Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Development of a Human Cancer Cell Line Inducing Cachexia
2.3. Animal Experiments
2.4. Cell Proliferation Assay
2.5. Assessment of Muscle Cross-Sectional Area
2.6. RNA Sequencing and Differential Gene Expression (DEG) Analysis
2.7. ddPCR
2.8. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) Analysis
2.9. The Cancer Genome Atlas Program (TCGA) and GTEx Data Analysis
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Quantitative Mass Spectrometry Analysis of PC3 and PC3* Proteomes
2.12. Clinical Samples
2.13. Data Presentation and Statical Analysis
3. Results
3.1. PC3* Preclinical Cancer Cachexia Model
3.2. Screening of Cachexia Related DEGs Based on RNA-Seq
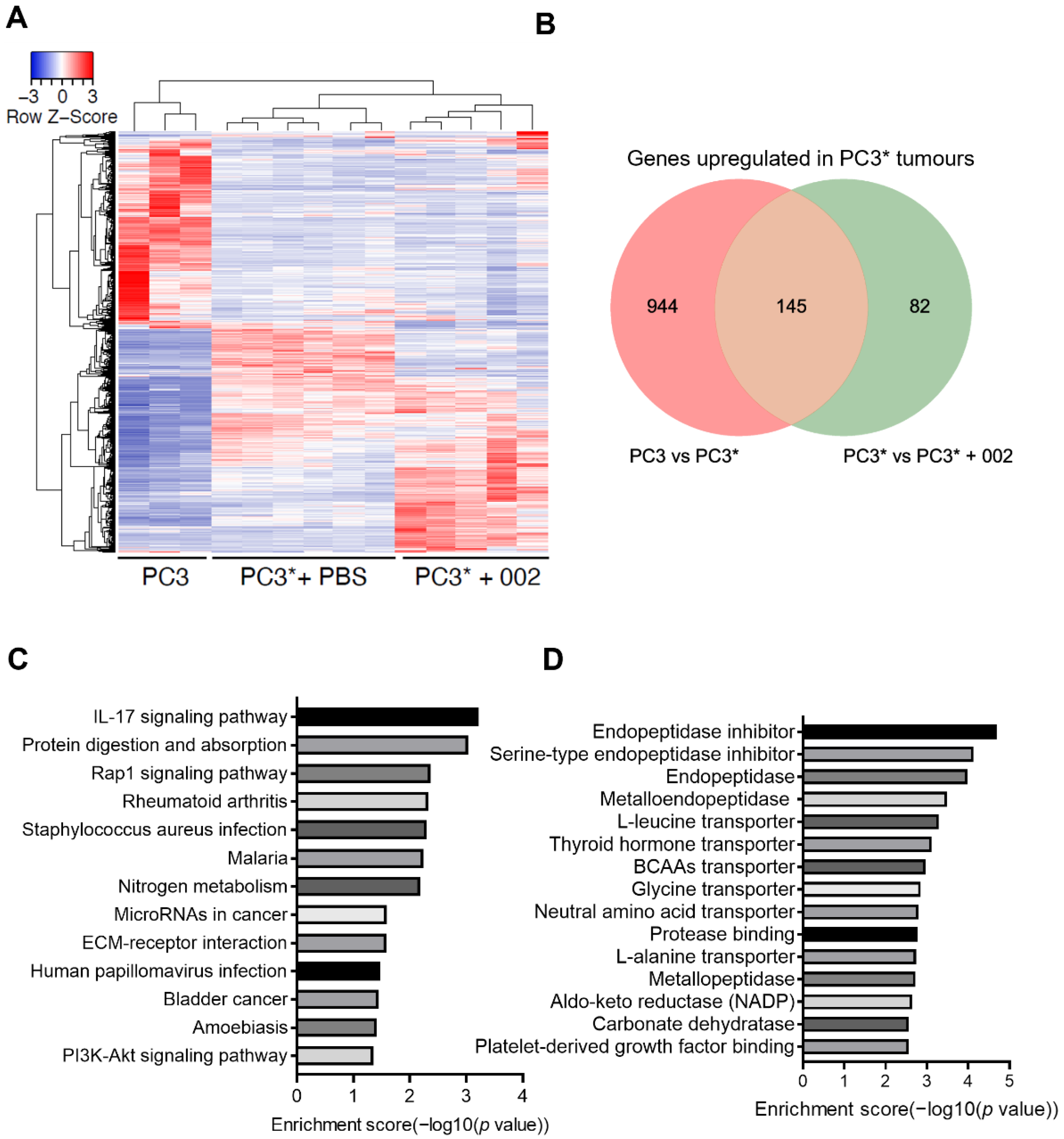
3.3. Secretomic and Transcriptomic Analysis of Cachexia Related DEGs
3.4. The Tumour Expression Profile of Potential Biomarkers in Different Cancers Is Correlated with Risk of Cachexia and Cancer Outcomes
3.5. Validation of Circulating LCN2 as a Biomarker for Cancer Cachexia
4. Discussion
5. Study Limitations
6. Conclusions
7. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.K.; Makuch, R.; Clamon, G.H.; Feld, R.; Weiner, R.S.; Moran, E.; Blum, R.; Shepherd, F.A.; Jeejeebhoy, K.N.; DeWys, W.D. Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res. 1985, 45, 3347–3353. [Google Scholar] [PubMed]
- Cao, Z.; Scott, A.M.; Hoogenraad, N.J.; Osellame, L.D. Mediators and clinical treatment for cancer cachexia: A systematic review. JCSM Rapid Commun. 2021, 4, 166–186. [Google Scholar] [CrossRef]
- Crawford, J. Cancer cachexia: Are we ready to take a step forward? Cancer 2018, 124, 456–458. [Google Scholar] [CrossRef]
- Yoshikwa, T.; Takano, M.; Kouta, H.; Horikoshi, M.; Asakawa, T.; Kudoh, K.; Kita, T.; Furuya, K.; Kikuchi, Y. Can serum IL-6 be a sentinel biomarker of cancer cachexia in gynecologic cancer patients? J. Clin. Oncol. 2018, 36, e17544. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for Cancer Cachexia: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4501. [Google Scholar] [CrossRef]
- Johnston, A.J.; Murphy, K.T.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G.; et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef]
- Cortazar, A.R.; Oguiza, J.A.; Aransay, A.M.; Lavín, J.L. VerSeDa: Vertebrate secretome database. Database 2017, 2017, baw171. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Sukari, A.; Muqbil, I.; Mohammad, R.M.; Philip, P.A.; Azmi, A.S. F-BOX proteins in cancer cachexia and muscle wasting: Emerging regulators and therapeutic opportunities. Semin. Cancer Biol. 2016, 36, 95–104. [Google Scholar] [CrossRef]
