Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Immune Mechanisms in Breast Cancer and Importance of Immune Responses in TNBC Prognosis and Treatment
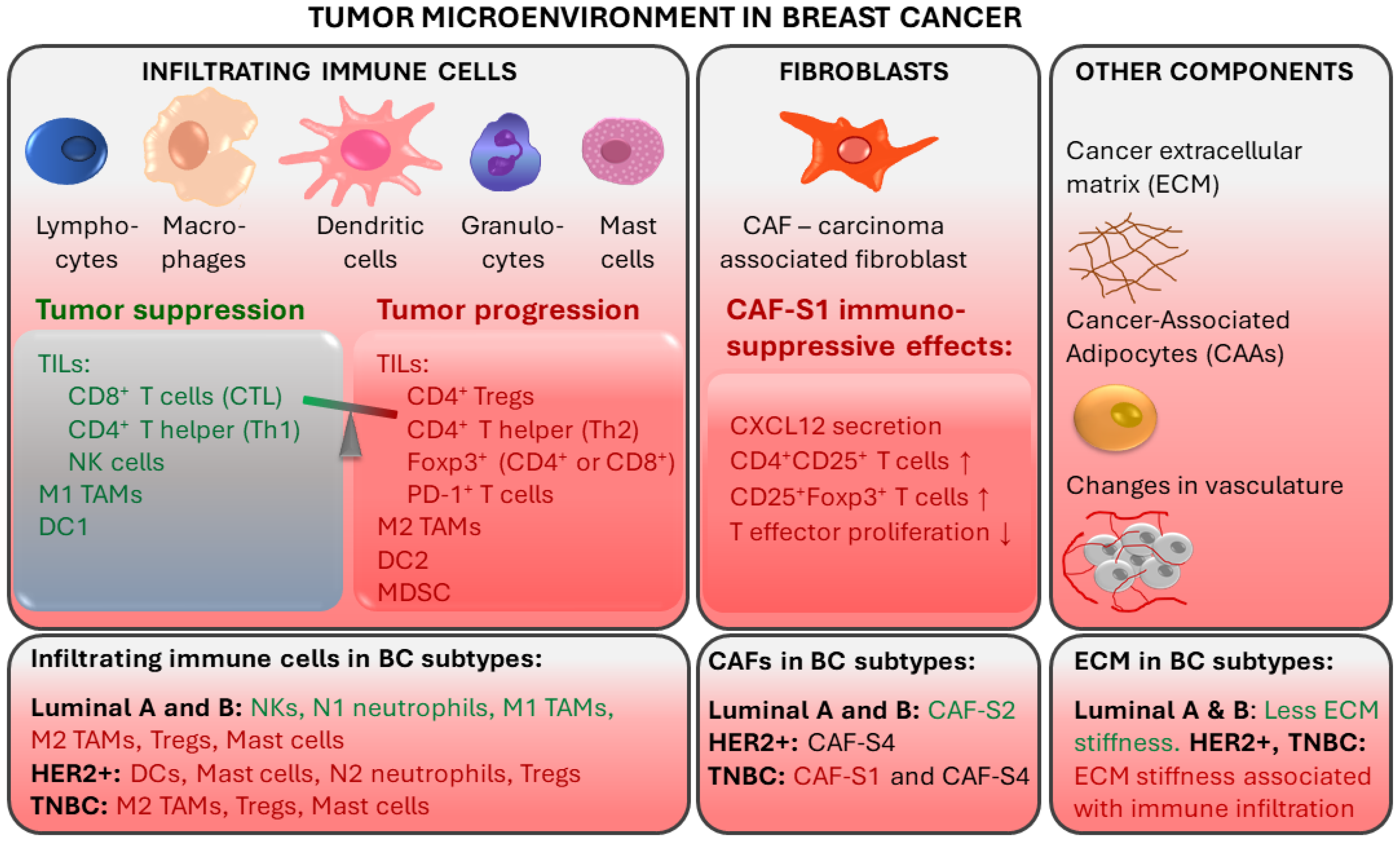
2.1. Tumor-Infiltrating Lymphocytes
2.2. Tumor-Associated Macrophages
2.3. Other TME Components
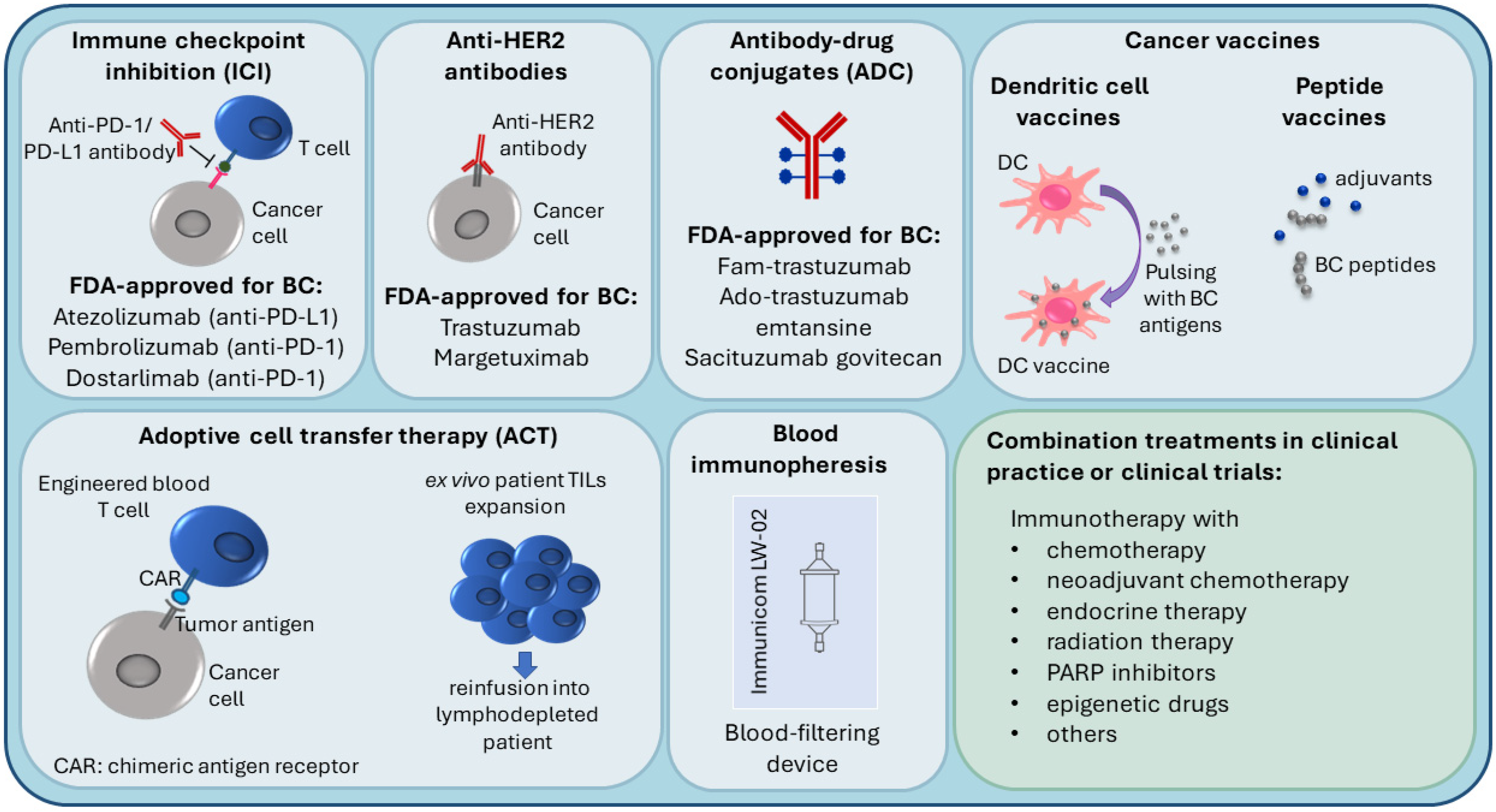
3. Immunotherapy in Breast Cancer
3.1. Immune Checkpoint Inhibition (ICI), Anti-HER2 Antibodies and Antibody-Drug Conjugates (ADC)
3.2. Personalized Immunotherapy Approaches
4. Transitioning toward Less Invasive Immune-Related Biomarkers for Cancer Detection and Prognosis
5. Circulating Blood Cell Transcriptome as a Source of Less Invasive Breast Cancer Biomarkers
6. PBMC Gene Expression Biomarkers for Classification of Novel BC Subtypes
7. Gene Expression Biomarkers in Nucleated Blood Cells of TNBC Patients
7.1. PBMC Transcriptome of TNBC (ER-/PR-/HER2-) versus Hormone-Dependent BC (ER+/PR+/HER2-)
7.2. PBMC Transcriptome of TNBC (ER-/PR-/HER2-) versus Her2-Overexpressing BC (ER-/PR-/HER2+)
8. Peripheral Blood Cell Transcriptome and Therapy Response and Prognosis in TNBC Patients
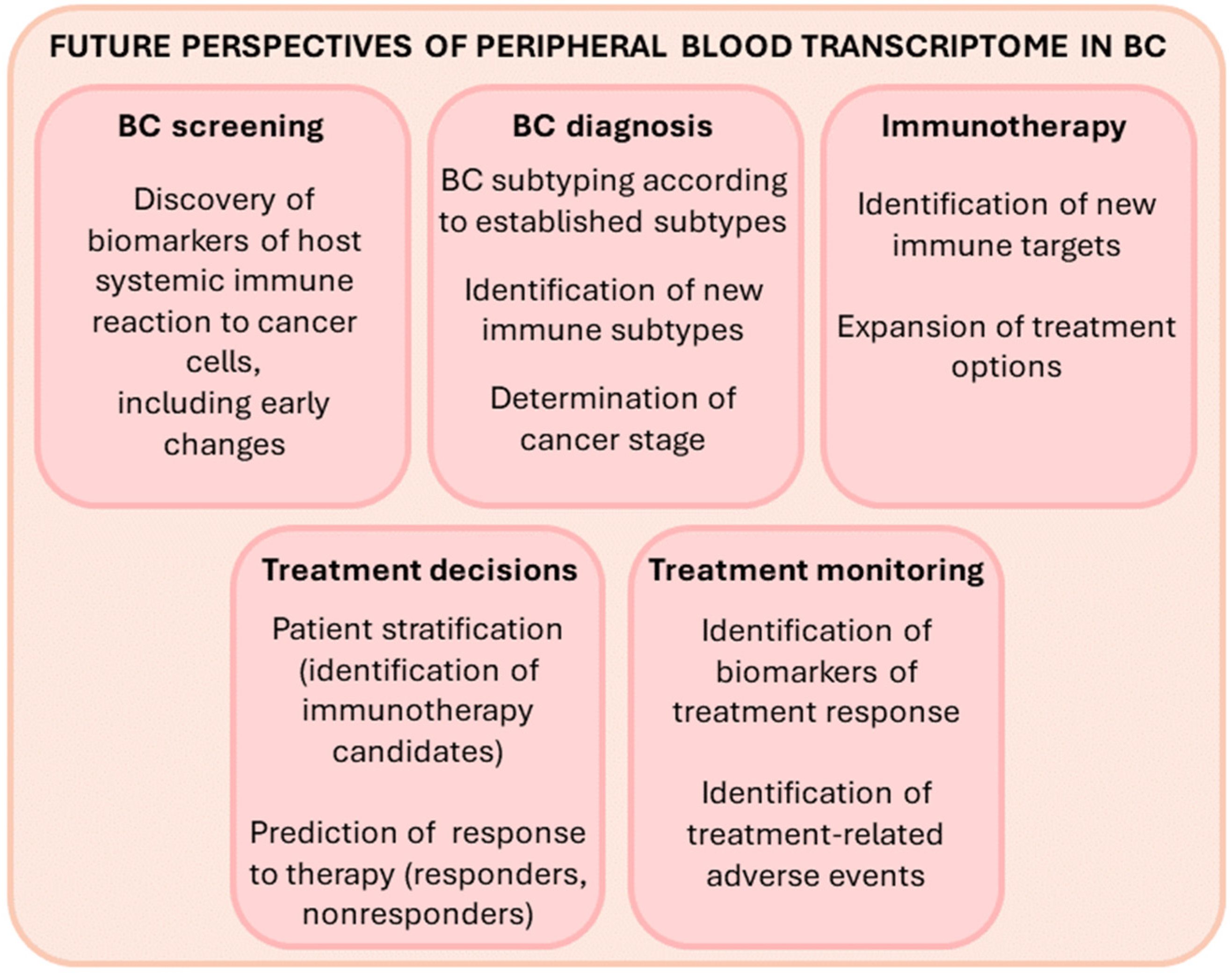
9. Future Outlook and Perspective
10. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Szymiczek, A.; Lone, A.; Akbari, M.R. Molecular intrinsic versus clinical subtyping in breast cancer: A comprehensive review. Clin. Genet. 2020, 99, 613–637. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular Characterization of Basal-Like and Non-Basal-Like Triple-Negative Breast Cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Balacescu, O.; Balacescu, L.; Virtic, O.; Visan, S.; Gherman, C.; Drigla, F.; Pop, L.; Bolba-Morar, G.; Lisencu, C.; Fetica, B.; et al. Blood Genome-Wide Transcriptional Profiles of HER2 Negative Breast Cancers Patients. Mediat. Inflamm. 2016, 3239167. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Cortes, J.; Pare, L.; Galván, P.; Bermejo, B.; Martínez, N.; Vidal, M.; Pernas, S.; López, R.L.; Munoz, M.; et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): An open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 2017, 18, 545–554. [Google Scholar] [CrossRef]
- Guarneri, V.; Dieci, M.V.; Griguolo, G.; Miglietta, F.; Girardi, F.; Bisagni, G.; Generali, D.G.; Cagossi, K.; Sarti, S.; Frassoldati, A.; et al. Trastuzumab-lapatinib as neoadjuvant therapy for HER2-positive early breast cancer: Survival analyses of the CHER-Lob trial. Eur. J. Cancer 2021, 153, 133–141. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.U.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef]
- Li, J.; Gonzalez-Angulo, A.M.; Allen, P.K.; Yu, T.K.; Woodward, W.A.; Ueno, N.T.; Lucci, A.; Krishnamurthy, S.; Gong, Y.; Bondy, M.L.; et al. Triple-Negative Subtype Predicts Poor Overall Survival and High Locoregional Relapse in Inflammatory Breast Cancer. Oncologist 2011, 16, 1675–1683. [Google Scholar] [CrossRef]
- Tudoran, O.; Virtic, O.; Balacescu, L.; Pop, L.; Dragla, F.; Eniu, A.; Fetica, B.; Balacescu, O.; Berindan-Neagoe, I. Differential Peripheral Blood Gene Expression Profile Based on Her2 Expression on Primary Tumors of Breast Cancer Patients. PLoS ONE 2014, 9, e102764. [Google Scholar] [CrossRef]
- Tudoran, O.; Virtic, O.; Balacescu, L.; Lisencu, C.; Fetica, B.; Balacescu, O.; Berindan-Neagoe, I.; Gherman, C. Baseline blood immunological profiling differentiates between Her2– breast cancer molecular subtypes: Implications for immunomediated mechanisms of treatment response. OncoTargets Ther. 2015, 8, 3415–3423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treat-ment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Radosevic-Robin, N.; Selenica, P.; Zhu, Y.; Won, H.H.; Berger, M.F.; Ferrando, L.; Cocco, E.; Privat, M.; Ponelle-Chachuat, F.; Abrial, C.; et al. Recurrence biomarkers of triple negative breast cancer treated with neoadjuvant chemotherapy and anti-EGFR antibodies. NPJ Breast Cancer 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Oner, G.; Altintas, S.; Canturk, Z.; Tjalma, W.; Verhoeven, Y.; Van Berckelaer, C.; Berneman, Z.; Peeters, M.; Pauwels, P.; van Dam, P.A. Triple-negative breast cancer—Role of immunology: A systemic review. Breast J. 2020, 26, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, L.L.; Gorris, M.A.J.; Halilovic, A.; Figdor, C.G.; de Vries, I.J.M. Migrating into the Tumor: A Roadmap for T Cells. Trends Cancer 2017, 3, 797–808. