Simple Summary
Bowel resection is often required to obtain complete removal of ovarian cancer. A major complication of this operation is anastomotic leakage, which has been shown to increase morbidity and mortality in this population. Numerous original research studies have assessed the risk factors for anastomotic leaks. We aimed to conduct a systematic review and meta-analysis to identify statistically significant risk factors. This meta-analysis identified multiple bowel resections as the only significant risk factor. With further research to identify additional risk factors, new management guidelines could be implemented to minimize the risk of anastomotic leaks and improve patient outcomes.
Abstract
Introduction: Anastomotic leaks (AL) following ovarian cytoreduction surgery could be detrimental, leading to significant delays in commencing adjuvant chemotherapy, prolonged hospital stays and increased morbidity. The aim of this study was to investigate risk factors associated with anastomotic leaks after ovarian cytoreduction surgery. Material and methods: The MEDLINE (via PubMed), Cochrane Library, EMBASE and Scopus bibliographical databases were searched. Original clinical studies investigating risk factors for AL in ovarian cytoreduction surgery were included. Results: Eighteen studies with non-overlapping populations reporting on patients undergoing cytoreduction surgery for ovarian cancer (n = 4622, including 344 cases complicated by AL) were included in our analysis. Patients undergoing ovarian cytoreduction surgery complicated by AL had a significantly higher rate of 30-day mortality but no difference in 60-day mortality. Multiple bowel resections were associated with an increased risk of postoperative AL, while no association was observed with body mass index (BMI), American Society of Anesthesiologists (ASA) score, age, smoking, operative approach (primary versus interval cytoreductive, stapled versus hand-sewn anastomoses and formation of diverting stoma), neoadjuvant chemotherapy and use of hyperthermic intraperitoneal chemotherapy (HIPEC). Discussion: Multiple bowel resections were the only clinical risk factor associated with increased risk for AL after bowel surgery in the ovarian cancer population. The increased 30-day mortality rate in patients undergoing ovarian cytoreduction complicated by AL highlights the need to minimize the number of bowel resections in this population. Further studies are required to clarify any association between neoadjuvant chemotherapy and decreased AL rates.
1. Introduction
Ovarian cancer remains the most lethal gynecological malignancy, with a five-year survival of 43% in the United Kingdom [1]. Optimal cytoreductive surgery, resulting in no residual disease, is the mainstay of treatment. Patients with advanced ovarian cancer, who have optimal cytoreduction, have prolonged 5-year survival compared to patients who have residual disease greater than 1 cm [2]. Therefore, ultra-radical surgery is often undertaken. These procedures traditionally involve resection of other abdominopelvic tissue that has likely been invaded by primary ovarian cancer [3]. In addition to surgery, the use of hyperthermic intraperitoneal chemotherapy (HIPEC) and neoadjuvant or adjuvant chemotherapy have also been shown to improve survival rates [4].
Bowel resection is frequently required to achieve optimal cytoreduction, particularly in advanced ovarian cancer [4]. The most common type of bowel resection performed in this context is rectosigmoid resection [5]. Primary bowel anastomosis, compared to permanent stoma formation, is the preferred method of repair following bowel resection [6]. A commonly reported severe complication of bowel anastomosis is an anastomotic leak (AL), which occurs in 8–14% of patients [7,8] and is associated with significant morbidity and mortality [9].
Given the detrimental effects of AL on patient outcomes, the identification of risk factors for AL is of utmost importance to improve prognosis in the ovarian cancer population. The risk factors associated with AL in patients undergoing colorectal surgery have been well described in the literature and include a prolonged operating time, the use of neoadjuvant chemotherapy, anastomotic level (i.e., low versus high rectal anastomosis) and pre-operative hypoalbuminemia [10,11,12]. However, given the surgical and pathological differences between ovarian and bowel cancer, the risk factors for AL are expected to differ in ovarian cancer cytoreduction surgery. This study aims to synthesize the most current primary evidence to identify the preoperative and intra-operative risk factors for AL in the ovarian cancer population undergoing cytoreductive surgery with bowel resection and anastomosis.
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and in line with the protocol developed and agreed a priori by all authors. Studies investigating risk factors for anastomotic leaks in patients undergoing ovarian cytoreduction surgery were deemed eligible for analysis. Exclusion criteria were: (i) articles published in languages other than English, (ii) narrative or systematic reviews and meta-analyses, (iii) case reports, errata, comments, perspectives, letters to the editor and editorials that did not provide any extractable data, (iv) published abstracts with no available full text and (v) non-comparative studies (single-arm studies). No publication date, sample size restrictions or any other search filters were applied. This study is registered with PROSPERO, number CRD42022364076.
2.2. Search Strategy
Eligible studies were identified by searching through the MEDLINE (via PubMed), Cochrane Library, Embase and Scopus databases (end-of-search date: 28 November 2021) by two independent researchers. The search strategies used are described in more detail in supplementary file S1. Any disagreements were resolved by a third reviewer. The reference lists and all previously published systematic reviews were thoroughly searched for missed studies eligible for inclusion based on the “snowball” methodology [13].
2.3. Data Extraction
A standardized, pre-piloted form was used for data tabulation and extraction. Two reviewers extracted the data independently, and any disagreements were identified and resolved by a third reviewer. We extracted the following data from the included studies: (i) study characteristics (first author, year of publication, study design, study center, country, study period and number of patients), (ii) patient characteristics (BMI, smoking, age and American Society of Anesthesiologists (ASA) physical status classification), (iii) operation (primary, secondary), (iv) intraoperative outcomes (type of anastomosis, stoma formation), (v) mortality outcomes and (vi) chemotherapy administration (bevacizumab, HIPEC).
2.4. Risk of Bias Assessment
We assessed the risk of bias using the Risk of Bias tool of the National Heart, Lung and Blood Institute (NHLBI). The tool examines eight domains as possible sources of bias: (i) study objectives, (ii) study population, (iii) consecutiveness of the population, (iv) comparability of the subjects, (v) intervention, (vi) measurement of outcomes, (vii) follow-up and (viii) statistical analysis results. For each domain, questions are answered with “yes” or “no”. Based on these answers, an overall risk of bias assessment was calculated for each included study [14].
2.5. Statistical Analysis
Available data were handled according to the principles stated in the Cochrane Handbook [13]. Data on outcomes of interest were summarized and analyzed cumulatively. Categorical variables were reported as the number of events among the total cases. Based on the extracted data, the odds ratio (OR) and 95% confidence interval (CI) were calculated by means of 2 × 2 tables for categorical events; OR > 1 indicated that the trait was more frequently present in the AL group. Between-study heterogeneity was assessed by estimating the I2 statistic. Continuous variables were summarized as means and standard deviations (SDs). Weighted mean differences (WMD) and 95% CIs were estimated for each continuous outcome; WMD > 0 corresponded to larger values in the normal group. High heterogeneity was confirmed with a significance level of p < 0.05 and I2 ≥ 50%. The random-effects model was used to calculate the pooled effect when heterogeneity was high, while the fixed-effects model was used when low heterogeneity was encountered. All statistical analyses and forest plots were performed with the use of Reviewer Manager 5.4.1 software (Review Manager (RevMan) [Computer Program]. Version 5.4.1, Copenhagen: The Nordic Cochrane Centre, Denmark, The Cochrane Collaboration, 2020).
