
| Gemcitabine | Biliary tract cancers, pulmonary adenocarcinoma, non-small-cell lung carcinoma (NSCLC), pancreatic adenocarcinoma | Healthy newborn (T1–T2) [43] IUGR (T2–T3) [43] |
| Capecitabine | Colorectal carcinoma, breast cancer | Healthy children (T1) [44] |
| 5-Fluoruracil | Colorectal carcinoma, breast cancer | Spontaneous abortion (T1) [45] Multiple congenital malformations (T1) [46] |
| 6-Mercaptopurine | Acute lymphocytic leukemia | No congenital malformations (T1–T2–T3) [30] IUGR, intrauterine and neonatal death (T1–T2–T3) [30] |
| Antitumor antibiotics | ||
| Daunorubicin | Acute lymphocytic and myeloid leukemia | Teratogenic effects (T1–T2–T3) [47] |
| Doxorubicin | Hematological cancers, various solid tumors | Skeletal malformations, imperforate anus and rectovaginal fistula (T1) [48,49] No congenital malformations, no cardiotoxicity (T2, T3) [49] |
| Epirubicin | Breast cancer | Intrauterine death, micrognathia, syndactyly, other fingers/metatarsal abnormalities (T1) [50] Polycystic kidney, clubfoot and rectal atresia (T2–T3) [51] |
| Idarubicin | Acute myeloid leukemia | No congenital malformations (T1) [52] Dilated cardiomyopathy, fingers and limbs malformations and micrognathia (T2–T3) [53] |
| Bleomycin | Hodgkin’s lymphoma, ovarian cancer | No congenital malformations (T1–T2–T3) [54] Floating thumb malformation (T1) [31] Plagiocephaly and syndactyly (T2–T3) [31] |
| Topoisomerase inhibitor | ||
| Irinotecan | Colorectal cancer, ovarian cancer | No congenital malformations (T2–T3) [55,56] |
| Etoposide | Ovarian cancer, hemophagocytic lymphohistiocytosis | No congenital malformations except one case of ventriculomegaly with cerebral atrophy (T2–T3) [57] |
| Antimitotic agents | ||
| Vincristine | Acute lymphoblastic leukemia, Hodgkin’s lymphoma, other lymphomas | Atrial septum defect, bilateral radius and fifth digit absence, hydrocephalus, renal and cardiac abnormalities (T1) [58] |
| Vinblastine | Acute lymphoblastic leukemia, Hodgkin’s lymphoma, other lymphomas | Hydrocephalus, spontaneous miscarriage, cleft palate (T1) [59] |
| Paclitaxel, Docetaxel | Breast cancer, cervical cancer, ovarian cancer | Healthy children (T1–T2–T3) [60] Pyloric stenosis [60] |
| Targeted agents | ||
| Rituximab | Cell B-lymphoproliferative diseases, autoimmune disorders | Cardiac malformation, clubfoot, transient hematologic abnormalities (peripheral B-cell depletion, neutropenia, lymphopenia, thrombocytopenia and anemia), neonatal infections [61] |
| Trastuzumab | HER-2 positive breast cancer | Oligo/anhydramnios with consequent fetal renal insufficiency [62] |
| Bevacizumab | Various solid tumors | Possible IUGR, miscarriage [63] |
| Imatinib, Nilotinib, Dasatinib | Chronic myeloid leukemia | Teratogenic effects (T1) [64] No congenital malformations (T2–T3) [65,66,67] |
| Gefitinib, Erlotinib | EGFR-mutated lung cancer | No congenital malformations (T3) [68,69] |
| Vemurafenib | Melanoma | Healthy children (T2–T3) [70] |
| Antineoplastic Agents | Relatively Safe (After 1st Trimester) | Absolutely Controindicated |
|---|---|---|
| Platinum compounds | Cisplatin, Carboplatin, Oxaliplatin | - |
| Alkylating agents | Cyclophosphamide, Ifosphamide, Dacarbazine, Procarbazine, Chlorambucile | Busulphan |
| Antimetabolites | Gemcitabine, 5-Fluoruracile, Capecitabine, 6-Mercaptopurine | Methotrexate, Cytarabine |
| Antitumor antibiotics | Doxorubicine, Epirubicin, Bleomycin, Actinomycin-D | Daunorubicine, Idarubicin |
| Topoisomerase inhibitors | Irinotecan, Etoposide | - |
| Antimitotic agents | Vincristine, Vinblastine, Docetaxel, Paclitaxel | - |
| Targeted agents | Rituximab, Lapatinib, Imatinib, Nilotinib, Dasatinib, Gefitinib, Erlotinib, Vemurafenib | Trastuzumab, Bevacizumab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triarico, S.; Rivetti, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Ruggiero, A. Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy. Cancers 2022, 14, 3103. https://doi.org/10.3390/cancers14133103
Triarico S, Rivetti S, Capozza MA, Romano A, Maurizi P, Mastrangelo S, Attinà G, Ruggiero A. Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy. Cancers. 2022; 14(13):3103. https://doi.org/10.3390/cancers14133103
Chicago/Turabian StyleTriarico, Silvia, Serena Rivetti, Michele Antonio Capozza, Alberto Romano, Palma Maurizi, Stefano Mastrangelo, Giorgio Attinà, and Antonio Ruggiero. 2022. "Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy" Cancers 14, no. 13: 3103. https://doi.org/10.3390/cancers14133103
APA StyleTriarico, S., Rivetti, S., Capozza, M. A., Romano, A., Maurizi, P., Mastrangelo, S., Attinà, G., & Ruggiero, A. (2022). Transplacental Passage and Fetal Effects of Antineoplastic Treatment during Pregnancy. Cancers, 14(13), 3103. https://doi.org/10.3390/cancers14133103