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune Tumor Microenvironment in Breast Cancer and the Participation of Estrogen and Its Receptors in Cancer Physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchiò, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef]
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S. Host Antitumor Immunity Plays a Role in the Survival of Patients With Newly Diagnosed Triple-Negative Breast Cancer. J. Clin. Oncol. 2014, 32, 2935–2937. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers From Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.B.; et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Dieci, M.; Miglietta, F.; Guarneri, V. Immune Infiltrates in Breast Cancer: Recent Updates and Clinical Implications. Cells 2021, 10, 223. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Yam, C.; Yen, E.-Y.; Chang, J.T.; Bassett, R.L.; Al-Atrash, G.; Garber, H.; Huo, L.; Yang, F.; Philips, A.V.; Ding, Q.-Q.; et al. Immune Phenotype and Response to Neoadjuvant Therapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Q.; Zhang, M.; Wang, H.; Wu, L.; Yang, J. Immune-related biomarkers in triple-negative breast cancer. Breast Cancer 2021, 28, 792–805. [Google Scholar] [CrossRef]
- Na Seo, A.; Lee, H.E.; Kim, E.J.; Kim, H.J.; Jang, M.H.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Park, S.Y. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br. J. Cancer 2013, 109, 2705–2713. [Google Scholar] [CrossRef]
- Tower, H.; Ruppert, M.; Britt, K. The Immune Microenvironment of Breast Cancer Progression. Cancers 2019, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Barhoush, E.; Tulbah, A.; Elkum, N.; Al-Tweigeri, T.; Dermime, S. FOXP3+ Tregs and B7-H1+/PD-1+T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer 2008, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lu, D.; Bai, Y.; Wang, Y.-P.; Bu, H.; Zheng, H. Immune Profiles of Tumor Microenvironment and Clinical Prognosis among Women with Triple-Negative Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Muenst, S.; Soysal, S.; Gao, F.; Obermann, E.C.; Oertli, D.; E Gillanders, W. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2013, 139, 667–676. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J. Clin. Oncol. 2020, 38 (Suppl. 15), 1000. [Google Scholar] [CrossRef]
- Schreiber, A.R.; Kagihara, J.A.; Weiss, J.A.; Nicklawsky, A.; Gao, D.; Borges, V.F.; Kabos, P.; Diamond, J.R. Clinical Outcomes for Patients With Metastatic Breast Cancer Treated With Immunotherapy Agents in Phase I Clinical Trials. Front Oncol. 2021, 11, 640690. [Google Scholar] [CrossRef]
- Schmid, P.; Salgado, R.; Park, Y.; Muñoz-Couselo, E.; Kim, S.; Sohn, J.; Im, S.-A.; Foukakis, T.; Kuemmel, S.; Dent, R.; et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: Results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann. Oncol. 2020, 31, 569–581. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 21, 44–59. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Barrios, C.H.; Diéras, V.; Iwata, H.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Winer, E.P.; Patel, S.; et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 2021, 32, 983–993. [Google Scholar] [CrossRef]