3. Results
3.1. Study Selection, Study Characteristics
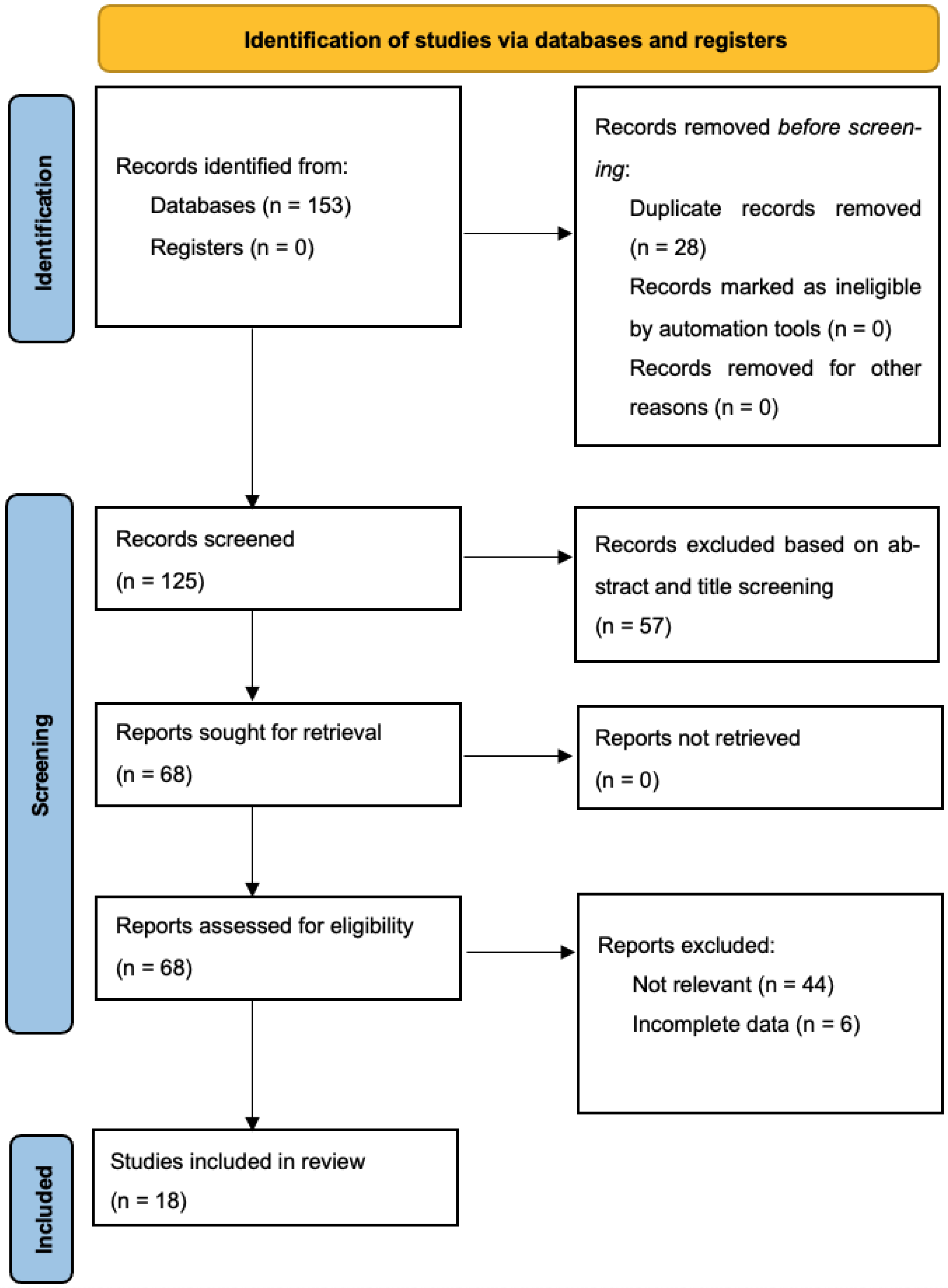
Through our systematic search, 125 unique articles were retrieved, 68 of which underwent full-text evaluation for eligibility (57 studies excluded based on title and abstract screening). Ultimately, 18 studies reporting on 4622 patients undergoing cytoreduction surgery for ovarian cancer (4278 non-AL and 344 AL patients) fulfilled the inclusion criteria and were included in our quantitative data synthesis [8,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] (Figure 1) [32]. Six of the studies were conducted in the United States of America, four in Germany, three in South Korea, one in the United Kingdom, one in Austria, one in Spain, one in France, and one was multicentric. All studies were retrospective. Baseline patient characteristics, including ovarian tumor histology, staging and CC-0 resection rate, are described in Table 1. Inclusion and exclusion criteria as well as the definition of anastomotic leak among the included studies, are summarized in Table 2.
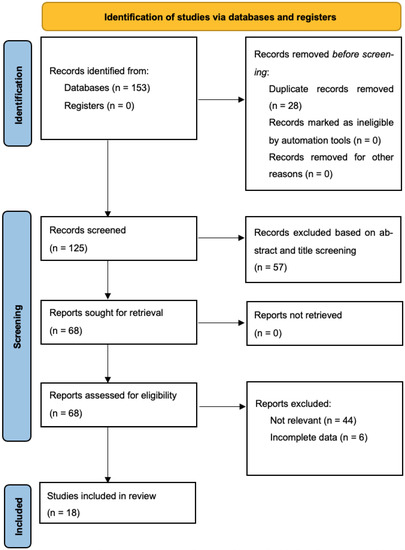
Figure 1.
PRISMA flowchart.

Table 1.
Basic characteristics of the included studies. Anastomotic leak (AL), Epithelial (E), Malignant Mixed Mullerian Tumor (MMT), Granulosa cell (G).

Table 2.
