Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
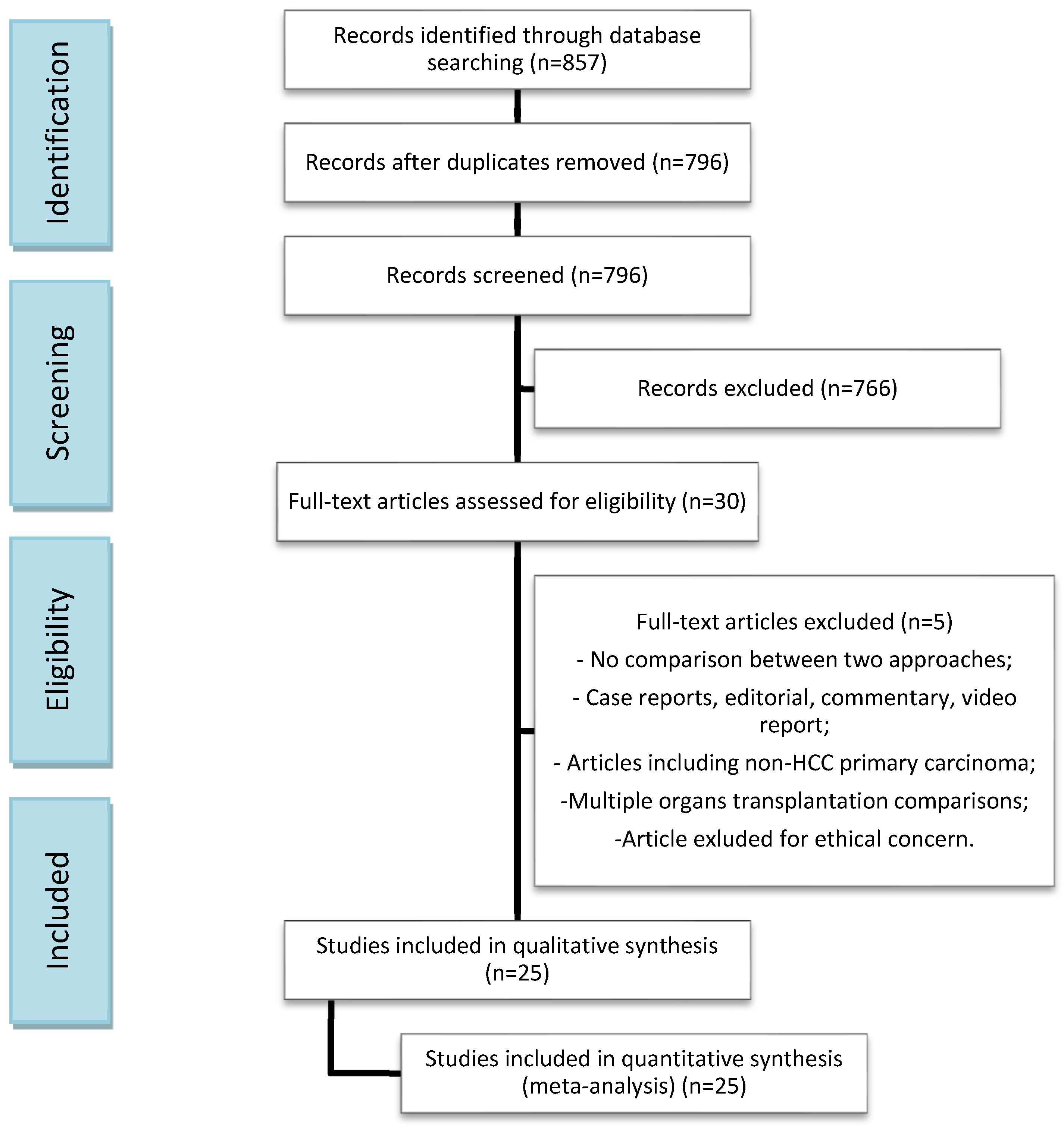
2. Materials and Methods
2.1. Study Design
2.2. Literature Search and Data Collection
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Studies and Patient Characteristics
3.2. Technical Outcomes
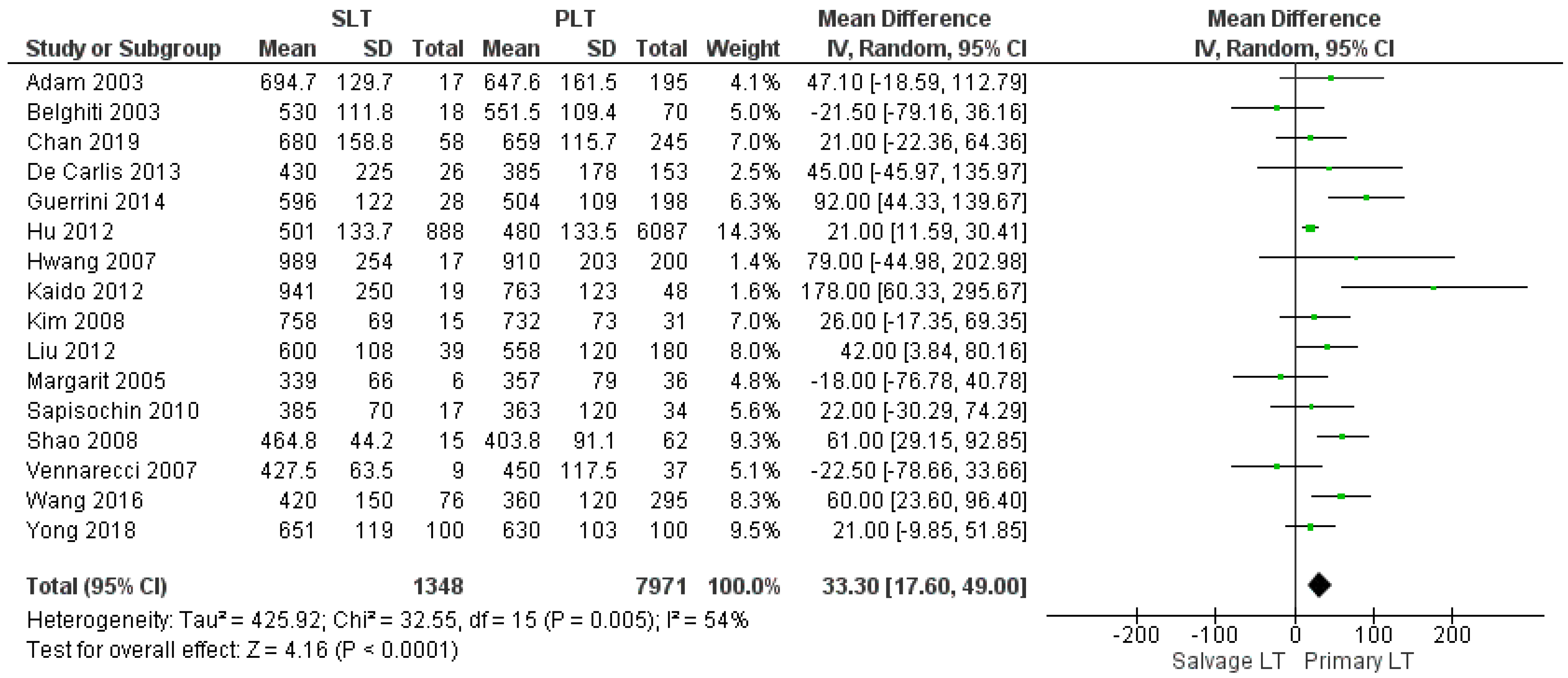
3.2.1. Duration of Surgery
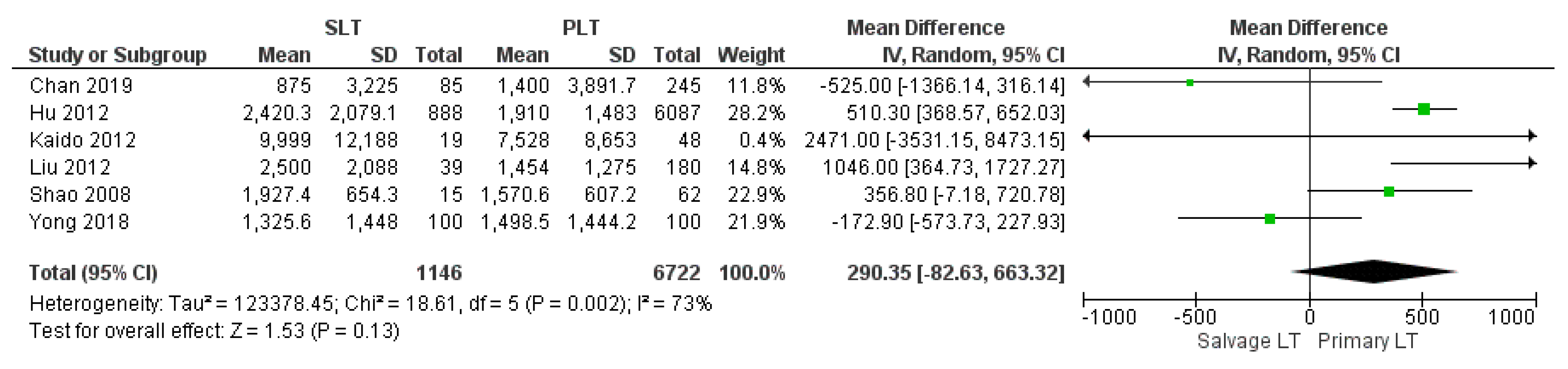
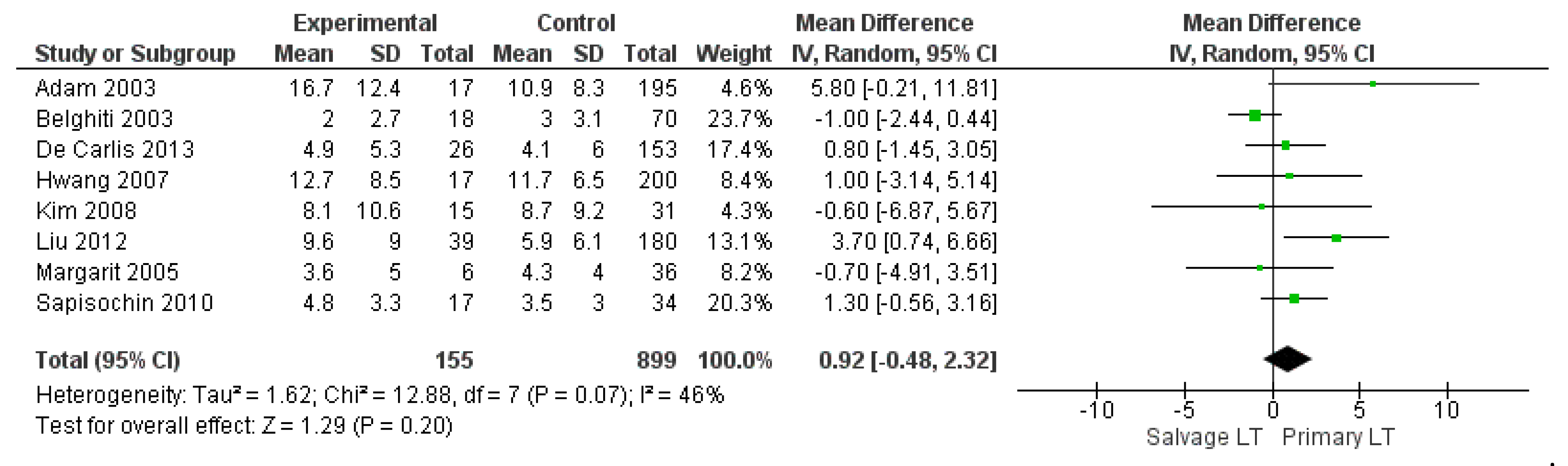
3.2.2. Intraoperative Blood Loss, Intraoperative Red Blood Cell (RBC), and Fresh Frozen Plasma (FFP) Transfusion
3.2.3. Reoperation Rate
3.2.4. Perioperative Mortality Rate
3.2.5. Retransplantation Rate
3.3. Postoperative Outcomes
3.3.1. Postoperative Bleeding
3.3.2. Intensive Care Unit Stay
3.3.3. Length of Hospitalization
3.3.4. Overall Vascular Complication
3.3.5. Arterial Thrombosis
3.3.6. Biliary Complications
3.3.7. Infection and Sepsis
3.4. Oncological and Survival Outcomes
3.4.1. Overall Survival Rates
3.4.2. HCC Recurrence Rate
3.4.3. Disease-Free Survival Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Pleguezuelo, M.; Maimone, S.; Calvaruso, V.; Xirouchakis, E.; Patch, D.; Rolando, N.; Davidson, B.; Rolles, K.; Burroughs, A. Impact of tips preliver transplantation for the outcome posttransplantation. Am. J. Transplant. 2009, 9, 192–200. [Google Scholar] [CrossRef] [PubMed]
- DuBray, B.J., Jr.; Chapman, W.C.; Anderson, C.D. Hepatocellular carcinoma: A review of the surgical approaches to management. Mo. Med. 2011, 108, 195–198. [Google Scholar]
- Abrams, P.; Marsh, J.W. Current approach to hepatocellular carcinoma. Surg. Clin. North Am. 2010, 90, 803–816. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Tarantino, G.; De Ruvo, N.; Cautero, N.; Montalti, R.; Guerrini, G.P.; Ballarin, R.; Spaggiari, M.; Smerieri, N.; Serra, V.; et al. University of Modena Experience in HIV-Positive Patients Undergoing Liver Transplantation. Transplant. Proc. 2011, 43, 1114–1118. [Google Scholar] [CrossRef]
- Lewin, S.M.; Mehta, N.; Kelley, R.K.; Roberts, J.P.; Yao, F.Y.; Brandman, D. Liver Transplant (LT) recipients with Nonalcoholic Steatohepatitis (NASH) Have Lower Risk Hepatocellular Carcinoma (HCC). Liver Transpl. 2017, 23, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology 2016, 63, 1707–1717. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Lencioni, R.; Majno, P. Early hepatocellular carcinoma on the procrustean bed of ablation, resection, and transplantation. Semin. Liver Dis. 2014, 34, 415–426. [Google Scholar]
- Alver, S.K.; Lorenz, D.J.; Washburn, K.; Marvin, M.R.; Brock, G.N. Comparison of two equivalent MELD scores for hepatocellular carcinoma patients using data from the United Network for Organ Sharing liver transplant waiting list registry. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2017, 30, 1098. [Google Scholar]
- Akamatsu, N.; Cillo, U.; Cucchetti, A.; Donadon, M.; Pinna, A.D.; Torzilli, G.; Kokudo, N. Surgery and Hepatocellular Carcinoma. Liver Cancer 2016, 6, 44–50. [Google Scholar] [CrossRef]
- Bruix, J.; Fuster, J. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations? An Observational Study of the HCC East-West Study Group. Ann. Surg. 2015, 262, e30. [Google Scholar] [CrossRef]