- Santoni, M.; Romagnoli, E.; Saladino, T.; Foghini, L.; Guarino, S.; Capponi, M.; Giannini, M.; Cognigni, P.D.; Ferrara, G.; Battelli, N. Triple negative breast cancer: Key role of Tumor-Associated Macrophages in reg-ulating the activity of anti-PD-1/PD-L1 agents. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, I.; Kang, S.H.; Shin, H.C.; Kwon, S.Y. Tumor-Associated Macrophages as Potential Prognostic Biomarkers of In-vasive Breast Cancer. J. Breast Cancer 2019, 22, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Gong, M.M.; Ayuso, J.M.; Tomko, L.A.; Beebe, D.J.; Virumbrales-Muñoz, M.; Ponik, S.M. Breast Fibroblasts and ECM Components Modulate Breast Cancer Cell Migration through the Secretion of MMPs in a 3D Microfluidic Co-Culture Model. Cancers 2020, 12, 1173. [Google Scholar] [CrossRef]
- Moccia, C.; Haase, K. Engineering Breast Cancer On-chip—Moving Toward Subtype Specific Models. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianou, A.; Papageorgis, P.; Polydorou, C.; Stylianopoulos, T. Remodeling Components of the Tumor Microenvironment to Enhance Cancer Therapy. Front. Oncol. 2015, 5, 214. [Google Scholar] [CrossRef]
- Emens, L.A. The dawn of immunotherapy for breast cancer. Clin. Adv. Hematol. Oncol. 2019, 17, 332–335. [Google Scholar] [PubMed]
- Li, Y.; Miao, W.; He, D.; Wang, S.; Lou, J.; Jiang, Y.; Wang, S. Recent Progress on Immunotherapy for Breast Cancer: Tumor Microenvironment, Nanotechnology and More. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Elghazaly, H.; Bousoik, E.; Elmazar, M.M.A.; Tolaney, S.M. Novel combinatorial strategies for boosting the efficacy of immune checkpoint inhibitors in advanced breast cancers. Clin. Transl. Oncol. 2021, 23, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P.; Wahby, S.; Gao, J.J.; Amiri-Kordestani, L.; Ibrahim, A.; Bloomquist, E.; Tang, S.; Xu, Y.; Liu, J.; Fu, W.; et al. FDA Approval Summary: Atezolizumab Plus Paclitaxel Protein-bound for the Treatment of Patients with Advanced or Metastatic TNBC Whose Tumors Express PD-L1. Clin. Cancer Res. 2020, 26, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Slater, H. FDA Approves Pembrolizumab + Chemotherapy Combination for Locally Recurrent Unresectable or Metastatic TNBC. Oncology 2020, 34, 547. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, S.; Tolaney, S.M.; Schwartzberg, L.; Mita, M.; McCann, G.; Tan, A.R.; Wahner-Hendrickson, A.E.; Forero, A.; Anders, C.; Wulf, G.M.; et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019, 5, 1132–1140. [Google Scholar] [CrossRef]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; King-Kallimanis, B.L.; Theoret, M.R.; Ibrahim, A.; et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-positive Early Breast Cancer. Clin. Cancer Res. 2020, 26, 4180–4185. [Google Scholar] [CrossRef]
- Gao, J.J.; Osgood, C.L.; Gong, Y.; Zhang, H.; Bloomquist, E.W.; Jiang, X.; Qiu, J.; Yu, J.; Song, P.; Rahman, N.A.; et al. FDA Approval Summary: Pertuzumab, Trastuzumab, and Hyaluronidase-zzxf Injection for Subcutaneous Use in Patients with HER2-positive Breast Cancer. Clin. Cancer Res. 2021, 27, 2126–2129. [Google Scholar] [CrossRef]
- Narayan, P.; Osgood, C.L.; Singh, H.; Chiu, H.-J.; Ricks, T.K.; Chow, E.C.Y.; Qiu, J.; Song, P.; Yu, J.; Namuswe, F.; et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 4478–4485. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef]
- Nagayama, A.; Vidula, N.; Ellisen, L.; Bardia, A. Novel antibody–drug conjugates for triple negative breast cancer. Ther. Adv. Med Oncol. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Wahby, S.; Fashoyin-Aje, L.; Osgood, C.L.; Cheng, J.; Fiero, M.H.; Zhang, L.; Tang, S.; Hamed, S.S.; Song, P.; Charlab, R.; et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin. Cancer Res. 2020, 27, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, M.; Akbari, V. Cancer vaccines as a targeted immunotherapy approach for breast cancer: An update of clinical evidence. Expert Rev. Vaccines 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Thomas, S.; Munster, P. Immunotherapy in breast cancer: A clinician’s perspective. J. Natl. Cancer Cent. 2021, 1, 47–57. [Google Scholar] [CrossRef]