Definition of anastomotic leak among the included studies as well as inclusion/exclusion criteria.
3.2. Patient Characteristics
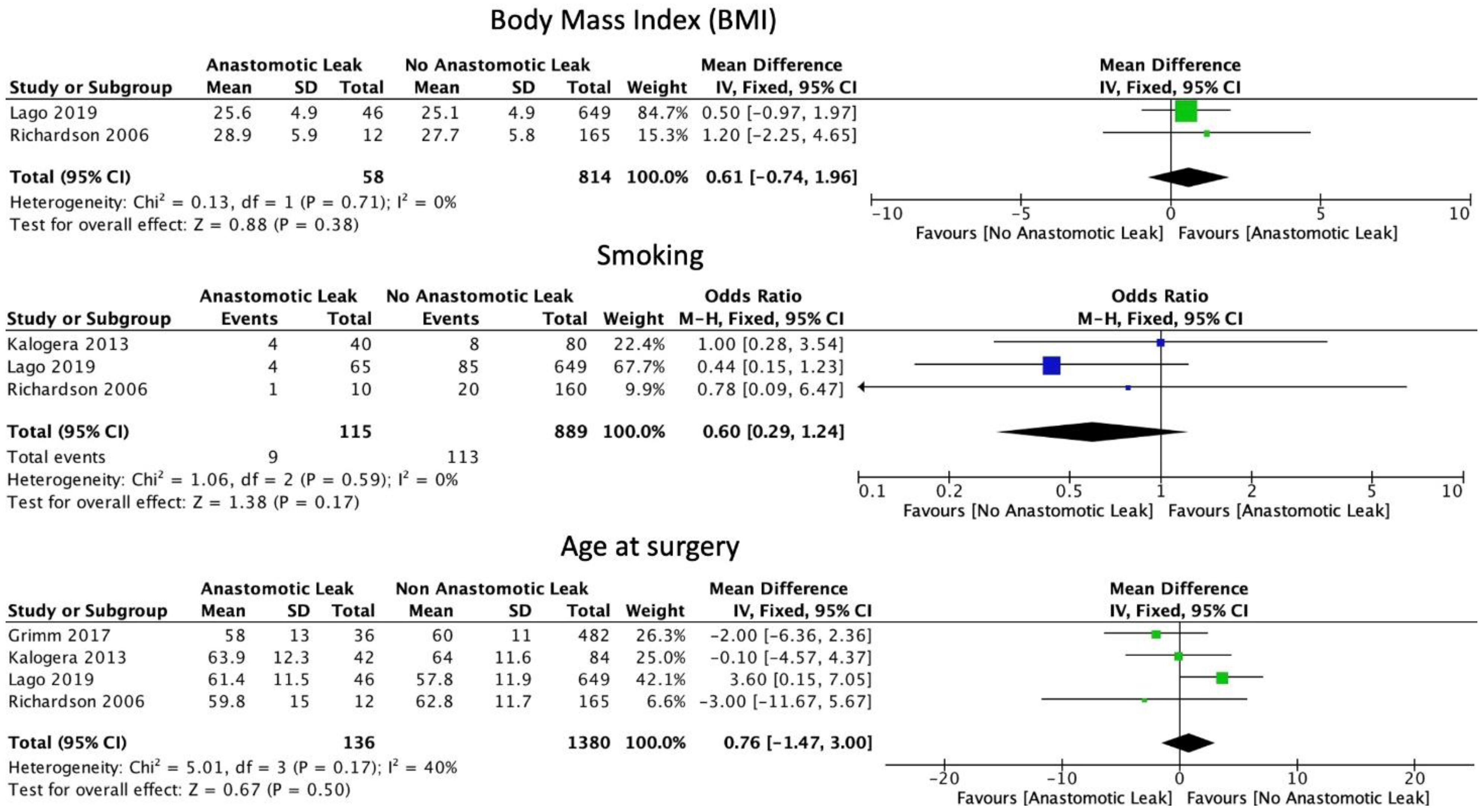
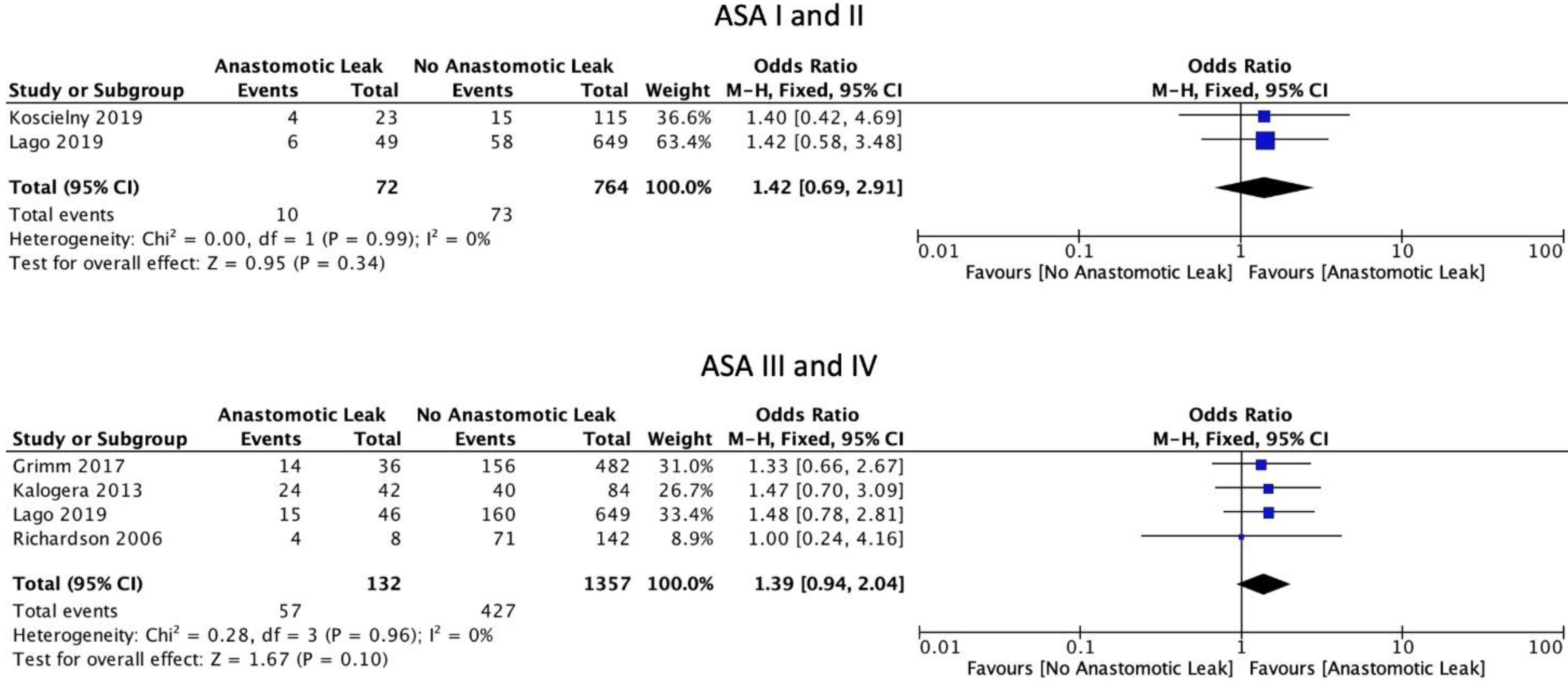
The FIGO staging system was used in the included studies. Eight studies included only those patients with ovarian cancer of stage 3 or above [6,15,16,17,19,22,29,30]. Five studies included patients of stage 2 and above [18,21,23,24,31]. Four studies included patients with all stages of ovarian cancer [8,25,26,27]. One study did not provide staging information [20]. Mean BMI (WMD 0.61, 95% CI: −0.74 to 1.96, p = 0.38, I2 = 0%) and current smoking status (OR: 0.60, 95% CI: 0.29 to 1.24, p = 0.12, I2 = 0%) were not associated with a significantly increased risk of AL, as reported by two and three studies, respectively [21,25]. Mean age at the time of surgery was not significantly associated with AL (WMD 0.76, 95% CI: −1.47 to 3.00, p = 0.5, I2 = 40%) (Figure 2). Neither the ASA I-II classification (OR: 1.42, 95% CI: 0.69 to 2.91, p = 0.34, I2 = 0%) nor the ASA III-IV classification (OR: 1.39, 95% CI: 0.94 to 2.04, p = 0.10, I2 = 0%) were significantly associated with AL (Figure 3).
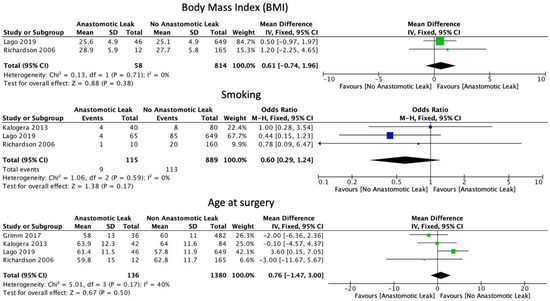
Figure 2.
Basic patient characteristics.
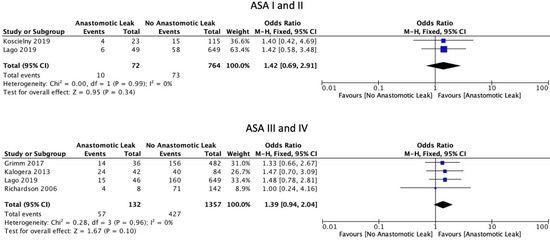
Figure 3.
ASA scores.