- Laurent, A.; Tayar, C.; Andréoletti, M.; Lauzet, J.Y.; Merle, J.C.; Cherqui, D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J. Hepatobiliary Pancreat. Surg. 2009, 16, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Aube, C.; Oberti, F.; Lonjon, J.; Pageaux, G.; Seror, O.; N’Kontchou, G.; Rode, A.; Radenne, S.; Cassinotto, C.; Vergniol, J.; et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017, 37, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Scatton, O.; Goumard, C.; Cauchy, F.; Fartoux, L.; Perdigao, F.; Conti, F.; Calmus, Y.; Boelle, P.Y.; Belghiti, J.; Rosmorduc, O.; et al. Early and resectable HCC: Definition and validation of a subgroup of patients who could avoid liver transplantation. J. Surg. Oncol. 2015, 111, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Facciuto, M.E.; Rochon, C.; Pandey, M.; Rodriguez-Davalos, M.; Samaniego, S.; Wolf, D.C.; Kim-Schluger, L.; Rozenblit, G.; Sheiner, P.A. Surgical dilemma: Liver resection or liver transplantation for hepatocellular carcinoma and cirrhosis. Intention-to-treat analysis in patients within and outwith Milan criteria. HPB 2009, 11, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Majno, P.E.; Sarasin, F.P.; Mentha, G.; Hadengue, A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: An outcome-oriented decision analysis. Hepatology 2000, 31, 899–906. [Google Scholar] [CrossRef]
- Goumard, C.; Scatton, O. Resectable HCC: Should salvage liver transplantation for HCC be discussed de principe? Clin. Res. Hepatol. Gastroenterol. 2020, 44, 117–118. [Google Scholar] [CrossRef]
- Muaddi, H.; Al-Adra, D.P.; Beecroft, R.; Ghanekar, A.; Moulton, C.A.; Doyle, A.; Selzner, M.; Wei, A.; McGilvary, I.D.; Gallinger, S.; et al. Liver Transplantation is Equally Effective as a Salvage Therapy for Patients with Hepatocellular Carcinoma Recurrence Following Radiofrequency Ablation or Liver Resection with Curative Intent. Ann. Surg. Oncol. 2018, 25, 991–999. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, J.; Wang, W.L.; Li, M.X.; Lu, Y. Short- and long-term outcomes after salvage liver transplantation versus primary liver transplantation for hepatocellular carcinoma: A meta-analysis. Transplant. Proc. 2013, 45, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Chen, W.; Bai, X.; Singh, A.; Li, G.; Ma, T.; Yu, X.; Xiao, Z.; Huang, B.; Liang, T. Salvage Liver Transplant versus Primary Liver Transplant for Patients with Hepatocellular Carcinoma. Ann. Transplant. 2018, 23, 524–545. [Google Scholar] [CrossRef]
- Xiong, Q.; Geng, T.T.; He, L.; Gao, H. Harm and Benefits of Salvage Transplantation for Hepatocellular Carcinoma: An Updated Meta-analysis. Transplant. Proc. 2016, 48, 3336–3347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Xie, Q.; Cheng, J. Salvage liver transplant for hepatocellular carcinoma: Rescues and benefits. Transl. Gastroenterol. Hepatol. 2018, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal literature search for systematic reviews in surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Adam, R.; Azoulay, D.; Castaing, D.; Eshkenazy, R.; Pascal, G.; Hashizume, K.; Samuel, D.; Bismuth, H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: A reasonable strategy? Ann. Surg. 2003, 238, 508–518; discussion 18–19. [Google Scholar] [CrossRef]
- Belghiti, J.; Cortes, A.; Abdalla, E.K.; Régimbeau, J.M.; Prakash, K.; Durand, F.; Sommacale, D.; Dondero, F.; Lesurtel, M.; Sauvanet, A.; et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann. Surg. 2003, 238, 885–893; discussion 92–93. [Google Scholar] [CrossRef]