- Yuan, L.; Han, J.; Meng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, J.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Talbert, E.E.; Cuitiño, M.C.; Ladner, K.J.; Rajasekerea, P.V.; Siebert, M.; Shakya, R.; Leone, G.W.; Ostrowski, M.C.; Paleo, B.; Weisleder, N.; et al. Modeling Human Cancer-induced Cachexia. Cell Rep. 2019, 28, 1612–1622.e1614. [Google Scholar] [CrossRef] [PubMed]
- Costelli, P.; Muscaritoli, M.; Bonetto, A.; Penna, F.; Reffo, P.; Bossola, M.; Bonelli, G.; Doglietto, G.B.; Baccino, F.M.; Fanelli, F.R. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur. J. Clin. Investig. 2008, 38, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar] [CrossRef]
- Laurencikiene, J.; Stenson, B.M.; Arvidsson Nordström, E.; Agustsson, T.; Langin, D.; Isaksson, B.; Permert, J.; Rydén, M.; Arner, P. Evidence for an important role of CIDEA in human cancer cachexia. Cancer Res. 2008, 68, 9247–9254. [Google Scholar] [CrossRef]
- Molfino, A.; Belli, R.; Imbimbo, G.; Carletti, R.; Amabile, M.I.; Tambaro, F.; di Gioia, C.R.T.; Belloni, E.; Ferraro, E.; Nigri, G.; et al. Evaluation of Browning Markers in Subcutaneous Adipose Tissue of Newly Diagnosed Gastrointestinal Cancer Patients with and without Cachexia. Cancers 2022, 14, 1948. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Kelly, R.; Yang, L.; Zhang, X.; Wortham, K.; Joseph, I.B. The anti-Fn14 antibody BIIB036 inhibits tumor growth in xenografts and patient derived primary tumor models and enhances efficacy of chemotherapeutic agents in multiple xenograft models. Cancer Biol. 2012, 13, 812–821. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Amatucci, A.; Kelly, R.; Su, L.; Garber, E.; Day, E.S.; Berquist, L.; Cho, S.; Li, Y.; Parr, M.; et al. Development of an Fn14 agonistic antibody as an anti-tumor agent. MAbs 2011, 3, 362–375. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, M.-K.; Lee, S.-Y.; Oh, H.-J.; Lim, M.-A.; Cho, W.-T.; Kim, E.-K.; Ju, J.-H.; Park, Y.-W.; Park, S.-H.; et al. TWEAK promotes the production of Interleukin-17 in rheumatoid arthritis. Cytokine 2012, 60, 143–149. [Google Scholar] [CrossRef]
- Tang, H.; Pang, S.; Wang, M.; Xiao, X.; Rong, Y.; Wang, H.; Zang, Y.Q. TLR4 Activation Is Required for IL-17–Induced Multiple Tissue Inflammation and Wasting in Mice. J. Immunol. 2010, 185, 2563–2569. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Wang, R.-C.; Cheng, K.; Ring, B.Z.; Su, L. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biol. Med. 2017, 14, 90–99. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Croft, M. Regulation of PI-3-Kinase and Akt Signaling in T Lymphocytes and Other Cells by TNFR Family Molecules. Front. Immunol. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, A.; Mirković, B.; Sosič, I.; Gobec, S.; Kos, J. Inhibition of endopeptidase and exopeptidase activity of cathepsin B impairs extracellular matrix degradation and tumour invasion. Biol. Chem. 2016, 397, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Obianyo, O.; Du, Y.; Fu, H.; Li, S.; Ye, K. Blockade of Asparagine Endopeptidase Inhibits Cancer Metastasis. J. Med. Chem. 2017, 60, 7244–7255. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Zhou, Z.; Stone, M.; Lu, B.; Flesken-Nikitin, A.; Nanus, D.M.; Nikitin, A.Y. Membrane metalloendopeptidase suppresses prostate carcinogenesis by attenuating effects of gastrin-releasing peptide on stem/progenitor cells. Oncogenesis 2020, 9, 38. [Google Scholar] [CrossRef]
- Digre, A.; Lindskog, C. The Human Protein Atlas—Spatial localization of the human proteome in health and disease. Protein Sci. 2021, 30, 218–233. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Anker, M.S.; Holcomb, R.; Muscaritoli, M.; von Haehling, S.; Haverkamp, W.; Jatoi, A.; Morley, J.E.; Strasser, F.; Landmesser, U.; Coats, A.J.S.; et al. Orphan disease status of cancer cachexia in the USA and in the European Union: A systematic review. J. Cachexia Sarcopenia Muscle 2019, 10, 22–34. [Google Scholar] [CrossRef]
- Leeman, M.; Choi, J.; Hansson, S.; Storm, M.U.; Nilsson, L. Proteins and antibodies in serum, plasma, and whole blood-size characterization using asymmetrical flow field-flow fractionation (AF4). Anal. Bioanal. Chem. 2018, 410, 4867–4873. [Google Scholar] [CrossRef] [PubMed]
- Echan, L.A.; Tang, H.Y.; Ali-Khan, N.; Lee, K.; Speicher, D.W. Depletion of multiple high-abundance proteins improves protein profiling capacities of human serum and plasma. Proteomics 2005, 5, 3292–3303. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.R.; da Silva Fernandes, R.; de Souza Pessôa, G.; Raimundo, I.M.; Arruda, M.A.Z. Depleting high-abundant and enriching low-abundant proteins in human serum: An evaluation of sample preparation methods using magnetic nanoparticle, chemical depletion and immunoaffinity techniques. Talanta 2017, 170, 199–209. [Google Scholar] [CrossRef]
- Hodge, K.; Have, S.T.; Hutton, L.; Lamond, A.I. Cleaning up the masses: Exclusion lists to reduce contamination with HPLC-MS/MS. J. Proteom. 2013, 88, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Sawazaki, S.; Oshima, T.; Sakamaki, K.; Aoyama, T.; Sato, T.; Shiozawa, M.; Yoshikawa, T.; Rino, Y.; Imada, T.; Masuda, M. Clinical Significance of Tensin 4 Gene Expression in Patients with Gastric Cancer. In Vivo 2017, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Y.; Wang, K.; Geng, L.; Sun, J.; Xu, W.; Liu, D.; Gong, S.; Zhu, Y. Identification of candidate diagnostic and prognostic biomarkers for pancreatic carcinoma. EBioMedicine 2019, 40, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of Cancer Cachexia: Attempting to Develop New Pharmacological Agents for New Effective Therapeutic Options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef]