3.3. Neoadjuvant, Bevacizumab and HIPEC Therapy
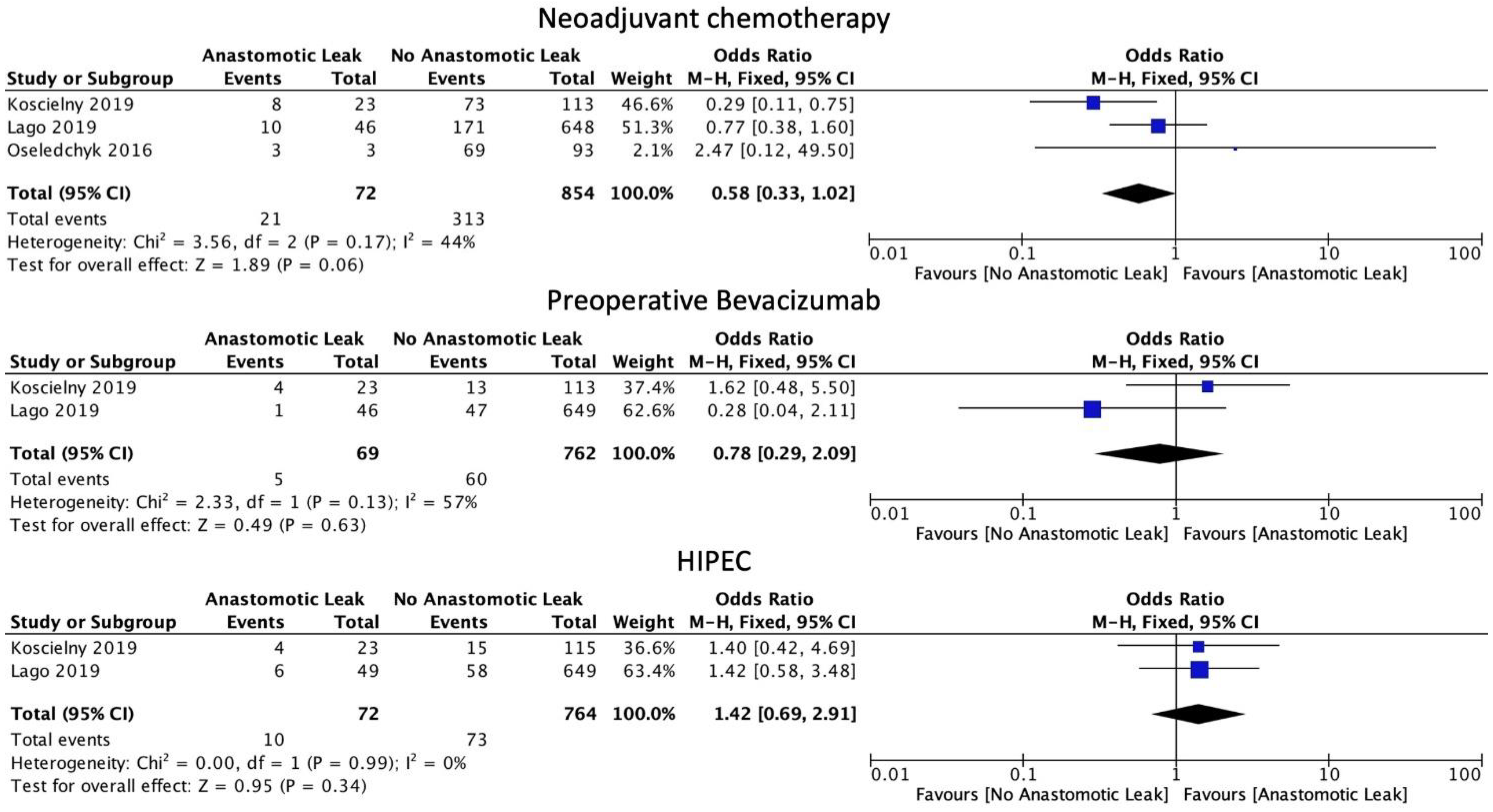
The use of neoadjuvant chemotherapy was associated with a statistically non-significant decrease in the rate of AL (OR 0.58, 95% CI: 0.33 to 1.02, p = 0.06, I2 = 44%). Similarly, in subgroup analysis, there was no difference in AL rate with the use of bevacizumab (OR: 0.78, 95% CI: 0.29 to 2.09, p = 0.63, I2 = 57%). The use of HIPEC was also not linked to a statistically significant increase in AL rates (OR: 1.42, 95% CI: 0.69 to 2.91, p = 0.34, I2 = 0%) when combined with surgical management (Figure 4).
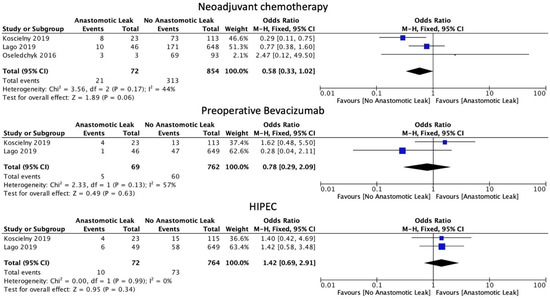
Figure 4.
Chemotherapy administration.
3.4. Intraoperative Considerations
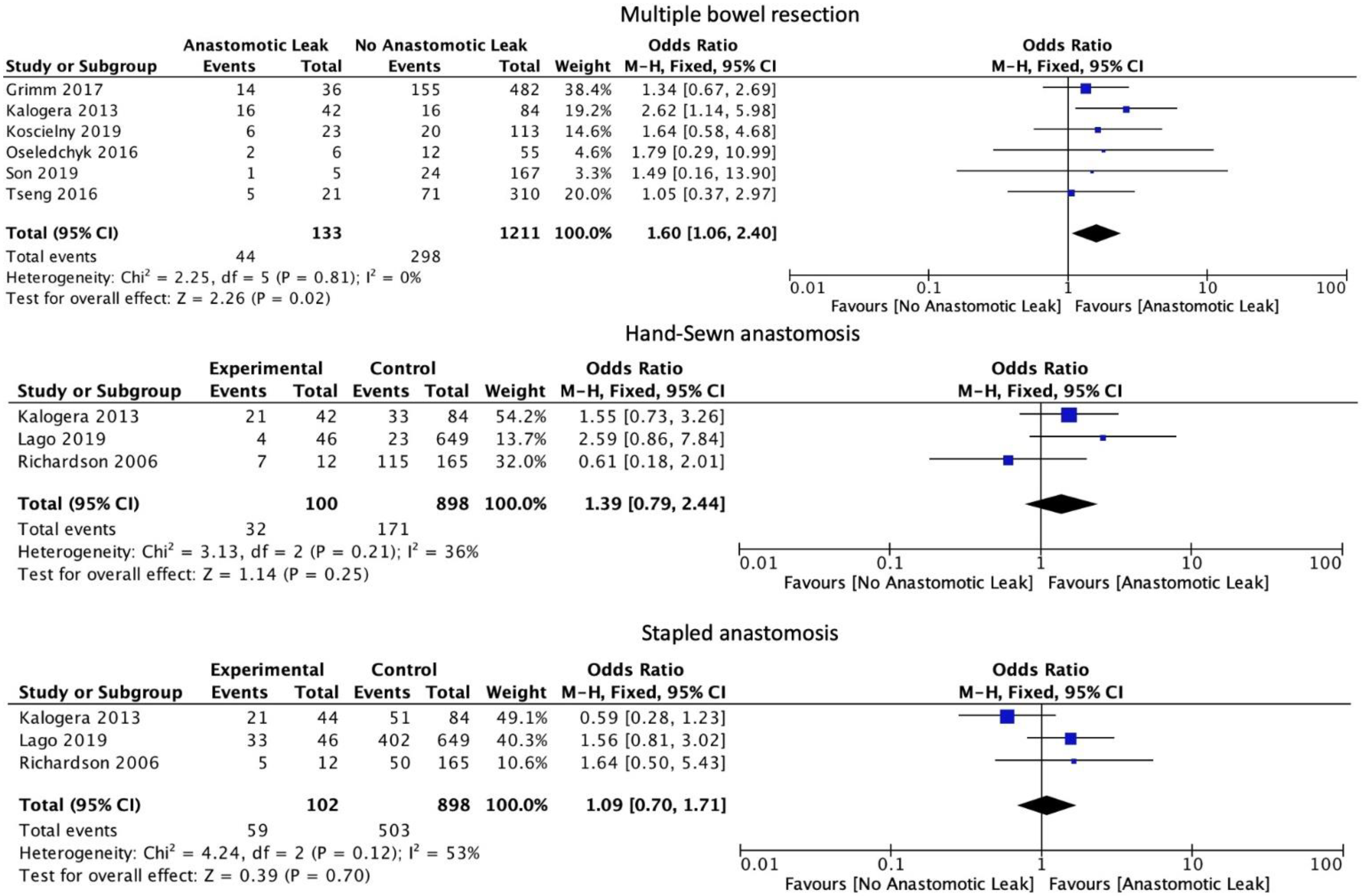
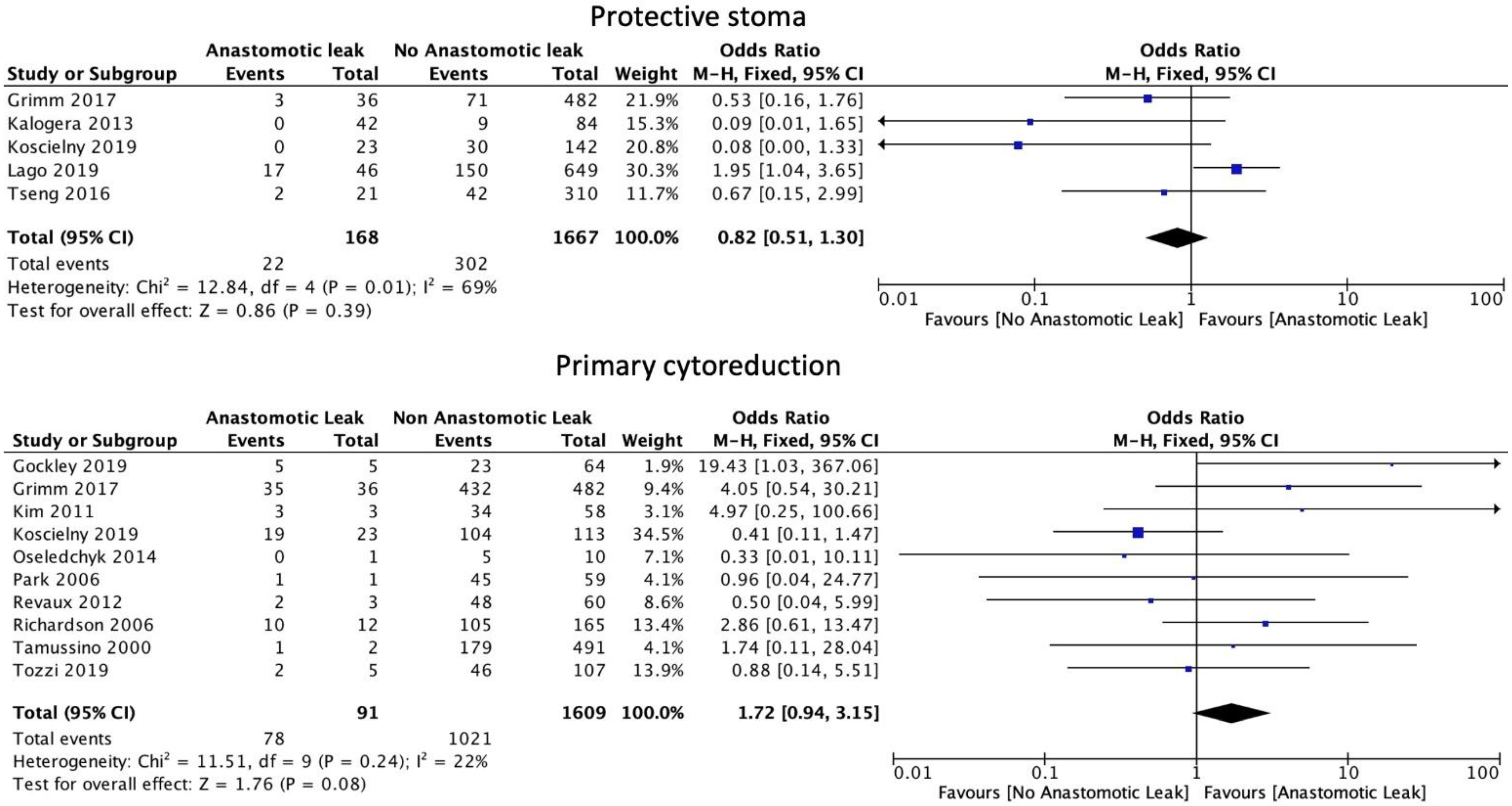
The number of anastomoses carried out intraoperatively (OR: 1.60, 95% CI: 1.06 to 2.40, p = 0.02, I2 = 0%) was associated with an increase in AL. The