- You, Z.; Zhou, W.; Weng, J.; Feng, H.; Liang, P.; Li, Y.; Shi, F. Application of HER2 peptide vaccines in patients with breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lowenfeld, L.; Mick, R.; Datta, J.; Xu, S.; Fitzpatrick, E.; Fisher, C.S.; Fox, K.R.; DeMichele, A.; Zhang, P.J.; Weinstein, S.P.; et al. Dendritic Cell Vaccination Enhances Immune Responses and Induces Regression of HER2pos DCIS Independent of Route: Results of Randomized Selection Design Trial. Clin. Cancer Res. 2016, 23, 2961–2971. [Google Scholar] [CrossRef]
- Roufarshbaf, M.; Esmaeil, N.; Akbari, V. Comparison of four methods of colon cancer cell lysates preparation for ex vivo matu-ration of dendritic cells. Res. Pharm. Sci. 2022, 17, 43–52. [Google Scholar] [CrossRef]
- Muranski, P.; Boni, A.; Wrzesinski, C.; Citrin, D.E.; Rosenberg, S.A.; Childs, R.; Restifo, N.P. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat. Clin. Prac. Oncol. 2006, 3, 668–681. [Google Scholar] [CrossRef]
- Roselli, E.; Frieling, J.; Thorner, K.; Ramello, M.C.; Lynch, C.C.; Abate-Daga, D. CAR-T Engineering: Optimizing Signal Transduction and Effector Mechanisms. BioDrugs 2019, 33, 647–659. [Google Scholar] [CrossRef]
- Wysocki, P.J.; Ostrowski, A.; Segal, R.; Potocki, P.; Konopka, K.W.; Kwinta, L.; Florin, L.; Prince, S. Preliminary data on antitumor activity of extracorporeal subtraction of soluble TNFRs to unleash the activity of endogenous TNFα combined with chemotherapy in treatment-refractory, advanced, triple-negative breast cancer patients. J. Clin. Oncol. 2021, 39 (Suppl. 15), e13052. [Google Scholar] [CrossRef]
- Goto, W.; Kashiwagi, S.; Asano, Y.; Takada, K.; Takahashi, K.; Hatano, T.; Takashima, T.; Tomita, S.; Motomura, H.; Ohsawa, M.; et al. Predictive value of improvement in the immune tumour microenvironment in patients with breast cancer treated with neoadjuvant chemotherapy. ESMO Open 2018, 3, e000305. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, M.; Liang, B.; Ge, S.; Peng, J.; Huang, H.; Xu, Y.; Tang, X.; Deng, L. Identification of human peripheral blood monocyte gene markers for early screening of solid tumors. PLoS ONE 2020, 15, e0230905. [Google Scholar] [CrossRef] [PubMed]
- Weedon-Fekjaer, H.; Lindqvist, B.H.; Vatten, L.J.; O Aalen, O.; Tretli, S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008, 10, R41. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors that Influence Them: An Analysis of 27,825 Patient Evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Foulds, G.A.; Vadakekolathu, J.; Abdel-Fatah, T.M.A.; Nagarajan, D.; Reeder, S.; Johnson, C.; Hood, S.; Moseley, P.; Chan, S.Y.T.; Pockley, A.; et al. Immune-Phenotyping and Transcriptomic Profiling of Peripheral Blood Mononuclear Cells From Patients With Breast Cancer: Identification of a 3 Gene Signature Which Predicts Relapse of Triple Negative Breast Cancer. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10. [Google Scholar] [CrossRef]
- Twine, N.C.; A Stover, J.; Marshall, B.; Dukart, G.; Hidalgo, M.; Stadler, W.; Logan, T.; Dutcher, J.; Hudes, G.; Dorner, A.J.; et al. Disease-associated expression profiles in peripheral blood mononuclear cells from patients with advanced renal cell carcinoma. Cancer Res. 2003, 63, 6069–6075. [Google Scholar]
- Stoiber, D.; Assinger, A. Platelet-leukocyte interplay in cancer development and progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef]
- Ward, M.P.; Kane, L.E.; Norris, L.A.; Mohamed, B.M.; Kelly, T.; Bates, M.; Clarke, A.; Brady, N.; Martin, C.M.; Brooks, R.D.; et al. Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Mol. Cancer 2021, 20, 59. [Google Scholar] [CrossRef]