- Margarit, C.; Escartín, A.; Castells, L.; Vargas, V.; Allende, E.; Bilbao, I. Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transplant. 2005, 11, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, S.-G.; Moon, D.-B.; Ahn, C.-S.; Kim, K.-H.; Lee, Y.-J.; Ha, T.-Y.; Song, G.-W. Salvage living donor liver transplantation after prior liver resection for hepatocellular carcinoma. Liver Transplant. 2007, 13, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Vennarecci, G.; Ettorre, G.M.; Antonini, M.; Santoro, R.; Maritti, M.; Tacconi, G.; Spoletini, D.; Tessitore, L.; Perracchio, L.; Visco, G.; et al. First-line liver resection and salvage liver transplantation are increasing therapeutic strategies for patients with hepatocellular carcinoma and child a cirrhosis. Transplant. Proc. 2007, 39, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, M.; Ercolani, G.; Ravaioli, M.; Cescon, M.; Lauro, A.; Vivarelli, M.; Zanello, M.; Cuccheti, A.; Vetrone, G.; Tuci, F.; et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am. J. Transplant. 2008, 8, 1177–1185. [Google Scholar] [CrossRef]
- Kim, B.W.; Park, Y.K.; Kim, Y.B.; Wang, H.J.; Kim, M.W. Salvage liver transplantation for recurrent hepatocellular carcinoma after liver resection: Feasibility of the Milan criteria and operative risk. Transplant. Proc. 2008, 40, 3558–3561. [Google Scholar] [CrossRef]
- Shao, Z.; Lopez, R.; Shen, B.; Yang, G.S. Orthotopic liver transplantation as a rescue operation for recurrent hepatocellular carcinoma after partial hepatectomy. World J. Gastroenterol. 2008, 14, 4370–4376. [Google Scholar] [CrossRef][Green Version]
- Cherqui, D.; Laurent, A.; Mocellin, N.; Tayar, C.; Luciani, A.; Van Nhieu, J.T.; Decaens, T.; Hurtova, M.; Memeo, R.; Mallat, A.; et al. Liver resection for transplantable hepatocellular carcinoma: Long-term survival and role of secondary liver transplantation. Ann. Surg. 2009, 250, 738–746. [Google Scholar] [CrossRef]
- Sapisochin, G.; Bilbao, I.; Balsells, J.; Dopazo, C.; Caralt, M.; Lázaro, J.L.; Castells, L.; Allende, H.; Charco, R. Optimization of liver transplantation as a treatment of intrahepatic hepatocellular carcinoma recurrence after partial liver resection: Experience of a single European series. World J. Surg. 2010, 34, 2146–2154. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, J.; Xu, X.; Li, Z.; Zhou, L.; Wu, J.; Zhang, M.; Zheng, S. Salvage liver transplantation is a reasonable option for selected patients who have recurrent hepatocellular carcinoma after liver resection. PLoS ONE 2012, 7, e36587. [Google Scholar] [CrossRef]
- Kaido, T.; Mori, A.; Ogura, Y.; Hata, K.; Yoshizawa, A.; Iida, T.; Yagi, S.; Uemoto, S. Living donor liver transplantation for recurrent hepatocellular carcinoma after liver resection. Surgery 2012, 151, 55–60. [Google Scholar] [CrossRef]
- Liu, F.; Wei, Y.; Wang, W.; Chen, K.; Yan, L.; Wen, T.; Zhao, J.; Xu, M.; Li, B. Salvage liver transplantation for recurrent hepatocellular carcinoma within UCSF criteria after liver resection. PLoS ONE 2012, 7, e48932. [Google Scholar] [CrossRef][Green Version]
- Moon, J.I.; Kwon, C.H.; Joh, J.W.; Choi, G.S.; Jung, G.O.; Kim, J.M.; Shin, M.; Choi, S.; Kim, S.; Lee, S.-K. Primary versus salvage living donor liver transplantation for patients with hepatocellular carcinoma: Impact of microvascular invasion on survival. Transplant. Proc. 2012, 44, 487–493. [Google Scholar] [CrossRef] [PubMed]