type of anastomosis, including hand-sewn (OR: 1.39, 95% CI: 0.79 to 2.44, p = 0.25, I2 = 36%) or stapled (OR: 1.09, 95% CI: 0.70 to 1.71, p = 0.7, I2 = 53%) was not associated with a significant increase in AL (Figure 5). Protective stoma (OR: 0.82, 95% CI: 0.51 to 1.30, p = 0.39, I2 = 69%) as well as primary (OR: 1.72, 95% CI: 0.94 to 3.15, p = 0.08, I2 = 22%) versus secondary cytoreductive or recurrent disease were not associated with a significantly altered risk of AL (Figure 6).
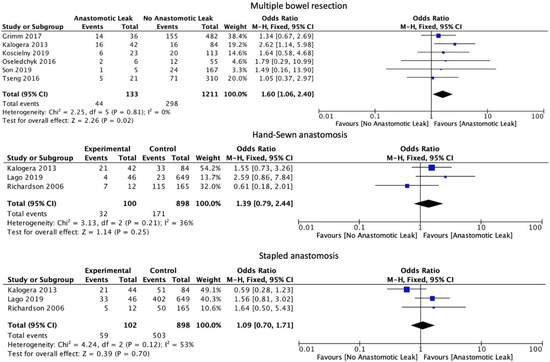
Figure 5.
Intraoperative outcomes.
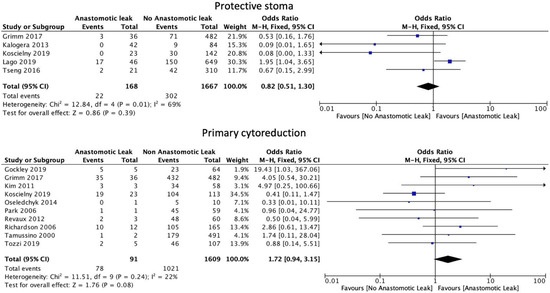
Figure 6.
Stoma formation and primary cytoreduction risk factors.
3.5. Mortality
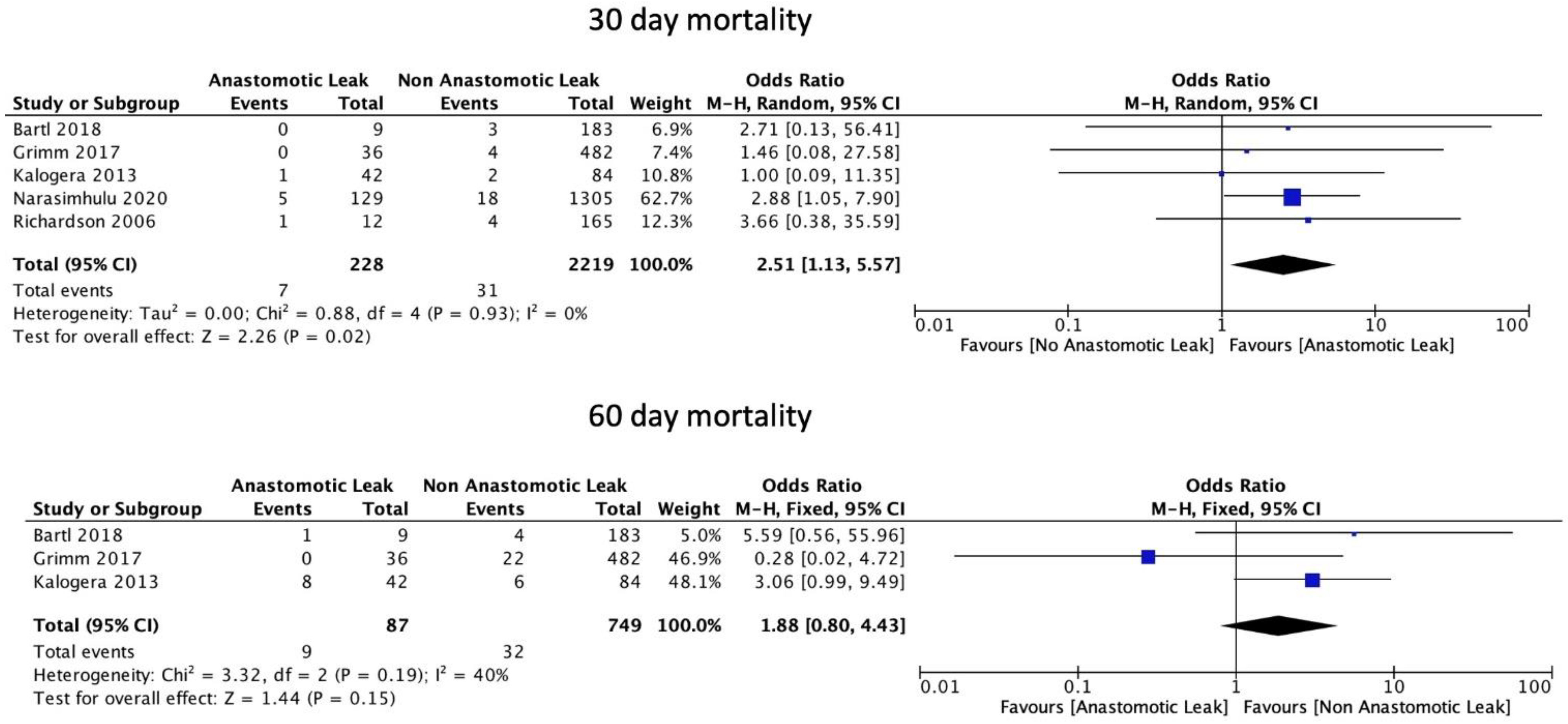
AL was significantly associated with an increase in 30-day mortality (OR: 2.51, 95% CI: 1.13 to 5.57, p = 0.02, I2 = 0%) but not with 60-day mortality (OR: 1.88, 95% CI: 0.80 to 4.43, p = 0.15, I2 = 40%) (Figure 7).