- Mosallaei, M.; Ehtesham, N.; Rahimirad, S.; Saghi, M.; Vatandoost, N.; Khosravi, S. PBMCs: A new source of diagnostic and prognostic biomarkers. Arch. Physiol. Biochem. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lusho, S.; Durando, X.; Mouret-Reynier, M.A.; Kossai, M.; Lacrampe, N.; Molnar, I.; Penault-Llorca, F.; Radosevic-Robin, N.; Abrial, C. Platelet-to-Lymphocyte Ratio Is Associated With Favorable Response to Neoadjuvant Chemotherapy in Triple Negative Breast Cancer: A Study on 120 Patients. Front. Oncol. 2021, 11, 678315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fang, Y.; Li, J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol. Lett. 2019, 18, 5399–5407. [Google Scholar] [CrossRef]
- Aarøe, J.; Lindahl, T.; Dumeaux, V.; Saebø, S.; Tobin, D.; Hagen, N.; Skaane, P.; Lönneborg, A.; Sharma, P.; Børresen-Dale, A.-L. Gene expression profiling of peripheral blood cells for early detection of breast cancer. Breast Cancer Res. 2010, 12, R7. [Google Scholar] [CrossRef] [PubMed]
- Dumeaux, V.; Fjukstad, B.; Fjosne, H.E.; Frantzen, J.-O.; Holmen, M.M.; Rodegerdts, E.; Schlichting, E.; Børresen-Dale, A.-L.; Bongo, L.A.; Lund, E.; et al. Interactions between the tumor and the blood systemic response of breast cancer patients. PLoS Comput. Biol. 2017, 13, e1005680. [Google Scholar] [CrossRef]
- Morisaki, T.; Kubo, M.; Umebayashi, M.; Yuan, Y.; Tsuyada, A.; Tanaka, H.; Koya, N.; Nakagawa, S.; Morisaki, T.; Nakamura, M. Usefulness of the nCounter Analysis System to Monitor Immune-related Biomarkers in PBMCs During Anti-PD-1 Therapy. Anticancer Res. 2019, 39, 4517–4523. [Google Scholar] [CrossRef]
- Wang, L.; Simons, D.L.; Lu, X.; Tu, T.Y.; Avalos, C.; Chang, A.Y.; Dirbas, F.M.; Yim, J.H.; Waisman, J.; Lee, P.P. Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine 2020, 52, 102631. [Google Scholar] [CrossRef]
- Dumeaux, V.; Ursini-Siegel, J.; Flatberg, A.; Fjosne, H.E.; Frantzen, J.; Holmen, M.M.; Rodegerdts, E.; Schlichting, E.; Lund, E. Peripheral blood cells inform on the presence of breast cancer: A population-based case–control study. Int. J. Cancer 2014, 136, 656–667. [Google Scholar] [CrossRef]
- Suzuki, E.; Sugimoto, M.; Kawaguchi, K.; Pu, F.; Uozumi, R.; Yamaguchi, A.; Nishie, M.; Tsuda, M.; Kotake, T.; Morita, S.; et al. Gene expression profile of peripheral blood mononuclear cells may contribute to the identification and immunological classification of breast cancer patients. Breast Cancer 2018, 26, 282–289. [Google Scholar] [CrossRef]
- Cui, G. TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front. Oncol. 2019, 9, 1002. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Hensler, M.; (Vančurová), I.R.; Becht, E.; Palata, O.; Strnad, P.; Tesarova, P.; Cabinakova, M.; Švec, D.; Kubista, M.; Bartůňková, J.; et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. OncoImmunology 2015, 5, e1102827. [Google Scholar] [CrossRef]
- Sharma, P.; Sahni, N.S.; Tibshirani, R.; Skaane, P.; Urdal, P.; Berghagen, H.; Jensen, M.; Kristiansen, L.; Moen, C.; Sharma, P.; et al. Early detection of breast cancer based on gene-expression patterns in peripheral blood cells. Breast Cancer Res. 2005, 7, R634. [Google Scholar] [CrossRef]
- Ciarloni, L.; Hosseinian, S.; Monnier-Benoit, S.; Imaizumi, N.; Dorta, G.; Ruegg, C.; On behalf of the DGNP-COL-0310 Study Group. Discovery of a 29-Gene Panel in Peripheral Blood Mononuclear Cells for the Detection of Colorectal Cancer and Adenomas Using High Throughput Real-Time PCR. PLoS ONE 2015, 10, e0123904. [Google Scholar] [CrossRef]
- Shaath, H.; Toor, S.M.; Nair, V.S.; Elkord, E.; Alajez, N.M. Transcriptomic Analyses Revealed Systemic Alterations in Gene Expression in Circulation and Tumor Microenvironment of Colorectal Cancer Patients. Cancers 2019, 11, 1994. [Google Scholar] [CrossRef]
- Nussbaumer, R.; Godau, J.; Zanetti-Dällenbach, R.; Faisst, A.; Schicht, O.; Barekati, Z.; Staedtler, F.; Woelnerhanssen, B. 25P Early detection of breast cancer by liquid biopsy exploiting the DNA damage sensitivity (DDS). Ann. Oncol. 2021, 32, S1353. [Google Scholar] [CrossRef]