- De Carlis, L.; Di Sandro, S.; Giacomoni, A.; Mangoni, I.; Lauterio, A.; Mihaylov, P.; Cusumano, C.; Rampoldi, A. Liver transplantation for hepatocellular carcinoma recurrence after liver resection: Why deny this chance of cure? J. Clin. Gastroenterol. 2013, 47, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Gerunda, G.E.; Montalti, R.; Ballarin, R.; Cautero, N.; De Ruvo, N.; Spaggiari, M.; Di Benedetto, F. Results of salvage liver transplantation. Liver Int. 2014, 34, e96–e104. [Google Scholar] [CrossRef]
- Abe, T.; Tashiro, H.; Teraoka, Y.; Hattori, M.; Tanimine, N.; Kuroda, S.; Tahara, H.; Ohira, M.; Tanaka, Y.; Kobayashi, T.; et al. Efficacy and Feasibility of Salvage Living Donor Liver Transplantation after Initial Liver Resection in Patients with Hepatocellular Carcinoma. Dig. Surg. 2015, 33, 8–14. [Google Scholar] [CrossRef]
- Bhangui, P.; Allard, M.A.; Vibert, E.; Cherqui, D.; Pelletier, G.; Cunha, A.S.; Guettier, C.; Valle, J.-C.D.; Saliba, F.; Bismuth, H.; et al. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann. Surg. 2016, 264, 155–163. [Google Scholar] [CrossRef]
- Bhavin Bhupendra Vasavada C-LC. Salvage transplantation for post-resection recurrence in hepatocellular carcinoma associated with hepatitis C virus. etiology: A feasible strategy? Hepatoma Res. 2015, 1, 36–40. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Pu, Y.; Li, H.; Shi, B.; Zheng, S.; Zhong, L. Prognosis for recipients with hepatocellular carcinoma of salvage liver transplantation versus those of primary liver transplantation: A retrospective single-center study. Springer Plus 2016, 5, 1809. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, L.; Xia, Q. Salvage Liver Transplantation Leads to Poorer Outcome in Hepatocellular Carcinoma Compared with Primary Liver Transplantation. Sci. Rep. 2017, 7, 44652. [Google Scholar] [CrossRef]
- Yong, C.C.; Elsarawy, A.M.; Wang, S.H.; Lin, T.S.; Wang, C.C.; Li, W.F.; Lin, T.-L.; Kuo, F.-Y.; Cheng, Y.-F.; Chen, C.-L.; et al. The surgical challenges of salvage living donor liver transplantation for Hepatocellular carcinoma; The cumulative experience of 100 cases-A retrospective cohort study and a propensity score analysis. Int. J. Surg. 2018, 54, 187–192. [Google Scholar] [CrossRef]
- Chan, K.M.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Lee, W.-C. Salvage living donor liver transplantation for posthepatectomy recurrence: A higher incidence of recurrence but promising strategy for long-term survival. Cancer Manag. Res. 2019, 11, 7295–7305. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tan, E.K.; Krishnamoorthy, T.L.; Tan, C.K.; Tan, B.H.; Tan, T.T.; Lee, S.-Y.; Chan, C.-Y.; Cheow, P.-C.; Chung, A.Y.F.; et al. Outcomes of salvage liver transplant for recurrent hepatocellular carcinoma: A comparison with primary liver transplant. Ann. Hepatobiliary Pancreat. Surg. 2019, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Song, G.W.; Ahn, C.S.; Kim, K.H.; Moon, D.B.; Ha, T.Y.; Jung, D.; Park, G.; Yoon, Y.; Lee, S. Salvage living donor liver transplantation for hepatocellular carcinoma recurrence after hepatectomy: Quantitative prediction using ADV score. J. Hepatobiliary Pancreat. Sci. 2021, 28, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Toniutto, P.; Zanetto, A.; Ferrarese, A.; Burra, P. Current challenges and future directions for liver transplantation. Liver Int. 2016, 37, 317–327. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, C.; Ha, T.; Moon, D.; Choi, K.; Song, G.; Chung, D.; Park, G.; Yu, Y.; Choi, N.; et al. Liver transplantation for hepatocellular carcinoma: Korean experience. J. Hepatobiliary Pancreat. Sci. 2010, 17, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.Z. Liver transplantation as a management of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1347–1354. [Google Scholar] [CrossRef]
- Cucchetti, A.; Cescon, M.; Bertuzzo, V.; Bigonzi, E.; Ercolani, G.; Morelli, M.C.; Ravaioli, M.; Pinna, A.D. Can the dropout risk of candidates with hepatocellular carcinoma predict survival after liver transplantation? Am. J. Transplant. 2011, 11, 1696–1704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Benedetto, F.; Tarantino, G.; De Ruvo, N.; Cautero, N.; Montalti, R.; Guerrini, G.P.; Ballarin, R.; Spaggiari, M.; Serra, V.; Guaraldi, G.; et al. Liver Transplantation for Hepatocellular Carcinoma in HIV Co-Infected Patients: A Single Centre Experience. Liver Transplant. 2011, 17, S273–S274. [Google Scholar]
- Guerrini, G.P.; Berretta, M.; Guaraldi, G.; Magistri, P.; Esposito, G.; Ballarin, R.; Serra, V.; Di Sandro, S.; Di Benedetto, F. Liver Transplantation for HCC in HIV-Infected Patients: Long-Term Single-Center Experience. Cancers 2021, 13, 4727. [Google Scholar] [CrossRef]
- Xu, D.W.; Wan, P.; Xia, Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: A review. World J. Gastroenterol. 2016, 22, 3325–3334. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Mimmo, A.; De Ruvo, N.; Montalti, R.; Cautero, N.; Guerrini, G.P.; Gerunda, G.E. Liver Transplantation Due to TIPS Complications. Liver Transplant. 2010, 16, S161. [Google Scholar]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transplant. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Jarnagin, W.R. Management of small hepatocellular carcinoma: A review of transplantation, resection, and ablation. Ann. Surg. Oncol. 2010, 17, 1226–1233. [Google Scholar] [CrossRef][Green Version]
- Morise, Z.; Kawabe, N.; Tomishige, H.; Nagata, H.; Kawase, J.; Arakawa, S.; Yoshida, R.; Isetani, M. Recent advances in liver resection for hepatocellular carcinoma. Front. Surg. 2014, 1, 21. [Google Scholar] [CrossRef]
- Hanish, S.I.; Knechtle, S.J. Liver transplantation for the treatment of hepatocellular carcinoma. Oncology 2011, 25, 752–757. [Google Scholar]
- Llovet, J.M.; Fuster, J.; Bruix, J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology 1999, 30, 1434–1440. [Google Scholar] [CrossRef]
- Colombo, M.; Sangiovanni, A. Treatment of hepatocellular carcinoma: Beyond international guidelines. Liver Int. 2015, 35 (Suppl. S1), 129–138. [Google Scholar] [CrossRef]
- Magistri, P.; Olivieri, T.; Assirati, G.; Guerrini, G.P.; Ballarin, R.; Tarantino, G.; Di Benedetto, F. Robotic Liver Resection Expands the Opportunities of Bridging Before Liver Transplantation. Liver Transpl. 2019, 25, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.H.; Ha, T.Y.; Jung, D.H.; Park, G.C.; Lee, S.G. Salvage living donor liver transplantation for recurrent hepatocellular carcinoma after prior laparoscopic hepatectomy. Hepatobiliary Pancreat Dis Int. 2018, 17, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Felli, E.; Baumert, T.; Pessaux, P. Is minimally invasive true anatomical HCC resection a future way to improve results in bridge or salvage liver transplantation? Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101396. [Google Scholar] [CrossRef]