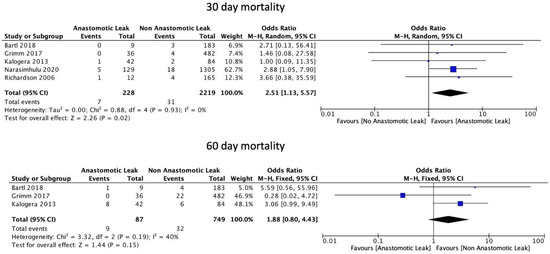
Figure 7.
Mortality rates.
3.6. Risk of Bias Assessment
The mean score of the NHLBI scale was 7.7 ± 1.1. These results highlight that the included studies were, on average, of good quality. This is outlined in Table 3.

Table 3.
The risk of bias assessment using the Risk of Bias tool of the National Heart, Lung and Blood Institute (NHLBI). Y = Yes. N = No.
4. Discussion
This systematic review and meta-analysis of 18 retrospective studies showed that multiple bowel resections increased the risk of AL following ovarian cytoreduction surgery. In addition, the presence of AL in ovarian cytoreduction surgery significantly increased the risk of 30-day mortality. These findings indicate that multiple bowel resections may be a major contributor to poor outcomes, including 30-day mortality, in patients with ovarian cancer who require bowel anastomosis. Historically, AL was thought to only impact 30-day mortality [33,34], given the well-established risk of associated postoperative morbidity [35] and reoperation following diagnosis [36]. However, this hypothesis is unlikely to accurately reflect the true extent of the physiological burden associated with AL based on recent studies recording higher absolute values of clinically, but not statistically, significant 60-day and 90-day mortality (11.1% to 19.1%) [23,36]. In support of these findings, 60-day mortality was not significantly increased following AL in our meta-analysis. Given that a key consequence of AL is a delay in starting adjuvant therapy [37], a study with long-term follow-up may be required to fully assess the long-term consequences of AL on morbidity and mortality and the safe interval of initiation of adjuvant chemotherapy in the setting of AL.
Our analysis revealed that the use of neoadjuvant chemotherapy was associated with a clinically significant decrease in the rate of AL that marginally failed to attain statistical significance. In the neoadjuvant setting, a typical regimen consists of two or three courses administered prior to interval cytoreductive surgery, followed by up to six courses of chemotherapy postoperatively [38]. This regimen is advocated for in the guidelines published by the National Comprehensive Cancer Network (NCCN) for patients who are poor surgical candidates or have a low likelihood of successful cytoreduction [39]. The European Society for Medical Oncology (ESMO) guidelines remain equivocal [40]. The aim of neoadjuvant chemotherapy is to downstage tumors with a low chance of optimal cytoreductivity with upfront surgery [16]. Randomized controlled trials have shown non-inferiority in terms of survival for patients receiving neoadjuvant therapy compared to primary surgical management [41,42]. Further analysis completed by Kehoe et al. [41] demonstrated that neoadjuvant therapy significantly reduced the risk of Clavien Dindo grade III and IV complications compared to adjuvant therapy as per the National Cancer Institute Common Terminology Criteria for Adverse Events. In contrast, a meta-analysis demonstrated decreased survival in individuals receiving neoadjuvant chemotherapy compared to those receiving upfront surgery [43]. This, in part, can be explained through the requirement of neoadjuvant chemotherapy only in those with advanced cancer where suboptimal surgical cytoreduction and reduced overall survival are anticipated.
Our analysis further confirmed that there is no significant difference in AL rates in patients receiving HIPEC. HIPEC consists of circulating warmed chemotherapeutic agents around the peritoneal cavity intraoperatively, following resection and prior to anastomosis or stoma formation, and aims to eliminate any remaining neoplastic cells. A recent phase III trial, randomizing patients with advanced ovarian cancer to either HIPEC or no HIPEC during cytoreductive surgery, demonstrated that HIPEC increases recurrence-free survival and overall survival [44]. Moreover, there was no increase in side effects. These findings demonstrated that the addition of HIPEC is safe and efficacious in patients with advanced ovarian cancer. Of note, the aforementioned study included only 24 individuals that underwent bowel resection, with the majority receiving a protective colostomy. This raises concerns regarding the potential for adverse events associated with bowel resection following HIPEC treatment in patients with advanced ovarian cancer. Subsequent analysis by Gruner et al. [7] investigated the incidence and associated risk factors for anastomotic failure following interval cytoreductive surgery with or without HIPEC and showed that there was no significant difference in AL rates between those who underwent interval cytoreductive surgery with or without HIPEC. These data are consistent with our findings and support the hypothesis that the anticipated risk of AL should not guide the use of HIPEC in ovarian cancer patients.
Protective stoma formation describes the process of creating a temporary diverting proximal stoma in patients at higher risk of complications, such as AL, to prevent postoperative morbidity [45]. Our analysis did not show any statistically significant difference in AL rates of patients undergoing the creation of a protective stoma. Koscielny et al. [8] found a statistically significant reduction in the AL rate, but this effect was not confirmed by larger studies. This overall result is in part due to the heterogeneity between studies; criteria for covering stoma formation vary between studies and between individual surgeons, and overall, the patient numbers are small. For example, most stomas reported in studies were created at the surgeon’s discretion based on the fulfilment of certain criteria. While Koscielny et al. [8] generated a stoma if expected blood loss exceeded 1000 mL, Grimm et al. [15] did not use a blood loss cut-off as a criterion. Protective stoma reduces the rate of AL, as well as the length of stay in the hospital and postoperative mortality [36]. A meta-analysis of 27 studies showed an association between a defunctioning stoma with significantly decreased AL rates in patients with rectal cancer [46]. However, a recent systematic review and meta-analysis of 17 studies with a total number of 2719 patients by Santana et al. revealed that protective stoma formation did not decrease AL rates in ovarian cancer cases, thus suggesting that its use is limited to only selected cases [47]. Finally, a study of 145 patients that underwent colorectal resection during cytoreduction surgery for ovarian cancer reported no differences in AL rates among patients with or without diverting ileostomy and ghost ileostomy [48].
Secondary debulking describes cytoreduction surgery in the setting of recurrence in patients that underwent primary surgery to remove the tumor found in the first place. Our analysis found no significant difference in AL rates between individuals undergoing primary or secondary recurrent disease cytoreduction procedures. However, in the literature, the eligibility criteria for secondary cytoreductive surgery seem to be more selective than for primary cytoreductive surgery [49,50]. Therefore, secondary cytoreductive surgery patients tend to be fitter for surgery and are at a reduced risk of postoperative complications prior to the cytoreduction surgery. Optimal cytoreductive surgery in residual disease is a matter of controversy. Harter et al. [50] suggested an improved prognosis only if complete resection can be achieved, while another meta-analysis demonstrated benefits for patients with microscopic residual disease [51].