- Ming, W.; Xie, H.; Hu, Z.; Chen, Y.; Zhu, Y.; Bai, Y.; Liu, H.; Sun, X.; Liu, Y.; Gu, W. Two Distinct Subtypes Revealed in Blood Transcriptome of Breast Cancer Patients with an Un-supervised Analysis. Front. Oncol. 2019, 9, 985. [Google Scholar] [CrossRef]
- Holsbø, E.; Olsen, K.S. Metastatic Breast Cancer and Pre-Diagnostic Blood Gene Expression Profiles—The Norwegian Women and Cancer (NOWAC) Post-Genome Cohort. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuang, M.; An, H.; Zhang, Y.; Zhang, K.; Feng, L.; Zhang, L.; Cheng, S. Peripheral blood transcriptome heterogeneity and prognostic potential in lung cancer revealed by RNA-Seq. J. Cell. Mol. Med. 2021, 25, 8271–8284. [Google Scholar] [CrossRef]
- Savino, M.; Parrella, P.; Copetti, M.; Barbano, R.; Murgo, R.; Fazio, V.M.; Valori, V.M.; Carella, M.; Garrubba, M.; Santini, S.A. Comparison between Real-Time Quantitative PCR Detection of HER2 mRNA Copy Number in Peripheral Blood and ELISA of Serum HER2 Protein for Determining HER2 Status in Breast Cancer Patients. Cell Oncol 2009, 31, 203–211. [Google Scholar] [CrossRef]
- Savino, M.; Garrubba, M.; Parrella, P.; Baorda, F.; Copetti, M.; Murgo, R.; Zelante, L.; Carella, M.; Valori, V.M.; Santini, S.A. Development of real-time quantitative reverse transcription-PCR for Her2 detection in peripheral blood from patients with breast cancer. Clin. Chim. Acta 2007, 384, 52–56. [Google Scholar] [CrossRef]
- Naik, A.; Al-Zeheimi, N.; Bakheit, C.S.; Al Riyami, M.; Al Jarrah, A.; Al Moundhri, M.S.; Al Habsi, Z.; Basheer, M.; Adham, S.A. Neuropilin-1 Associated Molecules in the Blood Distinguish Poor Prognosis Breast Cancer: A Cross-Sectional Study. Sci. Rep. 2017, 7, 3301. [Google Scholar] [CrossRef]
- Rachner, T.D.; Kasimir-Bauer, S.; Goebel, A.; Erdmann, K.; Hoffmann, O.; Rauner, M.; Hofbauer, L.C.; Kimmig, R.; Bittner, A.-K. Soluble Neuropilin-1 is an independent marker of poor prognosis in early breast cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2233–2238. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.N.; Xu, D.Q.; Huang, J.G.; Lv, D.; Shi, X.Y.; Liu, J.Y.; Ren, H.W.; Han, Z.X. Neuropilin1, a novel independent prognostic factor and therapeutic target in triple-negative breast cancer. Neoplasma 2021, 67, 1335–1345. [Google Scholar] [CrossRef]
- Tordjman, R.; Lepelletier, Y.; Lemarchandel, V.E.R.; Cambot, M.; Gaulard, P.; Hermine, O.; Romeo, P.-H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 2002, 3, 477–482. [Google Scholar] [CrossRef]
- Dumond, A.; Pagès, G. Neuropilins, as Relevant Oncology Target: Their Role in the Tumoral Microenvironment. Front. Cell Dev. Biol. 2020, 8, 662. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Nixon, M.J.; Gonzalez-Ericsson, P.I.; Bergman, R.E.; Pilkinton, M.A.; McDonnell, W.J.; Sanchez, V.; Opalenik, S.R.; Loi, S.; Zhou, J.; et al. Changes in Peripheral and Local Tumor Immunity after Neoadjuvant Chemotherapy Reshape Clinical Outcomes in Patients with Breast Cancer. Clin. Cancer Res. 2020, 26, 5668–5681. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Gonzalez-Ericsson, P.I.; Sun, X.; Bergman, R.E.; Donaldson, J.; Tolaney, S.M.; Krop, I.E.; Garrido-Castro, A.C.; Sanders, M.E.; Mayer, I.A.; et al. Abstract PD9-06: Peripheral blood gene signatures predict response to neo-adjuvant chemotherapy in breast cancer patients. Cancer Res. 2021, 81 (Suppl. 4), PD9-06. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Benincasa, G.; Ferraro, G.; Caliendo, G.; Nicoletti, G.F.; Napoli, C. DNA methylation and breast cancer: A way forward (Review). Int. J. Oncol. 2021, 59, 98. [Google Scholar] [CrossRef]
- Prajzendanc, K.; Domagała, P.; Hybiak, J.; Ryś, J.; Huzarski, T.; Szwiec, M.; Tomiczek-Szwiec, J.; Redelbach, W.; Sejda, A.; Gronwald, J.; et al. BRCA1 promoter methylation in peripheral blood is associated with the risk of triple-negative breast cancer. Int. J. Cancer 2019, 146, 1293–1298. [Google Scholar] [CrossRef]
- Tang, Q.; Cheng, J.; Cao, X.; Surowy, H.; Burwinkel, B. Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin. Epigenetics 2016, 8, 115. [Google Scholar] [CrossRef]