- Qu, W.; Zhu, Z.J.; Sun, L.Y.; Wei, L.; Liu, Y.; Zeng, Z.G. Salvage liver transplantation for hepatocellular carcinoma recurrence after primary liver resection. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Delman, K.A.; Vauthey, J.N.; Nagorney, D.M.; Ng, I.O.; Ikai, I.; Yamaoka, Y.; Belghiti, J.; Lauwers, G.Y.; Poon, R.T.; et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transplant. 2005, 11, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Pinelli, D.; Di Benedetto, F.; Marini, E.; Corno, V.; Guizzetti, M.; Aluffi, A.; Zambelli, M.; Fagiuoli, S.; Lucà, M.G.; et al. Predictive value of nodule size and differentiation in HCC recurrence after liver transplantation. Surg. Oncol. 2015, 25, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Fàbrega, J.; Forner, A.; Liccioni, A.; Miquel, R.; Molina, V.; Navasa, M.; Fondevila, C.; García-Valdecasas, J.C.; Bruix, J.; Fuster, J. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology 2016, 63, 839–849. [Google Scholar] [CrossRef]
- Sala, M.; Fuster, J.; Llovet, J.M.; Navasa, M.; Solé, M.; Varela, M.; Pons, F.; Rimola, A.; García-Valdecasas, J.C.; Brú, C.; et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: An indication for salvage liver transplantation. Liver Transplant. 2004, 10, 1294–1300. [Google Scholar] [CrossRef]
- Lee, S.G. Salvage living-donor liver transplantation to previously hepatectomized hepatocellular carcinoma patients: Is it a reasonable strategy? Hepatobiliary Pancreat. Dis. Int. 2013, 12, 10–11. [Google Scholar] [CrossRef]
- Fuks, D.; Dokmak, S.; Paradis, V.; Diouf, M.; Durand, F.; Belghiti, J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: An intention-to-treat analysis. Hepatology 2012, 55, 132–140. [Google Scholar] [CrossRef]
- Chok, K. Management of recurrent hepatocellular carcinoma after liver transplant. World J. Hepatol. 2015, 7, 1142–1148. [Google Scholar] [CrossRef]
- Tarantino, G.; Magistri, P.; Ballarin, R.; Di Francia, R.; Berretta, M.; Di Benedetto, F. Oncological Impact of M-Tor Inhibitor Immunosuppressive Therapy after Liver Transplantation for Hepatocellular Carcinoma: Review of the Literature. Front. Pharmacol. 2016, 7, 387. [Google Scholar] [CrossRef]
- Duvoux, C.; Toso, C. mTOR inhibitor therapy: Does it prevent HCC recurrence after liver transplantation? Transplant. Rev. 2015, 29, 168–174. [Google Scholar] [CrossRef]
- Perricone, G.; Mancuso, A.; Belli, L.S.; Mazzarelli, C.; Zavaglia, C. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation: Does mTOR inhibitors association augment toxicity? Eur. J. Gastroenterol. Hepatol. 2014, 26, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews of clinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
| n. | Author | Region | Year | Study Period | Study Design | Sample Size | Follow-Up (mo) | LDLT/DDLT | MINORS (Quality) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SLT | PLT | SLT | PLT | ||||||||
| 1 | Adam [29] | France | 2003 | 1984–2000 | OCS (R) | 17 | 195 | 49 | 51 | DDLT | 21 |
| 2 | Belghiti [30] | France | 2003 | 1991–2001 | OCS (R) | 18 | 70 | 56.2 | 56.2 | DDLT | 21 |