- Weisheng, B.; Nezhat, C.H.; Huang, G.F.; Mao, Y.-Q.; Sidell, N.; Huang, R.-P. Discovering endometriosis biomarkers with multiplex cytokine arrays. Clin. Proteom. 2019, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Talbert, E.E.; Lewis, H.L.; Farren, M.R.; Ramsey, M.L.; Chakedis, J.M.; Rajasekera, P.; Haverick, E.; Sarna, A.; Bloomston, M.; Pawlik, T.M.; et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Kir, S.; White, J.P.; Kleiner, S.; Kazak, L.; Cohen, P.; Baracos, V.E.; Spiegelman, B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014, 513, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yoon, H.-j.; Lee, Y.-h.; Kim, H.R.; Lee, B.W.; Rhee, Y.; Kang, E.S.; Cha, B.-S.; Lee, H.C. Serum PTHrP predicts weight loss in cancer patients independent of hypercalcemia, inflammation, and tumor burden. J. Clin. Endocrinol. Metab. 2016, 101, 1207–1214. [Google Scholar] [CrossRef]
- Lodge, W.; Zavortink, M.; Golenkina, S.; Froldi, F.; Dark, C.; Cheung, S.; Parker, B.L.; Blazev, R.; Bakopoulos, D.; Christie, E.L.; et al. Tumor-derived MMPs regulate cachexia in a Drosophila cancer model. Dev. Cell 2021, 56, 2664–2680.e2666. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Zhu, X.; Norgard, M.A.; Levasseur, P.R.; Butler, J.T.; Buenafe, A.; Burfeind, K.G.; Michaelis, K.A.; Pelz, K.R.; Mendez, H.; et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat. Commun. 2021, 12, 2057. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Burvenich, I.J.; Zhao, K.; Senko, C.; Glab, J.; Fogliaro, R.; Liu, Z.; Jose, I.; Puthalakath, H.; Hoogenraad, N.J.; et al. Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy. Cancers 2022, 14, 5533. https://doi.org/10.3390/cancers14225533
Cao Z, Burvenich IJ, Zhao K, Senko C, Glab J, Fogliaro R, Liu Z, Jose I, Puthalakath H, Hoogenraad NJ, et al. Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy. Cancers. 2022; 14(22):5533. https://doi.org/10.3390/cancers14225533
Chicago/Turabian StyleCao, Zhipeng, Ingrid J. Burvenich, Kening Zhao, Clare Senko, Jason Glab, Renee Fogliaro, Zhanqi Liu, Irvin Jose, Hamsa Puthalakath, Nick J. Hoogenraad, and et al. 2022. "Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy" Cancers 14, no. 22: 5533. https://doi.org/10.3390/cancers14225533
APA StyleCao, Z., Burvenich, I. J., Zhao, K., Senko, C., Glab, J., Fogliaro, R., Liu, Z., Jose, I., Puthalakath, H., Hoogenraad, N. J., Osellame, L. D., & Scott, A. M. (2022). Identification of Potential Biomarkers for Cancer Cachexia and Anti-Fn14 Therapy. Cancers, 14(22), 5533. https://doi.org/10.3390/cancers14225533