- Wong, E.M.; Southey, M.C.; Fox, S.B.; Brown, M.A.; Dowty, J.G.; Jenkins, M.A.; Giles, G.G.; Hopper, J.L.; Dobrovic, A. Constitutional Methylation of the BRCA1 Promoter Is Specifically Associated with BRCA1 Mutation-Associated Pathology in Early-Onset Breast Cancer. Cancer Prev. Res. 2011, 4, 23–33. [Google Scholar] [CrossRef]
- Wong, E.M.; Southey, M.C.; Terry, M.B. Integrating DNA methylation measures to improve clinical risk assessment: Are we there yet? The case of BRCA1 methylation marks to improve clinical risk assessment of breast cancer. Br. J. Cancer 2020, 122, 1133–1140. [Google Scholar] [CrossRef]
- Palomeras, S.; Diaz-Lagares, A.; Viñas, G.; Setien, F.; Ferreira, H.J.; Oliveras, G.; Crujeiras, A.B.; Hernández, A.; Lum, D.H.; Welm, A.L.; et al. Epigenetic silencing of TGFBI confers resistance to trastuzumab in human breast cancer. Breast Cancer Res. 2019, 21, 79. [Google Scholar] [CrossRef]
- Hamadneh, L.; Abu-Irmaileh, B.; Al-Majawleh, M.; Bustanji, Y.; Jarrar, Y.; Al-Qirim, T. Doxorubicin–paclitaxel sequential treatment: Insights of DNA methylation and gene expression changes of luminal A and triple negative breast cancer cell lines. Mol. Cell. Biochem. 2021, 476, 3647–3654. [Google Scholar] [CrossRef]
- Buocikova, V.; Rios-Mondragon, I.; Pilalis, E.; Chatziioannou, A.; Miklikova, S.; Mego, M.; Pajuste, K.; Rucins, M.; Yamani, N.E.; Longhin, E.M.; et al. Epigenetics in Breast Cancer Therapy—New Strategies and Future Nano-medicine Perspectives. Cancers 2020, 12, 3622. [Google Scholar] [CrossRef]
- Masuda, T.; Noda, M.; Kitagawa, A.; Hu, Q.; Fujii, A.; Ito, S.; Kosai, K.; Ando, Y.; Matsumoto, Y.; Ohtsu, H.; et al. The Expression Level of PD-L1 (CD274) mRNA in Peripheral Blood Is a Potential Biomarker for Predicting Recurrence in Breast Cancer. Anticancer Res. 2020, 40, 3733–3742. [Google Scholar] [CrossRef]
- Liyanage, U.K.; Moore, T.T.; Joo, H.G.; Tanaka, Y.; Herrmann, V.; Doherty, G.; Drebin, J.A.; Strasberg, S.M.; Eberlein, T.J.; Goedegebuure, P.S.; et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002, 169, 2756–2761. [Google Scholar] [CrossRef]
- Wong, L.; Jiang, K.; Chen, Y.; Hennon, T.; Holmes, L.; Wallace, C.A.; Jarvis, J.N. Limits of Peripheral Blood Mononuclear Cells for Gene Expression-Based Biomarkers in Juvenile Idiopathic Arthritis. Sci. Rep. 2016, 6, 29477. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 161–167. [Google Scholar]
- Russo, G.; Zegar, C.; Giordano, A. Advantages and limitations of microarray technology in human cancer. Oncogene 2003, 22, 6497–6507. [Google Scholar] [CrossRef]
- Chuah, S.; Chew, V. High-dimensional immune-profiling in cancer: Implications for immunotherapy. J. Immunother. Cancer 2019, 8, e000363. [Google Scholar] [CrossRef] [PubMed]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat. Rev. Nephrol. 2018, 14, 479–492. [Google Scholar] [CrossRef] [PubMed]
| Study Cohort | Methodology | Novel BC Immune Profiles Based on PBMC Transcriptome | Reference |
|---|---|---|---|
| 33 BC (8 TNBC, 16 luminal A, 6 luminal B, 3 HER2-positive) | RNA-seq | Two new BC subtypes differing in the activation of immune cells, regulation and response of innate and adaptive immune system, and antibody production; distinct immune cell proportions (lymphocytes and neutrophils); distinct immune patterns, with altered pathways including myeloid leukocyte activation, osteoclast differentiation and interleukin-10 signaling. Twenty-eight-gene signature enriched in one subtype: TYROBP, IFNGR1, GAB2, TNFRSF1A, PTGS2, NFKB2, NFKBIA, SIRPB1, NFKBIB, RELB, IL1A, IL1R1, IL1B, TLR4, TLR2, FCGR2A, IFNGR2, FCGR3B, JUNB, FOSL1, JUN, SOCS3, SIRPA, CR1, LILRB3, LILRA2, LILRA6, CSF1 | [97] |