| 3 | Margarit [31] | Spain | 2005 | 1988–2002 | OCS (P) | 6 | 36 | NA | NA | NA | 20 |
| 4 | Hwang [32] | Korea | 2007 | 1997–2006 | OCS (R) | 17 | 200 | 30.7 | 40.1 | LDLT | 22 |
| 5 | Vennarecci [33] | Italy | 2007 | 2001–2006 | OCS (P) | 9 | 37 | 26.3 | 26.3 | NA | 23 |
| 6 | Del Gadio [34] | Italy | 2008 | 1996–2005 | OCS (R) | 16 | 147 | 26.2 | 36 | DDLT | 23 |
| 7 | Kim [35] | Korea | 2008 | 2005–2007 | OCS (NA) | 15 | 31 | 18.3 | 18.7 | DDLT + LDLT | 20 |
| 8 | Shao [36] | China | 2008 | 2003–2005 | OCS (P) | 15 | 62 | 18 | 22.4 | DDLT | 22 |
| 9 | Cherqui [37] | France | 2009 | 1990–2007 | OCS (R) | 18 | 136 | 57.6 | 576 | DDLT | 21 |
| 10 | Sapisochin [38] | Spain | 2010 | 1990–2007 | OCS (P) | 17 | 34 | 70 | 70 | NA | 22 |
| 11 | Hu [39] | China | 2012 | 1999–2009 | OCS (R) | 888 | 6087 | 15.2 | 15 | DDLT + LDLT | 22 |
| 12 | Kaido [40] | Japan | 2012 | 1999–2009 | OCS (R) | 19 | 48 | 77 | 77 | LDLT | 22 |
| 13 | Liu [41] | China | 2012 | 2001–2011 | OCS (R) | 39 | 180 | 30 | 33 | DDLT + LDLT | 22 |
| 14 | Moon [42] | Korea | 2012 | 1996–2008 | OCS (R) | 17 | 169 | 27.3 | 39 | LDLT | 21 |
| 15 | De Carlis [43] | Italy | 2013 | 2000–2009 | OCS (R) | 26 | 153 | NA | NA | NA | 22 |
| 16 | Guerrini [44] | Italy | 2014 | 2000–2011 | OCS (P) | 28 | 198 | 44.2 | 44.2 | DDLT + LDLT | 22 |
| 17 | Abe [45] | Japan | 2015 | 2001–2011 | OCS (R) | 15 | 45 | 66.3 | 73.2 | LDLT | 22 |
| 18 | Bhangui [46] | France | 2015 | 1990–2012 | OCS (P) | 31 | 340 | 62 | 62 | DDLT | 23 |
| 19 | Vasavada [47] | China | 2015 | 2002–2012 | OCS (R) | 18 | 91 | NA | NA | LDLT | 22 |
| 20 | Whang [48] | China | 2016 | 2001–2011 | OCS (P) | 76 | 295 | 32.4 | 32.4 | DDLT | 23 |
| 21 | Shan [49] | China | 2017 | 2006–2015 | OCS (R) | 28 | 211 | 35 | 35 | DDLT + LDLT | 21 |
| 22 | Yong [50] | Taiwan | 2018 | 2000–2015 | OCS (R) | 100 | 100 | NA | NA | LDLT | 22 |
| 23 | Chan [51] | Taiwan | 2019 | 2001–2018 | OCS (R) | 58 | 245 | NA | NA | LDLT | 22 |
| 24 | Guo [52] | Singapore | 2019 | 2006–2017 | OCS (P) | 14 | 35 | 43.9 | 43.9 | DDLT + LDLT | 22 |
| 25 | Hwan [53] | Korea | 2020 | 2007–2018 | OCS (R) | 125 | 500 | NA | NA | LDLT | 23 |
| SLT | PLT | Patient (Studies) | |
|---|---|---|---|
| Total patients included | 1630 | 9645 | 11,275 (25) |
| Follow-up (months) | 41.3 | 43.8 | 19 |
| HBV infection (%) | 1166/1399 (83.3) | 7157/8652 (82.7) | 16 |
| HCV infection (%) | 103/1240 (8.3) | 786/7842 (10) | 10 |
| MELD score | 11 | 14 | 12 |
| AFP (ng/dl) pre-LT | 184.2 | 208.4 | 11 |
| MILAN in pre-Lt (%) | 264/419 (63) | 1683/2391 (70.4) | 15 |
| MILAN IN on explant (%) | 183/268 (68.2) | 702/948 (74) | 4 |
| Pre-LT Locoregional Treatments (%) | 812/1221 (66.5) | 2901/7600 (38.2) | 11 |
| Waiting list time (months) | 9.6 | 7.2 | 6 |
| Maximum tumor diameter pre LT (cm) | 2.6 | 2.6 | 4 |
| Maximum tumor diameter on explant (cm) | 2.6 | 2.9 | 12 |
| Number of HCC nodule pre LT | 2 | 1.6 | 4 |
| Number of HCC nodule on explant | 3.3 | 2 | 9 |
| Sum of tumor size on explant (cm) | 3.1 | 3.8 | 4 |
| Microvascular invasion (%) | 145/491 (29.5) | 394/1860 (21.2) | 13 |
| Technical and postoperative outcomes | ||||
| Surgical outcome | Type of surgery | Observations (n) | Mean or % | Studies included (n) |
| Operating time (min) | SLT | 1348 | 600.44 | 16 |
| PLT | 7971 | 547.12 | ||
| Blood loss (ml) | SLT | 1146 | 3174.55 | 6 |
| PLT | 6722 | 2342.02 | ||