The limitations of this study include the use of observational studies as opposed to randomized controlled trials. Other limitations include the small number of patients, with few studies primarily designed to investigate AL-associated risk factors, and many of these only consider AL in subgroup analysis. Furthermore, additional relevant factors, including the surgeon’s experience, blood loss and the need for transfusion, were not amenable to analysis due to limited available data. The role of preoperative hypoalbuminemia (albumin level < 3.4 g/dL) was not examined in this study as it is a well-established independent preoperative risk factor for AL after colorectal surgery [52,53,54,55] and more recently, a 2022 systematic review and meta-analysis of 3274 patients demonstrated that a preoperative albumin level of <3.0 g/dL is also a significant risk factor of AL after bowel resection and anastomosis for ovarian cancer [56,57]. The research regarding the AL rate for bowel cancer patients is more extensive, potentially offering valuable insight for further reduction of AL in the ovarian cancer population [52].
5. Conclusions
In conclusion, our study suggests that AL is associated with a significantly increased 30-day mortality rate in the ovarian cancer population undergoing a cytoreduction operation. Multiple bowel resections were a risk factor for AL in this patient population. However, larger prospectively-designed studies are required to increase the statistical power of any future analyses and more accurately assess a wider range of risk factors for an anastomotic leak in ovarian cytoreduction, similar to bowel cancer studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14215464/s1, supplementary file S1: Search Strategy.
Author Contributions
Conceptualization of the study was carried out by G.G.; methodology was completed by M.F., G.G., K.P., K.S.K. and K.K.T.; formal analysis was completed by P.G., G.K., M.F. and G.G.; data curation was completed by M.F., G.G., G.K., P.G., N.A.P., J.L., K.S.K. and K.K.T.; original draft preparation was completed by M.F. and G.G.; review and editing were completed by M.F., C.K., N.A.P., J.L., D.G. and G.G.; visualization was completed by G.G.; supervision was completed by G.G., D.G. and C.K.; project administration was completed by M.F. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any data reported have been appropriately referenced, with no original data being reported by the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cancer Research UK. Ovarian Cancer Survival Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/survival#heading-Zero (accessed on 29 September 2022).
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, C.R.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet. Gynecol. 2006, 107, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, P.P. ASO Author Reflections: Ultra-Radical Resection in Ovarian Cancer: Where Are We and Where Are We Going? Ann. Surg. Oncol. 2021, 28, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Del Carmen, M.G.; Kaufman, H.S.; Montz, F.J. Radical oophorectomy with primary stapled colorectal anastomosis for resection of locally advanced epithelial ovarian cancer. J. Am. Coll. Surg. 2003, 197, 565–574. [Google Scholar] [CrossRef]
- Peiretti, M.; Bristow, R.E.; Zapardiel, I.; Gerardi, M.; Zanagnolo, V.; Biffi, R.; Landoni, F.; Bocciolone, L.; Aletti, G.D.; Maggioni, A. Rectosigmoid resection at the time of primary cytoreduction for advanced ovarian cancer. A multi-center analysis of surgical and oncological outcomes. Gynecol. Oncol. 2012, 126, 220–223. [Google Scholar] [CrossRef]
- Maguire, B.; Clancy, C.; Connelly, T.M.; Mehigan, B.J.; McCormick, P.; Altomare, D.F.; Gosselink, M.P.; Larkin, J.O. Quality of life meta-analysis following coloanal anastomosis versus abdominoperineal resection for low rectal cancer. Colorectal Dis. 2022, 24, 811–820. [Google Scholar] [CrossRef]
- Gruner, M.; Chambers, L.M.; Yao, M.; Chichura, A.; Morton, M.; Costales, A.B.; Horowitz, M.; Rose, P.G.; Debernardo, R.; Michener, C.M. Anastomotic leak following interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in women with advanced epithelial ovarian Cancer. Gynecol. Oncol. 2021, 162, 645–651. [Google Scholar] [CrossRef]
- Koscielny, A.; Ko, A.; Egger, E.K.; Kuhn, W.; Kalff, J.C.; Keyver-Paik, M.D. Prevention of anastomotic leakage in ovarian cancer debulking surgery and its impact on overall survival. Anticancer Res. 2019, 39, 5209–5218. [Google Scholar] [CrossRef]
- Kingham, P.T.; Pachter, L.H. Colonic anastomotic leak: Risk factors, diagnosis, and treatment. J. Am. Coll. Surg. 2009, 208, 269–278. [Google Scholar] [CrossRef]
- Kwak, H.D.; Kim, S.H.; Kang, D.W.; Baek, S.J.; Kwak, J.M.; Kim, J. Risk factors and oncologic outcomes of anastomosis leakage after laparoscopic right colectomy. Surg. Laparosc. Endosc. Percutan. Tech. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Shimura, T.; Toiyama, Y.; Hiro, J.; Imaoka, H.; Fujikawa, H.; Kobayashi, M.; Ohi, M.; Inoue, Y.; Mohri, Y.; Kusunoki, M. Monitoring perioperative serum albumin can identify anastomotic leakage in colorectal cancer patients with curative intent. Asian J. Surg. 2018, 1, 30–38. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, J.K.; Ko, Y.T.; Jang, H.J.; Shin, H.K.; Lee, Y.C.; Lim, C.H.; Jeong, S.K.; Yang, H.K. Risk factors for anastomotic leakage after laparoscopic rectal resection. J. Korean Soc. Coloproctol. 2010, 26, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools, Quality Assessment of Case Control Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 31 August 2022).
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 5.1.0. Available online: www.handbook.cochrane.org (accessed on 31 August 2022).