| 13 BC (4 ER+/HER2-, 2 ER+/HER2+, 3 ER-/HER2+, 4 ER-/HER2-) and 3 healthy subjects | RNA-seq | Two new BC subsets differing in B-cell receptor immunological pathways (Bcl-XL, EGR1, p70 S6 kinase 1, Bcl-10, calcineurin A (catalytic), SOS, calmodulin, SHIP, PI3K reg class IA, IKK-alpha, and TAK1 (MAP3K7)) and CRTH2 signaling in Th2 cells (Bcl-XL, calcineurin A (catalytic), calmodulin, PKC, Apaf-1, and G-protein). Additionally, based on the subset of immune activation- and immune checkpoint-related genes, 4 immunological subgroups suggested: (1) monocyte-activating (CD14, CD40, CD80, Siglec14, NRP1, and TIM3) (included 3 of 4 ER-HER2- patients), (2) lymphocyte retention (CD8A, CD4, CD248, IDO1, and IDO2 (included all healthy controls), (3) T-cell inhibitory (PD-L1, PD1, CTLA4, FOXP3, and CCR3), (4) other | [89] |
| 23 BC (14 TNBC and 9 luminal-A) | Pan-Cancer Immune Profiling Panel, 770 genes | Among all BC patients, a distinct group of 3 patients in the TNBC cohort showed changes in transcripts predominantly involved in inflammation; upregulated: IL1R2, THBS1, CD163, FLT3, MFGE8, IFNGR1, IL1RAP, CXCR4, TXNIP, TFRC, CD1D, CCND3, MAP2K1, HMGB1; downregulated: CLEC4C, TLR7, LTB, IL21R, IFIH1, PIK3CD | [76] |
| Study Cohort | Methodology | Gene Signatures in TNBC Patients | Reference |
|---|---|---|---|
| 13 BC (4 ER+/HER2-, 2ER+/HER2+, 3 ER-/HER2+, 4 ER-/HER2-) and 3 healthy subjects | RNA-seq | Distinct transcriptome in 3 ER-HER2- patients (i.e., monocyte activating immune subgroup): upregulated CD14, CD40 (TNFRSF5), CD80, Siglec14, NRP1, TIM3 | [89] |
| 29 BC (14 TNBC and 15 hormone-dependent (ER+/PR+/HER2-)) and 7 healthy subjects | Microarray analysis | Thirty-four-gene TNBC signature (distinguishing TNBC from both ER+/PR+/HER2- and healthy controls); downregulated genes: RNU105A, SCARNA5, CD200, SNORA53, BICC1, KLHL31, SNORA81, FAM86DP, SNORD3B-1, RNU2-2, LMAN1, STXBP4, MGC57346, MAP7D2, CCDC39, SNORD15A, SNORD3B-1, ZNF3, SNORD17, SNORA12, NT5E, SNORA74A, NT5E, SCARNA6; upregulated genes: PLAU, LOC100128175, ITPK1-AS1, ALPK3, C10orf105, ASAP1-IT1 | [5] |
| 23 BC (14 TNBC and 9 luminal-A) | Pan-Cancer Immune Profiling Panel, 770 genes | Distinct transcriptome in 3 out of 14 TNBC patients; upregulated genes: IL1R2, THBS1, CD163, FLT3, MFGE8, IFNGR1, IL1RAP, CXCR4, TXNIP, TFRC, CD1D, CCND3, MAP2K1, HMGB1; downregulated genes: CLEC4C, TLR7, LTB, IL21R, IFIH1, PIK3CD | [76] |
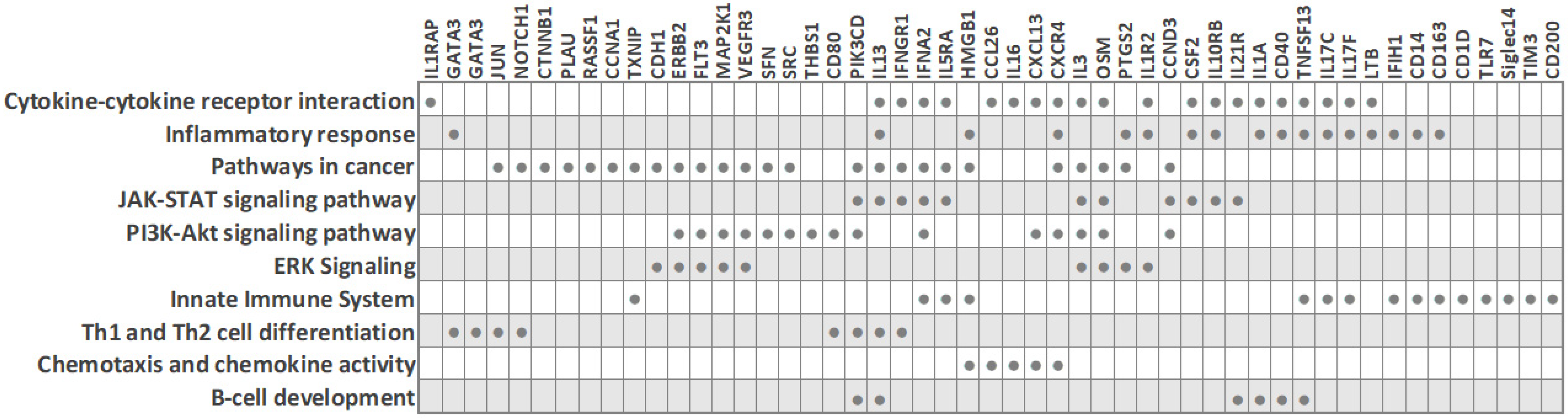
| 40 BC (23 TNBC and 17 luminal (ER+/PR+/HER2-)) | Human Inflammatory Cytokines and Receptors PCR Array, 84 genes | Downregulated in TNBC: interleukins (IL13, IL16, IL17C (IL21), IL17F, IL1A, IL3), interleukin receptor (IL5RA), cytokines (CSF2, OSM, TNSF13), chemokine (CCL26); upregulated in TNBC: interleukin receptor (IL10RB), chemokine (CXCL13), cytokine (IFNA2) | [12] |
| 30 BC (18 TNBC and 12 ER-/PR-/HER2+) | Human Breast Cancer PCR Array, 84 genes | Downregulated in TNBC: ERBB2, RASSF1, CDH1, MKI67, GATA3, GLI1, SFN, PTGS2, JUN, NOTCH1, CTNNB1, KRT8, SRC, and HIC1; upregulated in TNBC: CCNA1 | [11] |
| 70 BC (8 TNBC, 5 luminal A, 20 luminal B, 19 luminal B-like, 13 HER2, 5 unknown) and 50 healthy controls | qRT-PCR | Upregulated in TNBC compared to non-TNBC subtypes: VEGFR3 and PLXNA1 (co-receptors of NRP-1) | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čelešnik, H.; Potočnik, U. Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer. Cancers 2022, 14, 591. https://doi.org/10.3390/cancers14030591
Čelešnik H, Potočnik U. Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer. Cancers. 2022; 14(3):591. https://doi.org/10.3390/cancers14030591
Chicago/Turabian StyleČelešnik, Helena, and Uroš Potočnik. 2022. "Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer" Cancers 14, no. 3: 591. https://doi.org/10.3390/cancers14030591
APA StyleČelešnik, H., & Potočnik, U. (2022). Peripheral Blood Transcriptome in Breast Cancer Patients as a Source of Less Invasive Immune Biomarkers for Personalized Medicine, and Implications for Triple Negative Breast Cancer. Cancers, 14(3), 591. https://doi.org/10.3390/cancers14030591