| RBC transfusion | SLT | 155 | 7.8 | 8 |
| PLT | 899 | 6.5 | ||
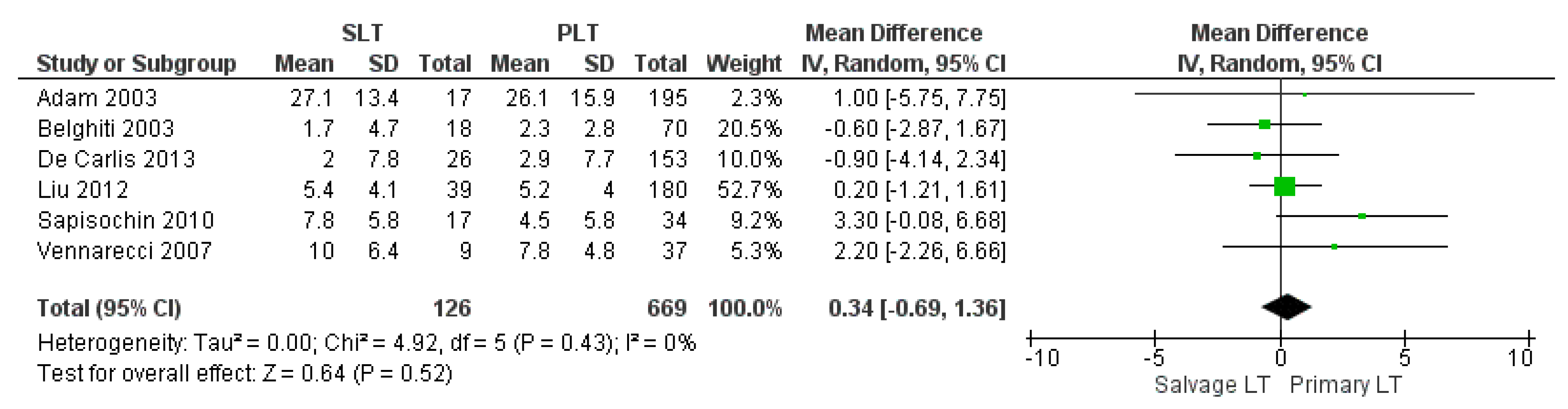
| FFP transfusion | SLT | 126 | 9 | 6 |
| PLT | 669 | 8 | ||
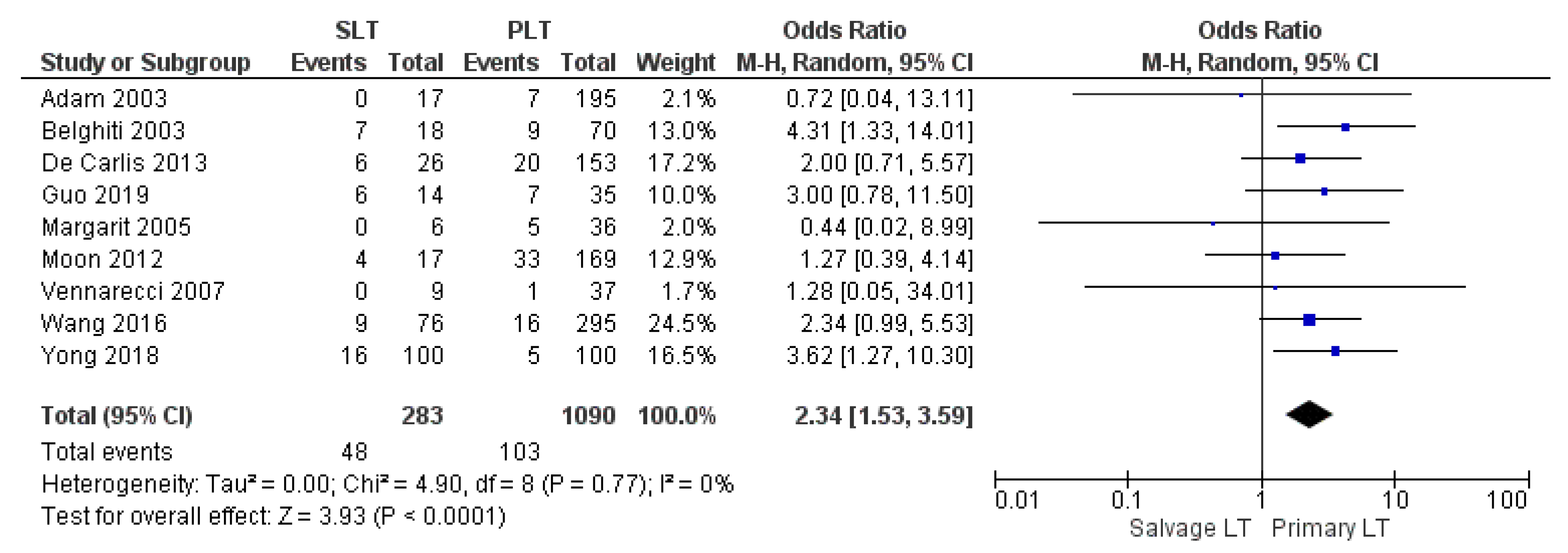
| Reoperation rate | SLT | 48/283 | 16.9% | 9 |
| PLT | 103/1090 | 9.4% | ||
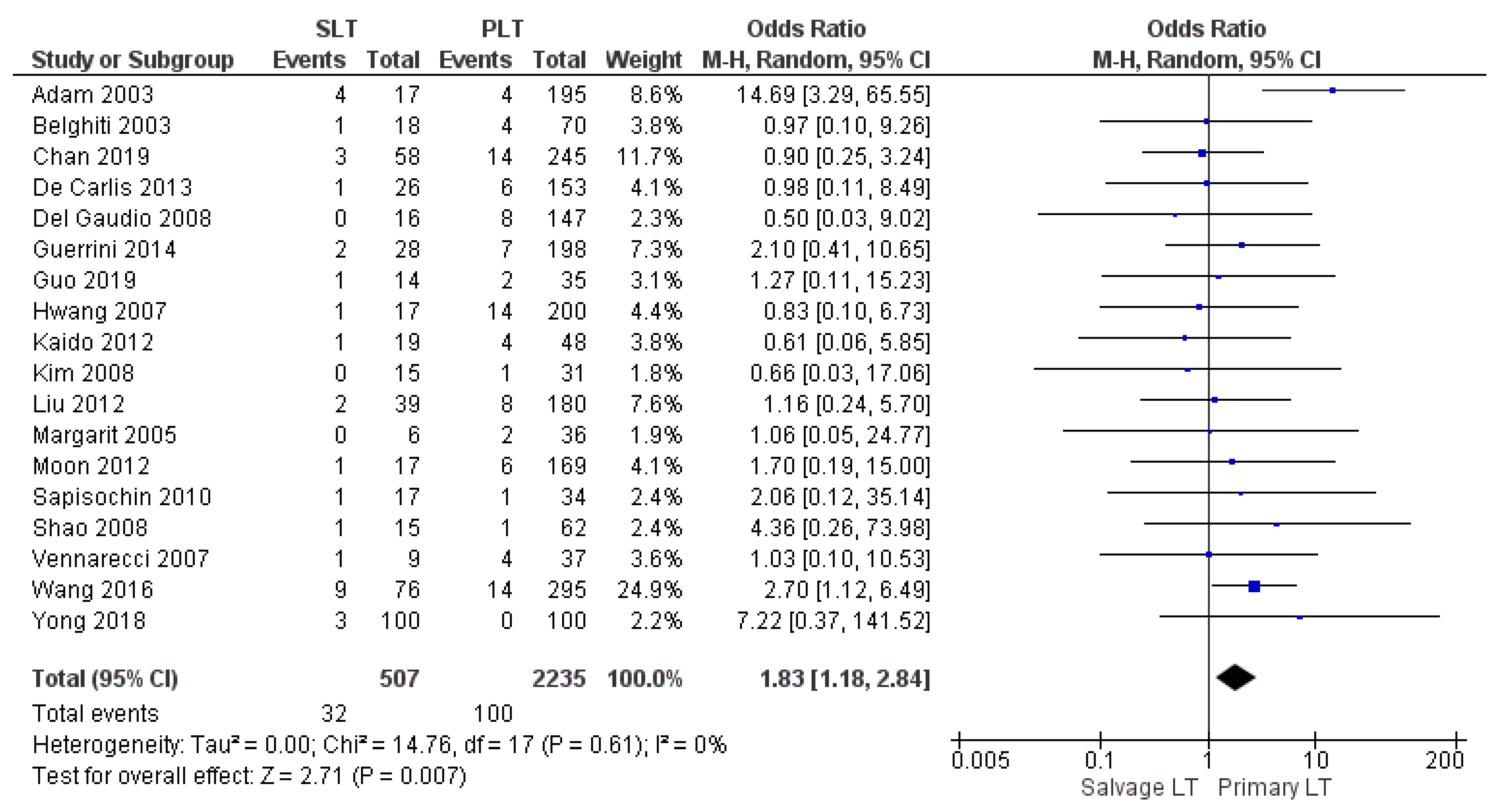
| Mortality rate | SLT | 32/507 | 6.3% | 18 |
| PLT | 100/2235 | 4.5% | ||
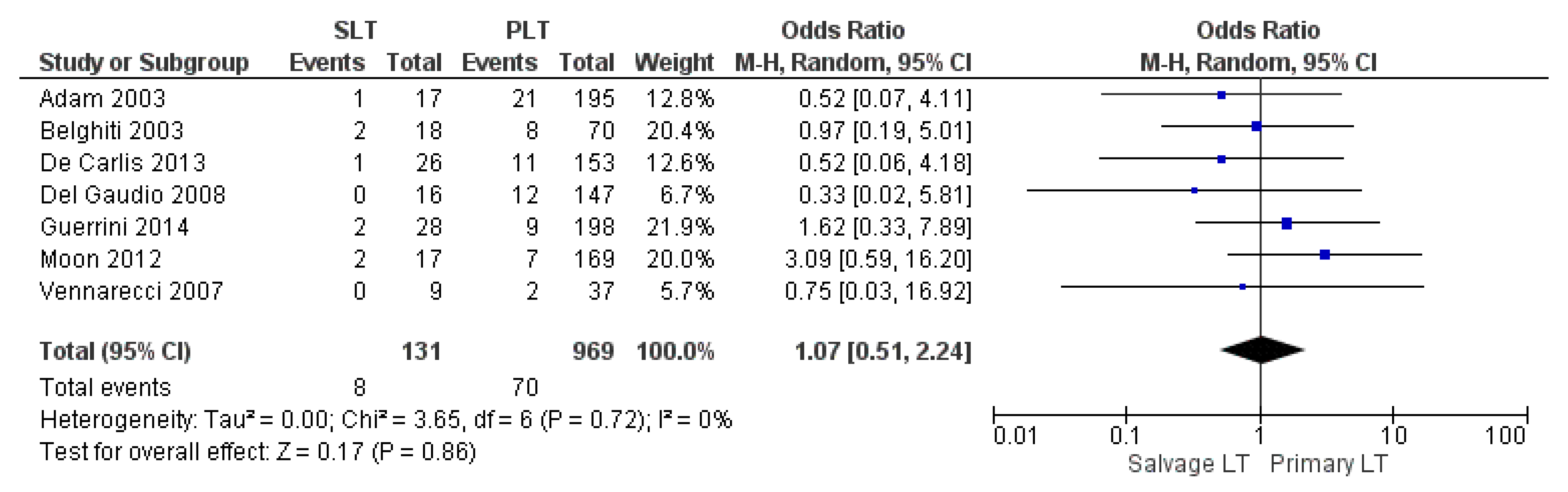
| Re-transplantation rate | SLT | 8/131 | 6.1% | 7 |
| PLT | 70/969 | 7.2% | ||
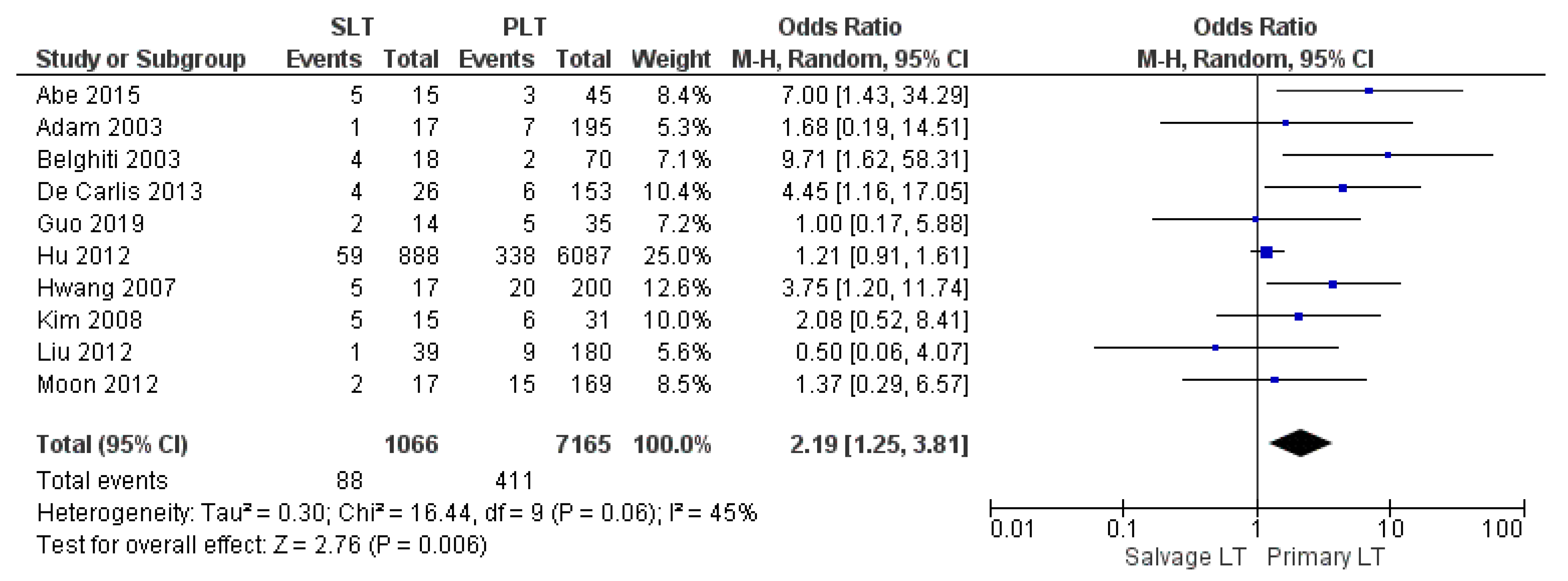
| Postoperative bleeding | SLT | 88/1066 | 8.25% | 10 |
| PLT | 411/7165 | 5.73% | ||
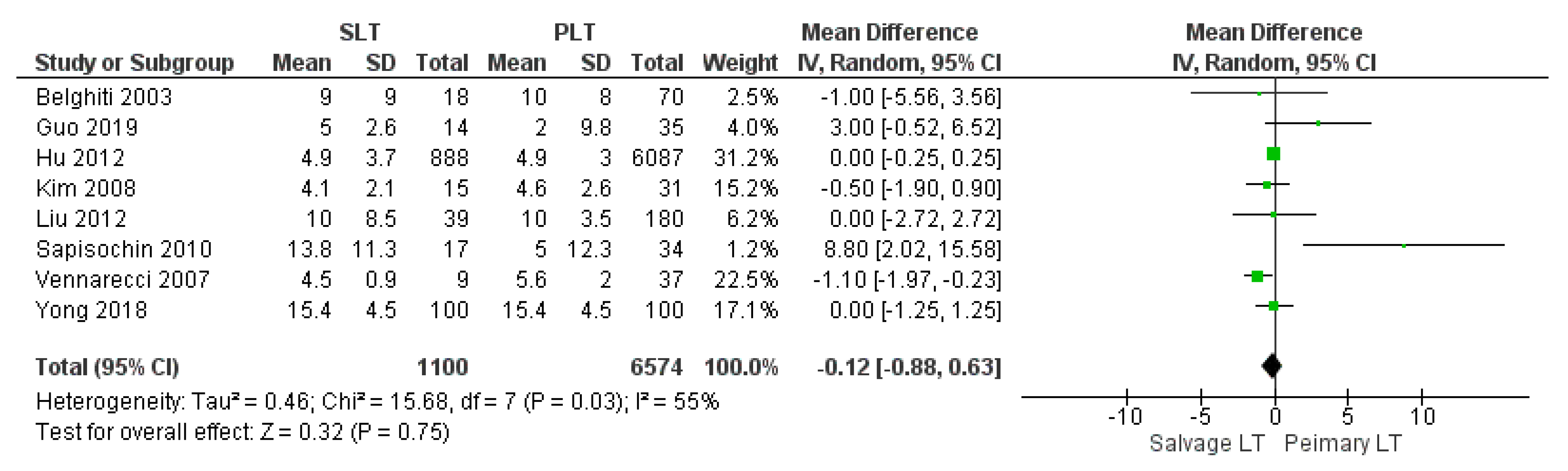
| ICU stay (days) | SLT | 1100 | 8.34 | 8 |
| PLT | 6574 | 5.44 | ||
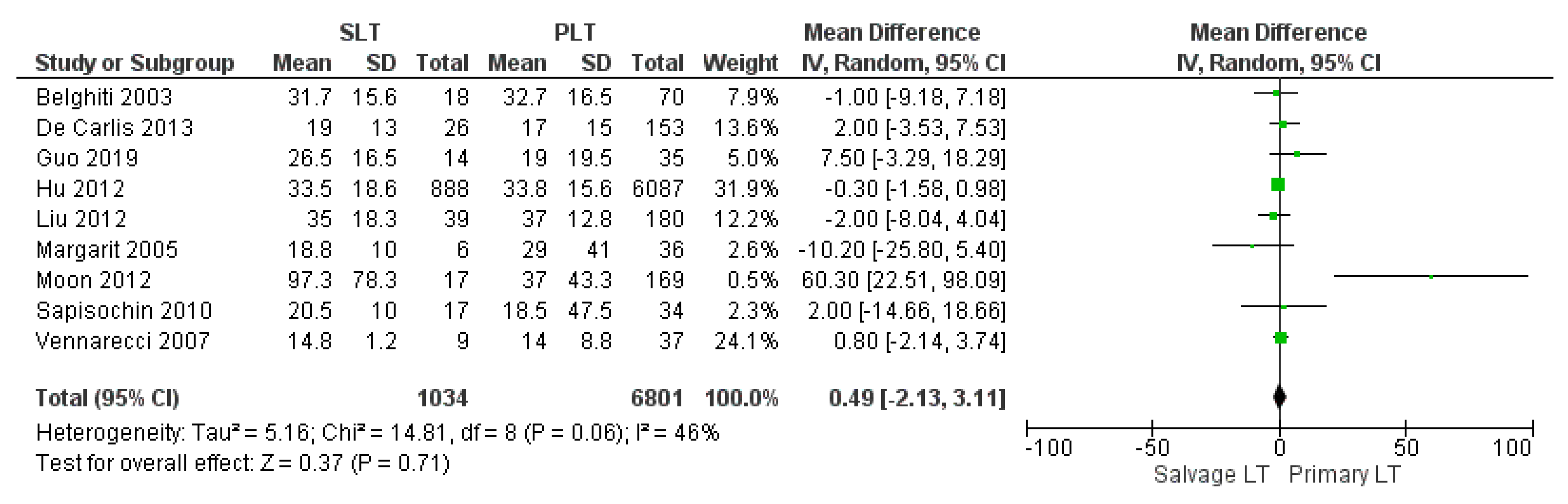
| Hospital stay (days) | SLT | 1034 | 33.01 | 9 |
| PLT | 6801 | 26.44 | ||
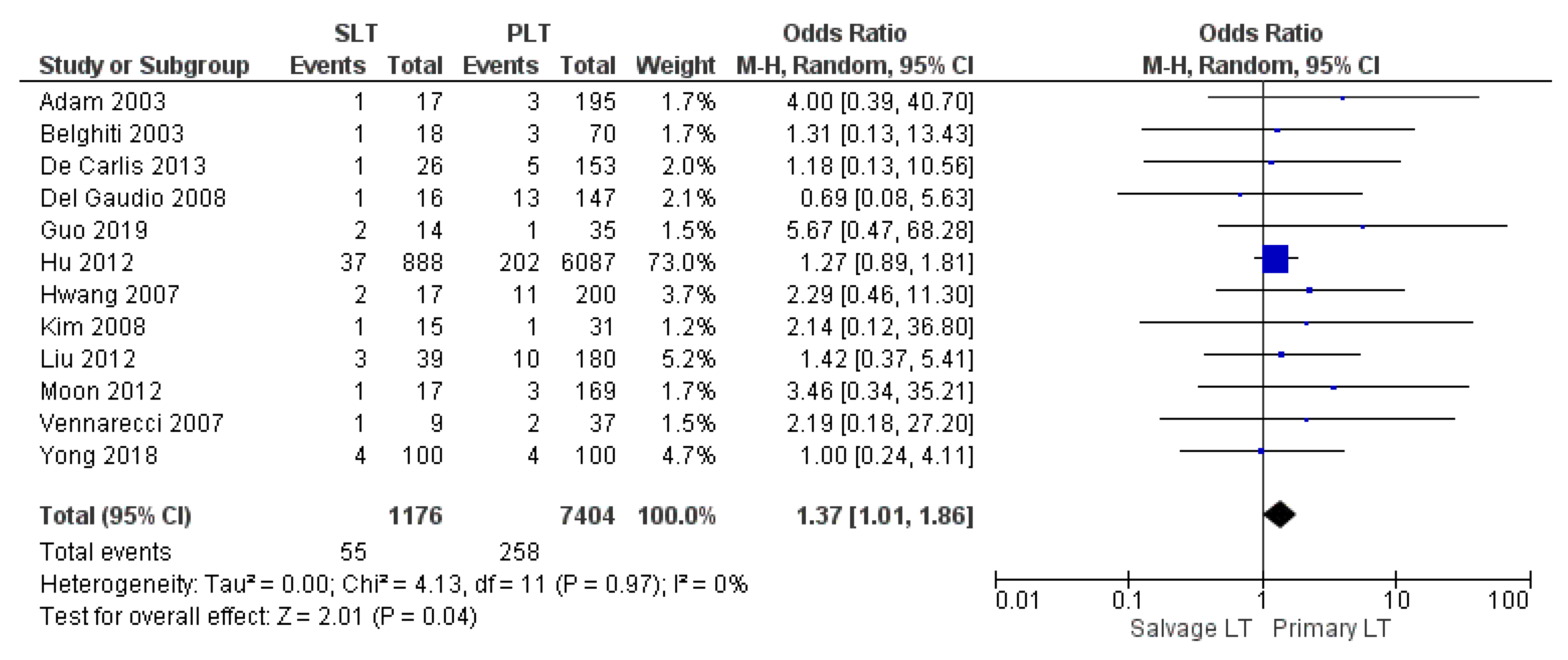
| Vascular complication | SLT | 55/1176 | 4.68% | 12 |
| PLT | 258/7404 | 3.48% | ||
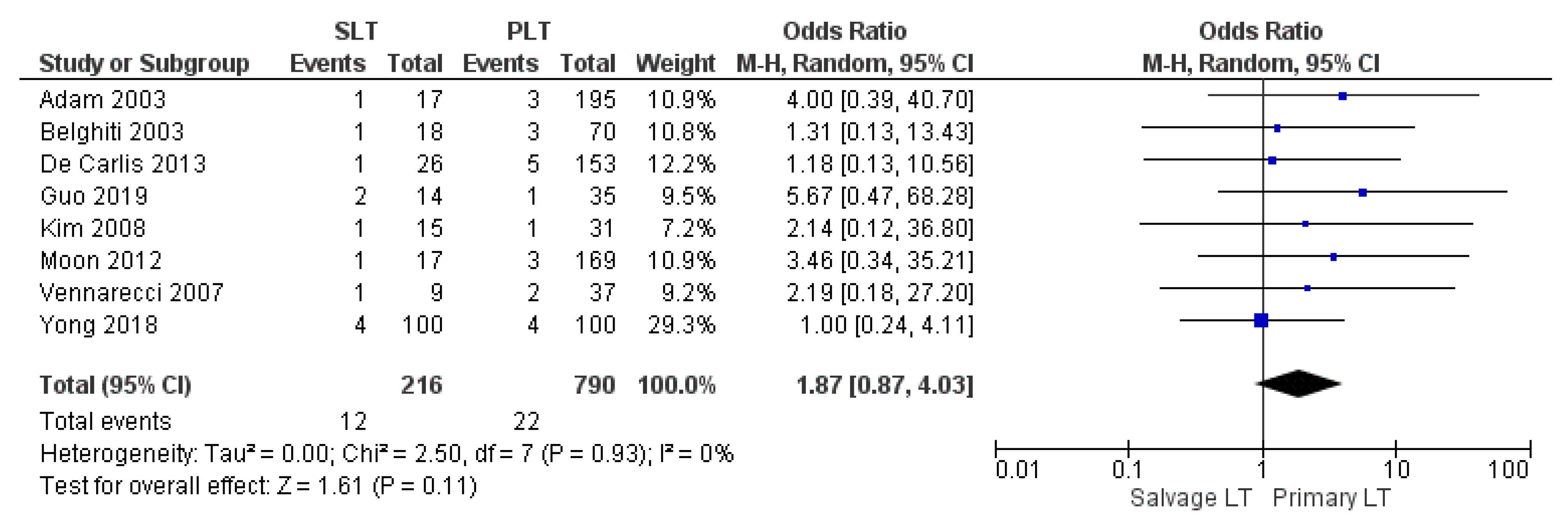
| Arterial thrombosis | SLT | 12/216 | 5.56% | 8 |
| PLT | 22/790 | 2.78% | ||
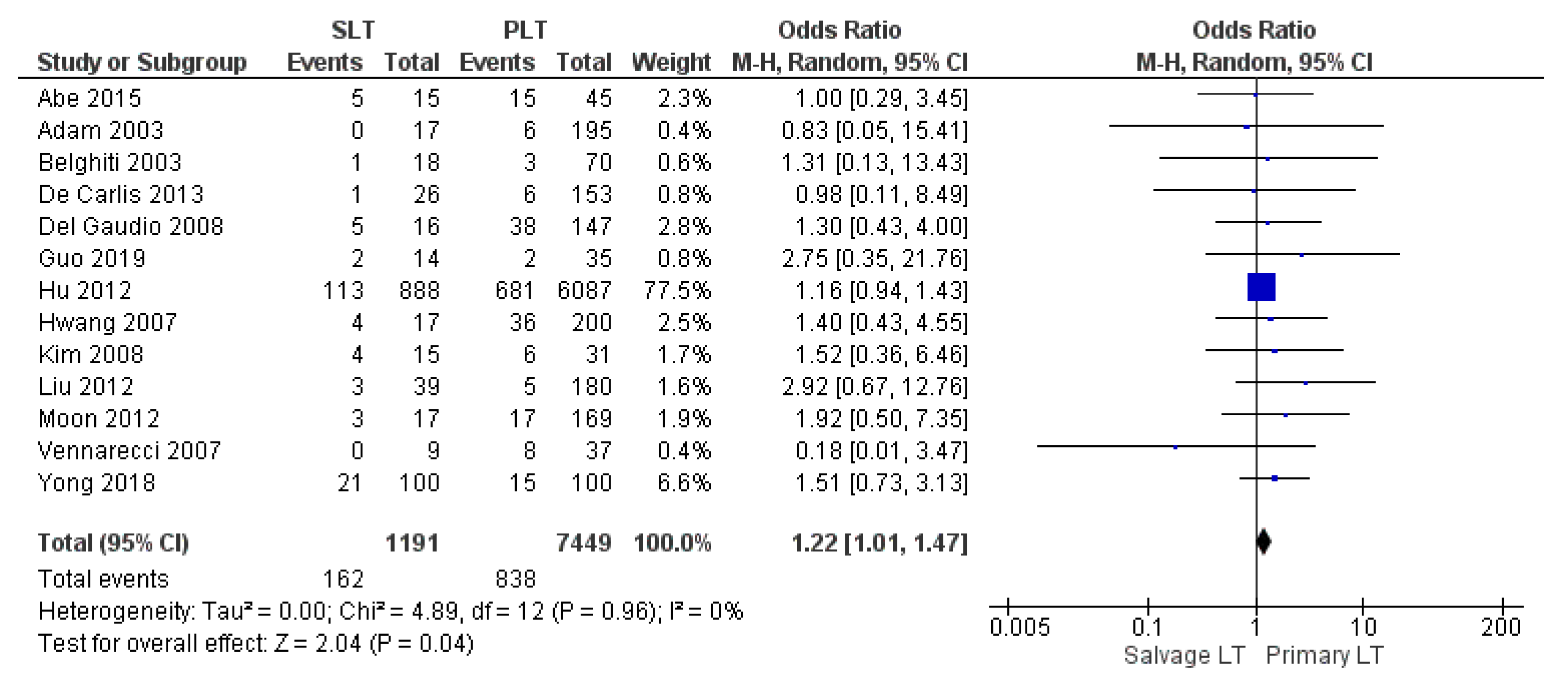
| Biliary complication | SLT | 162/1191 | 13.6% | 13 |
| PLT | 838/7449 | 11.2% | ||
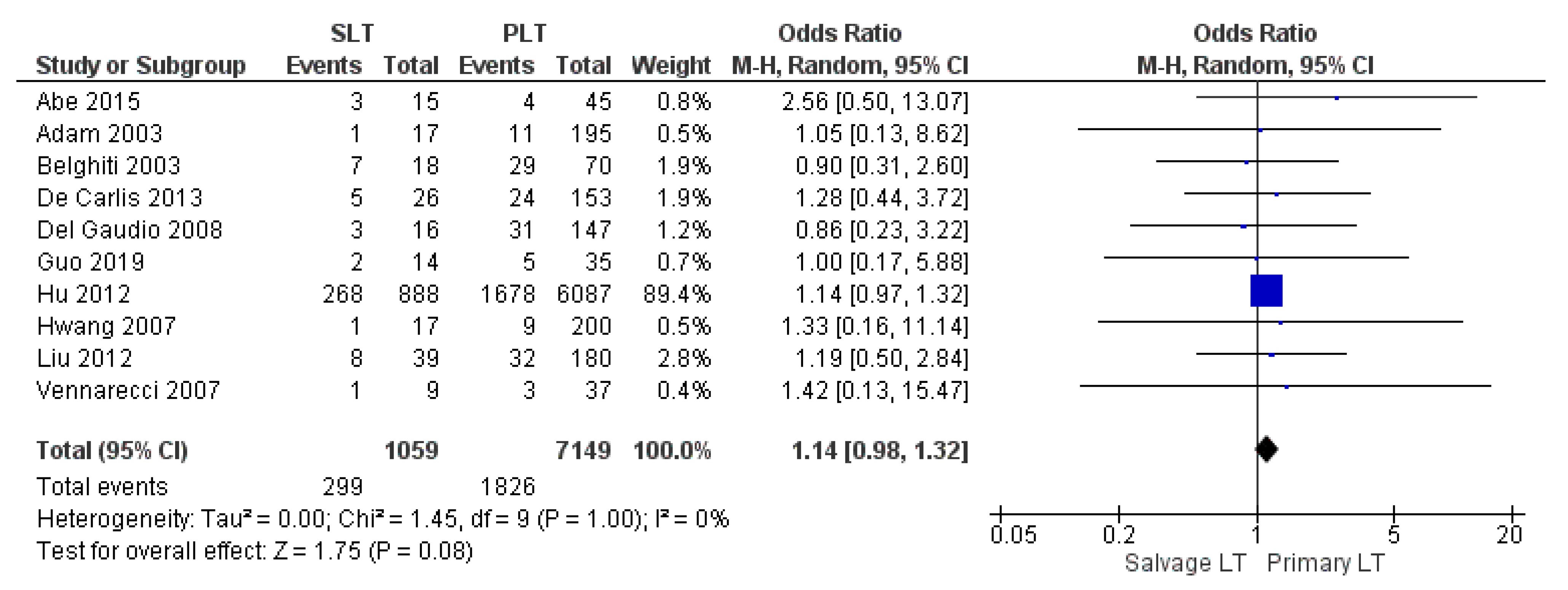
| Infection and sepsis | SLT | 299/1059 | 28.2% | 10 |
| PLT | 1826/7149 | 25.5% | ||
| Oncological and survival outcomes | ||||
| Oncological outcome | Type of surgery | Observations (n) | % | Studies included (n) |
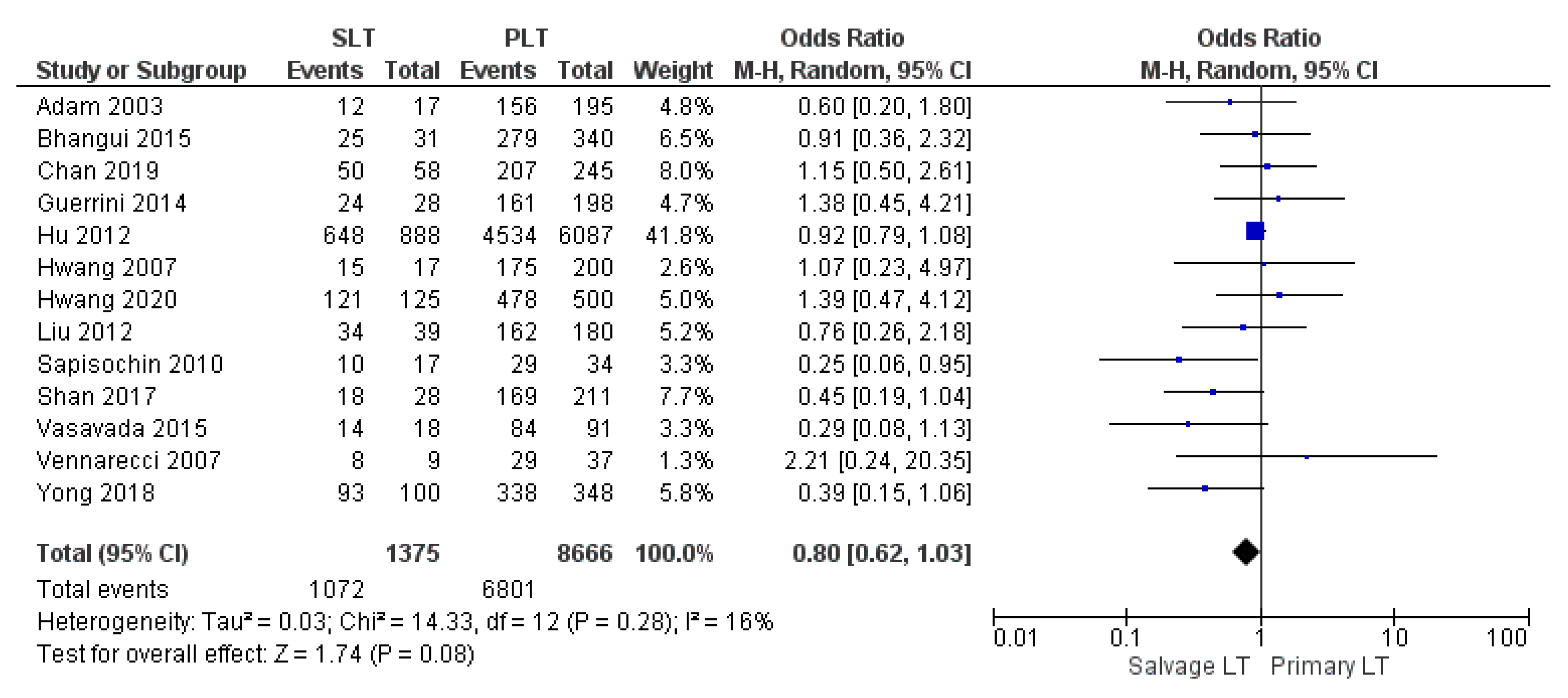
| 1-yr OS | SLT | 1072/1375 | 77.9% | 13 |
| PLT | 6801/8666 | 78.5% | ||
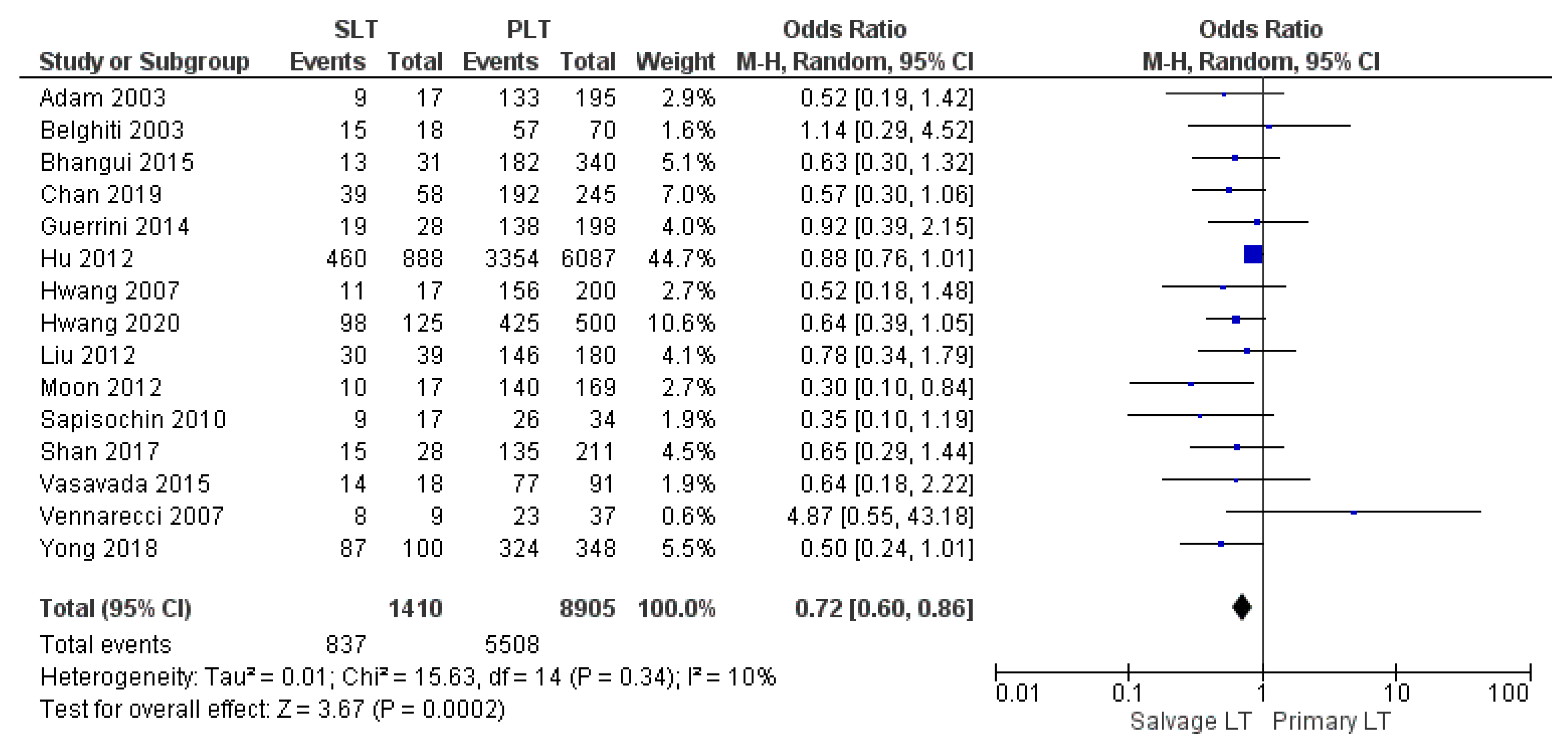
| 3-yr OS | SLT | 837/1410 | 59.3% | 15 |
| PLT | 5508/8950 | 61.9% | ||
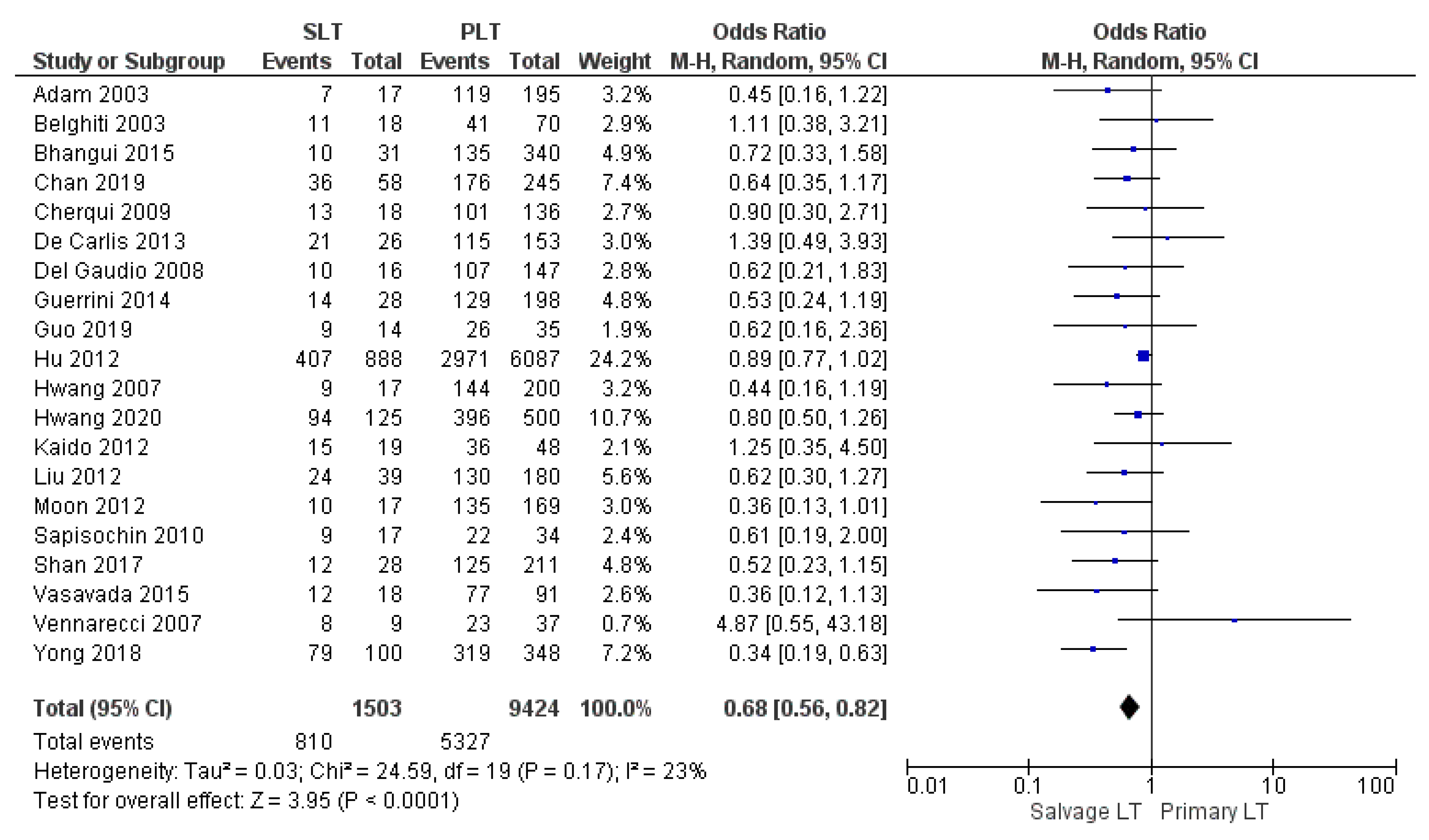
| 5-yr OS | SLT | 810/1503 | 53.9% | 20 |
| PLT | 5327/9424 | 56.5% | ||
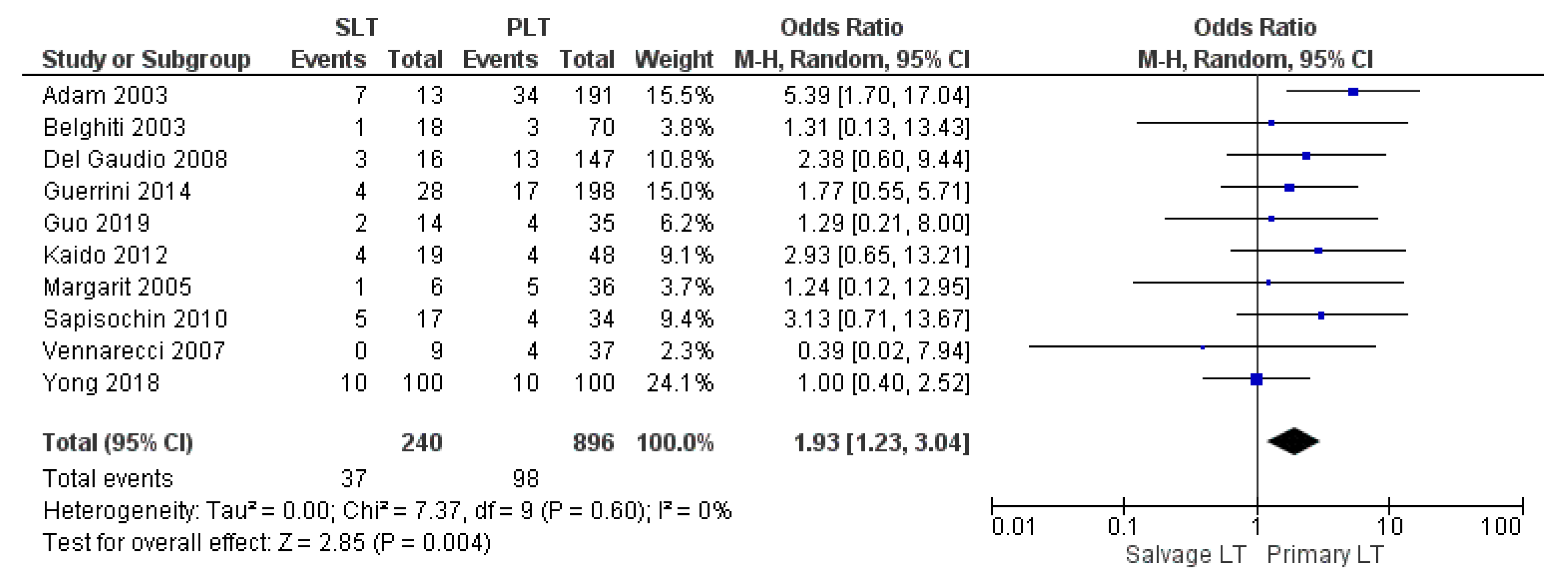
| HCC recurrence | SLT | 37/240 | 15.4% | 10 |
| PLT | 98/896 | 10.9% | ||
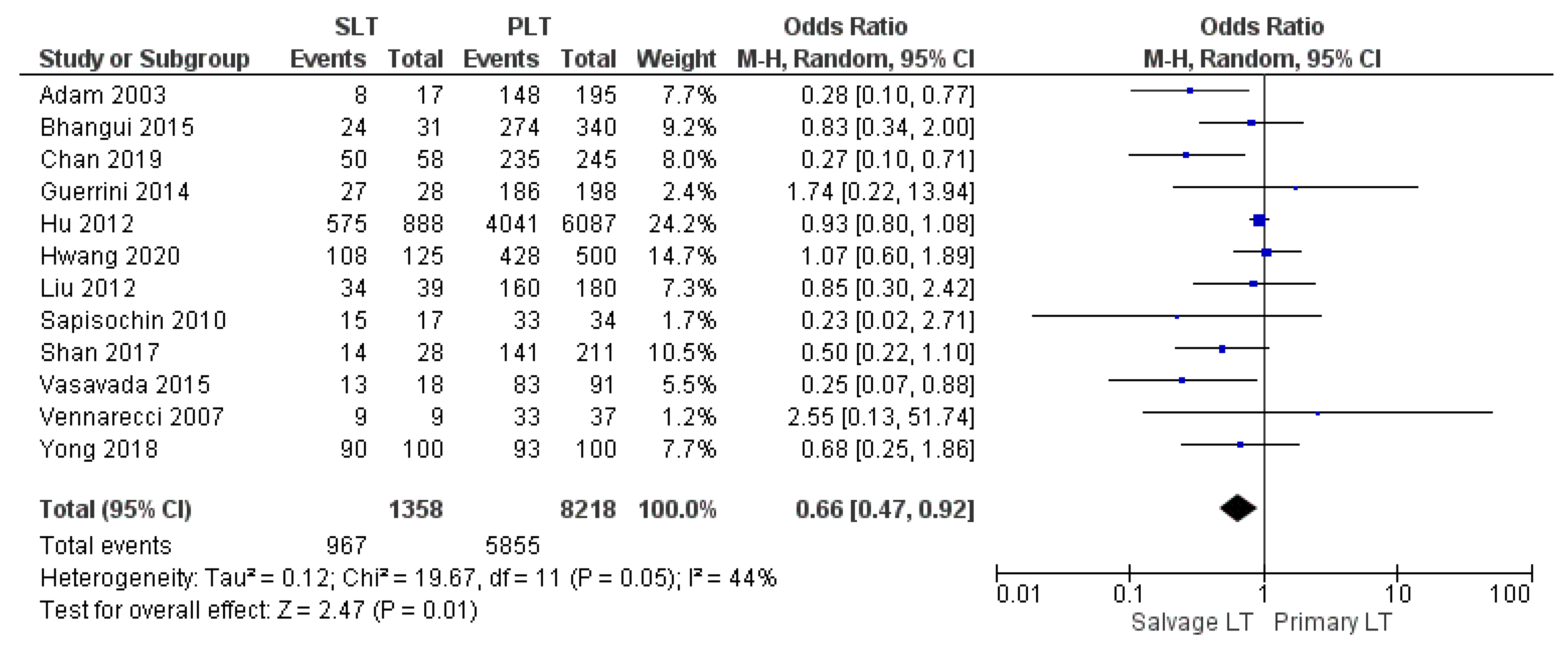
| 1-yr DFS | SLT | 967/1358 | 71.2% | 12 |
| PLT | 5855/8218 | 71.2% | ||
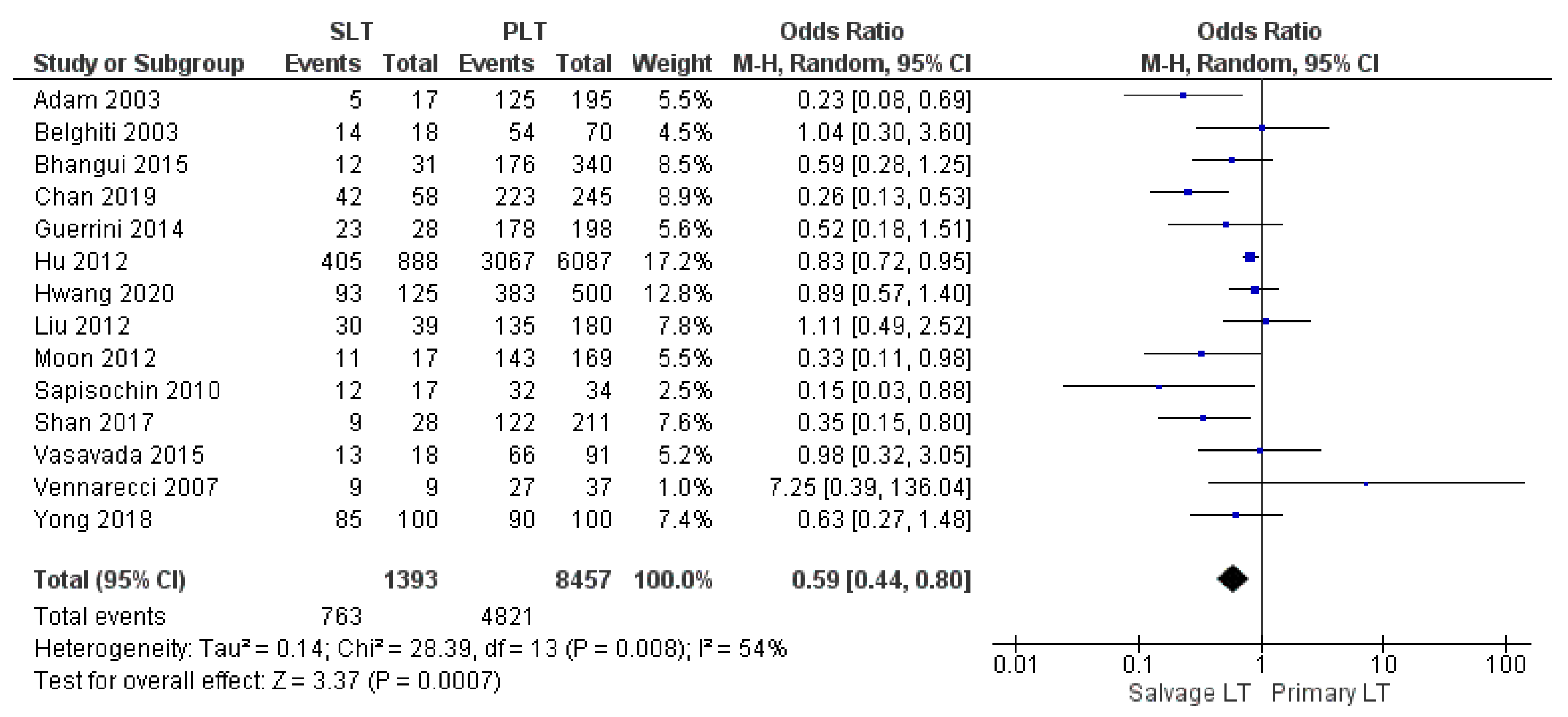
| 3-yr DFS | SLT | 763/1393 | 54.8% | 14 |
| PLT | 4821/8457 | 57% | ||
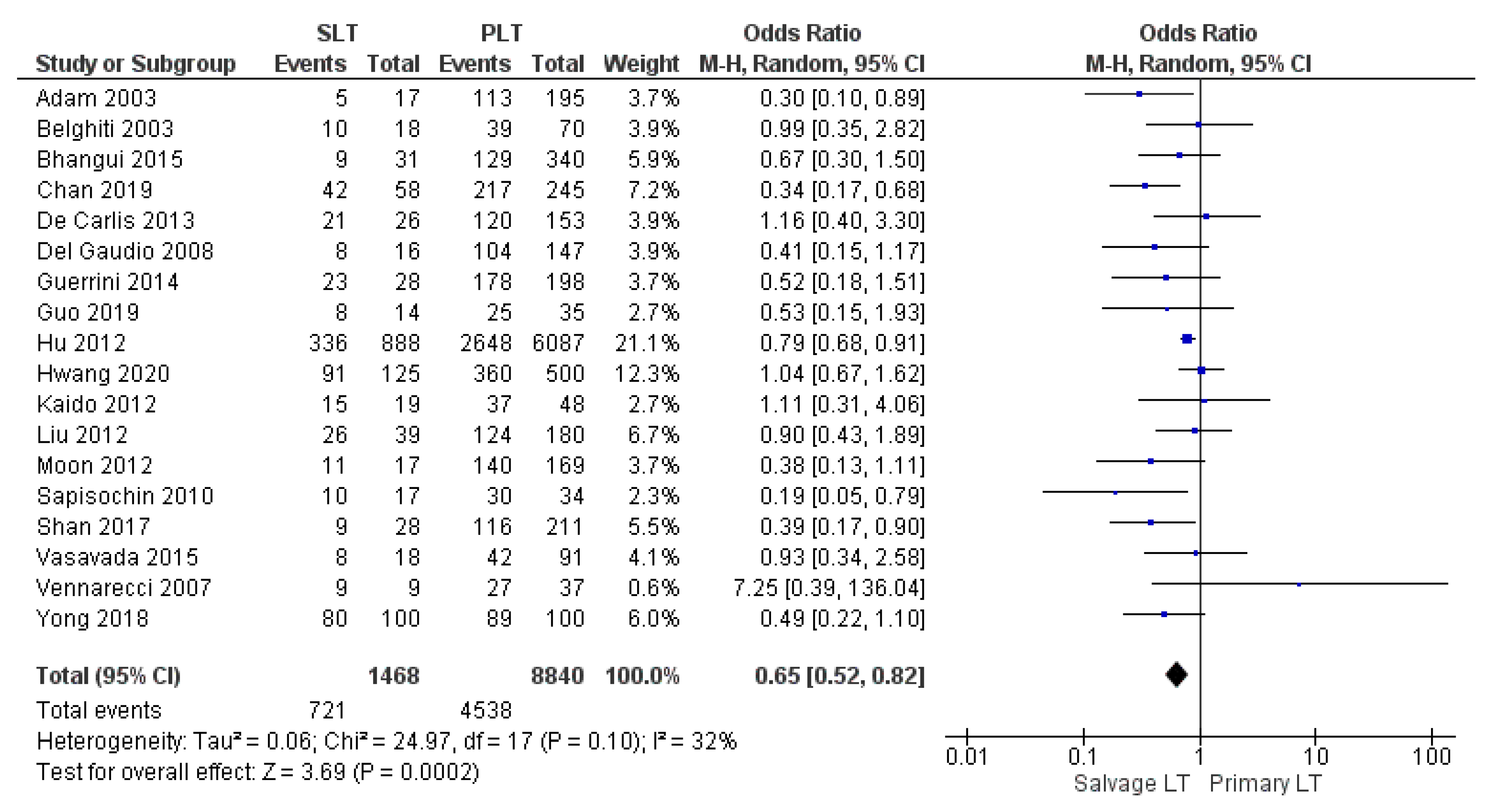
| 5-yr DFS | SLT | 721/1468 | 49.1% | 18 |
| PLT | 4538/8840 | 51.3% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, G.P.; Esposito, G.; Olivieri, T.; Magistri, P.; Ballarin, R.; Di Sandro, S.; Di Benedetto, F. Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers 2022, 14, 3465. https://doi.org/10.3390/cancers14143465
Guerrini GP, Esposito G, Olivieri T, Magistri P, Ballarin R, Di Sandro S, Di Benedetto F. Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers. 2022; 14(14):3465. https://doi.org/10.3390/cancers14143465
Chicago/Turabian StyleGuerrini, Gian Piero, Giuseppe Esposito, Tiziana Olivieri, Paolo Magistri, Roberto Ballarin, Stefano Di Sandro, and Fabrizio Di Benedetto. 2022. "Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis" Cancers 14, no. 14: 3465. https://doi.org/10.3390/cancers14143465
APA StyleGuerrini, G. P., Esposito, G., Olivieri, T., Magistri, P., Ballarin, R., Di Sandro, S., & Di Benedetto, F. (2022). Salvage versus Primary Liver Transplantation for Hepatocellular Carcinoma: A Twenty-Year Experience Meta-Analysis. Cancers, 14(14), 3465. https://doi.org/10.3390/cancers14143465