- Grimm, C.; Harter, P.; Alesina, P.F.; Prader, S.; Schneider, S.; Ataseven, B.; Meier, B.; Brunkhorst, V.; Hinrichs, J.; Kurzeder, C.; et al. The impact of type and number of bowel resections on anastomotic leakage risk in advanced ovarian cancer surgery. Gynecol. Oncol. 2017, 146, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Oseledchyk, A.; Abramian, A.; Kaiser, C.; Debald, M.; Domroese, C.; Kiefer, N.; Putensen, C.; Pantelis, D.; Kuhn, W.; Schäfer, N.; et al. Total or subtotal colectomy in patients undergoing surgery for primary or recurrent epithelial ovarian cancer. Oncol. Res. Treat. 2014, 37, 448–454. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Hunold, L.E.; Mallmann, M.R.; Domröse, C.M.; Abramian, A.; Debald, M.; Kaiser, C.; Kiefer, N.; Putensen, C.; Pantelis, D.; et al. Impact of extended primary surgery on suboptimally operable patients with advanced ovarian cancer. Int. J. Gynecol. Cancer 2016, 26, 873–883. [Google Scholar] [CrossRef]
- Revaux, A.; Rouzier, R.; Ballester, M.; Selle, F.; Daraï, E.; Chéreau, E. Comparison of morbidity and survival between primary and interval cytoreductive surgery in patients after modified posterior pelvic exenteration for advanced ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 1349–1354. [Google Scholar] [CrossRef]
- Tozzi, R.; Casarin, J.; Baysal, A.; Valenti, G.; Kilic, Y.; Majd, H.S.; Morotti, M. Bowel resection rate but not bowel related morbidity is decreased after interval debulking surgery compared to primary surgery in patents with stage IIIC–IV ovarian cancer. J. Gynecol. Oncol. 2019, 30, e25. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, D.M.; Bews, K.A.; Hanson, K.T.; Chang, Y.H.H.; Dowdy, S.C.; Cliby, W.A. Using evidence to direct quality improvement efforts: Defining the highest impact complications after complex cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2020, 156, 278–283. [Google Scholar] [CrossRef]
- Lago, V.; Fotopoulou, C.; Chiantera, V.; Minig, L.; Gil-Moreno, A.; Cascales-Campos, P.A.; Jurado, M.; Tejerizo, A.; Padilla-Iserte, P.; Malune, M.E.; et al. Risk factors for anastomotic leakage after colorectal resection in ovarian cancer surgery: A multi-centre study. Gynecol. Oncol. 2019, 153, 549–554. [Google Scholar] [CrossRef]
- Son, J.H.; Kong, T.W.; Paek, J.; Chang, S.J.; Ryu, H.S. Perioperative outcomes of extensive bowel resection during cytoreductive surgery in patients with advanced ovarian cancer. J. Surg. Oncol. 2019, 119, 1011–1015. [Google Scholar] [CrossRef]
- Bartl, T.; Schwameis, R.; Stift, A.; Bachleitner-Hofmann, T.; Reinthaller, A.; Grimm, C.; Polterauer, S. Predictive and prognostic implication of bowel resections during primary cytoreductive surgery in advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2018, 28, 1664–1671. [Google Scholar] [CrossRef]
- Tseng, J.H.; Suidan, R.S.; Zivanovic, O.; Gardner, G.J.; Sonoda, Y.; Levine, D.A.; Abu-Rustum, N.R.; Tew, W.P.; Chi, D.S.; Roche, K.L. Diverting ileostomy during primary debulking surgery for ovarian cancer: Associated factors and postoperative outcomes. Gynecol. Oncol. 2016, 142, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.L.; Mariani, A.; Cliby, W.A. Risk factors for anastomotic leak after recto-sigmoid resection for ovarian cancer. Gynecol. Oncol. 2006, 103, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Tamussino, K.F.; Lim, P.C.; Webb, M.J.; Lee, R.A.; Lesnick, T.G. Gastrointestinal surgery in patients with ovarian cancer. Gynecol. Oncol. 2001, 80, 79–84. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, S.S.; Kang, S.; Lee, K.B.; Lim, S.Y.; Choi, H.S.; Park, S.Y. The benefits of low anterior en bloc resection as part of cytoreductive surgery for advanced primary and recurrent epithelial ovarian cancer patients outweigh morbidity concerns. Gynecol. Oncol. 2006, 103, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, E.N.; Jeong, S.Y.; Chung, H.H.; Kim, Y.B.; Kim, J.W.; Park, K.J.; Park, N.H.; Song, Y.S.; Park, J.G.; et al. Comparison of the efficacy of low anterior resection with primary anastomosis and Hartmann’s procedure in advanced primary or recurrent epithelial ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kalogera, E.; Dowdy, S.C.; Mariani, A.; Weaver, A.L.; Aletti, G.; Bakkum-Gamez, J.N.; Cliby, W.A. Multiple large bowel resections: Potential risk factor for anastomotic leak. Gynecol. Oncol. 2013, 130, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.M.; Leath, C.A., III; Straughn, J.M., Jr.; Rocconi, R.P.; Kirby, T.O.; Huh, W.K.; Barnes, M.N., III. Bowel resection at the time of primary debulking for epithelial ovarian carcinoma: Outcomes in patients treated with platinum and taxane-based chemotherapy. J. Am. Coll. Surg. 2006, 203, 527–532. [Google Scholar] [CrossRef]
- Lago, V.; Flor, B.; Matute, L.; Padilla-Iserte, P.; García-Granero, A.; Bustamante, M.; Domingo, S. Ghost ileostomy in advanced ovarian cancer: A reliable option. Int. J. Gynecol. Cancer 2018, 28, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Phillips, R.K.S.; Hittinger, R.; Blesovsky, L.; Fry, J.S.; Fielding, L.P. Local recurrence following ‘curative’ surgery for large bowel cancer: I. The overall picture. Br. J. Surg. 1984, 71, 12–16. [Google Scholar] [CrossRef]
- Sauven, P.; Playforth, M.J.; Evans, M.; Pollock, A.V. Early infective complications and late recurrent cancer in stapled colonic anastomoses. Dis. Colon Rectum 1989, 32, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.L.; Hyman, N.H.; Alverdy, J.C. Prevention of perioperative anastomotic healing complications: Anastomotic stricture and anastomotic leak. Adv. Surg. 2016, 50, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Gessler, B.; Eriksson, O.; Angenete, E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef]
- Joseph, N.; Clark, R.M.; Dizon, D.S.; Lee, M.S.; Goodman, A.; Boruta, D., Jr.; Schorge, J.O.; Del Carmen, M.G.; Growdon, W.B. Delay in chemotherapy administration impacts survival in elderly patients with epithelial ovarian cancer. Gynecol. Oncol. 2015, 137, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Pölcher, M.; Mahner, S.; Ortmann, O.; Hilfrich, J.; Diedrich, K.; Breitbach, G.P.; Höss, C.; Leutner, C.; Braun, M.; Möbus, V.; et al. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer—A prospective multicenter phase II trial (PRIMOVAR). Oncol. Rep. 2009, 22, 605–613. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN guidelines insights: Ovarian cancer, version 1.2019: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Bristow, R.E.; Chi, D.S. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol. Oncol. 2006, 103, 1070–1076. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Cristian, D.A.; Grama, F.A.; Burcoş, T.; Bordea, A. Temporary protective loop ileostomy in open low rectal resection—An alternative technique. Chirurgia 2014, 109, 238–242. [Google Scholar]
- Hüser, N.; Michalski, C.W.; Erkan, M.; Schuster, T.; Rosenberg, R.; Kleeff, J.; Friess, H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann. Surg. 2008, 248, 52–60. [Google Scholar] [CrossRef]
- Santana, B.N.; Torralba, E.G.; Soriano, J.V.; Laseca, M.; Martinez, A.M. Protective ostomies in ovarian cancer surgery: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e21. [Google Scholar] [CrossRef] [PubMed]
- Lago, V.; Sanchez-Migallón, A.; Flor, B.; Padilla-Iserte, P.; Matute, L.; García-Granero, Á.; Bustamante, M.; Domingo, S. Comparative study of three different managements after colorectal anastomosis in ovarian cancer: Conservative management, diverting ileostomy, and ghost ileostomy. Int. J. Gynecol. Cancer 2019, 29, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Reuss, A.; Hasenburg, A.; Scambia, G.; Cibula, D.; Mahner, S.; Vergote, I.; Reinthaller, A.; Burges, A.; et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: The Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int. J. Gynecol. Cancer 2011, 21, 289–295. [Google Scholar] [CrossRef]
- Harter, P.; Hahmann, M.; Lueck, H.J.; Poelcher, M.; Wimberger, P.; Ortmann, O.; Canzler, U.; Richter, B.; Wagner, U.; Hasenburg, A.; et al. Surgery for recurrent ovarian cancer: Role of peritoneal carcinomatosis: Exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 1324–1330. [Google Scholar] [CrossRef]
- Bristow, R.E.; Puri, I.; Chi, D.S. Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis. Gynecol. Oncol. 2009, 112, 265–274. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Parthasarathy, M.; Greensmith, M.; Bowers, D.; Groot-Wassink, T. Risk factors for anastomotic leakage after colorectal resection: A retrospective analysis of 17 518 patients. Color. Dis. 2017, 19, 288–298. [Google Scholar] [CrossRef]
- Ionescu, D.; Tibrea, C.; Puia, C. Pre-operative hypoalbuminemia in colorectal cancer patients undergoing elective surgery—A major risk factor for postoperative outcome. Chirurgia 2013, 108, 822–828. [Google Scholar] [PubMed]
- Telem, D.A.; Chin, E.H.; Nguyen, S.Q.; Divino, C.M. Risk factors for anastomotic leak following colorectal surgery: A case-control study. Arch Surg. 2010, 145, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Vitagliano, A.; Morotti, M.; Giorda, G.; Sopracordevole, F.; Sapia, F.; Lo Presti, V.; Chiofalo, B.; Forte, S.; Lo Presti, L.; et al. Risks factors for anastomotic leakage in advanced ovarian cancer: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 269, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Liu, Y.; Bi, D.S. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: A systematic review and meta-analysis. Surg. Endosc. 2015, 29, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).