Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter?
Abstract
:Simple Summary
Abstract
1. Introduction
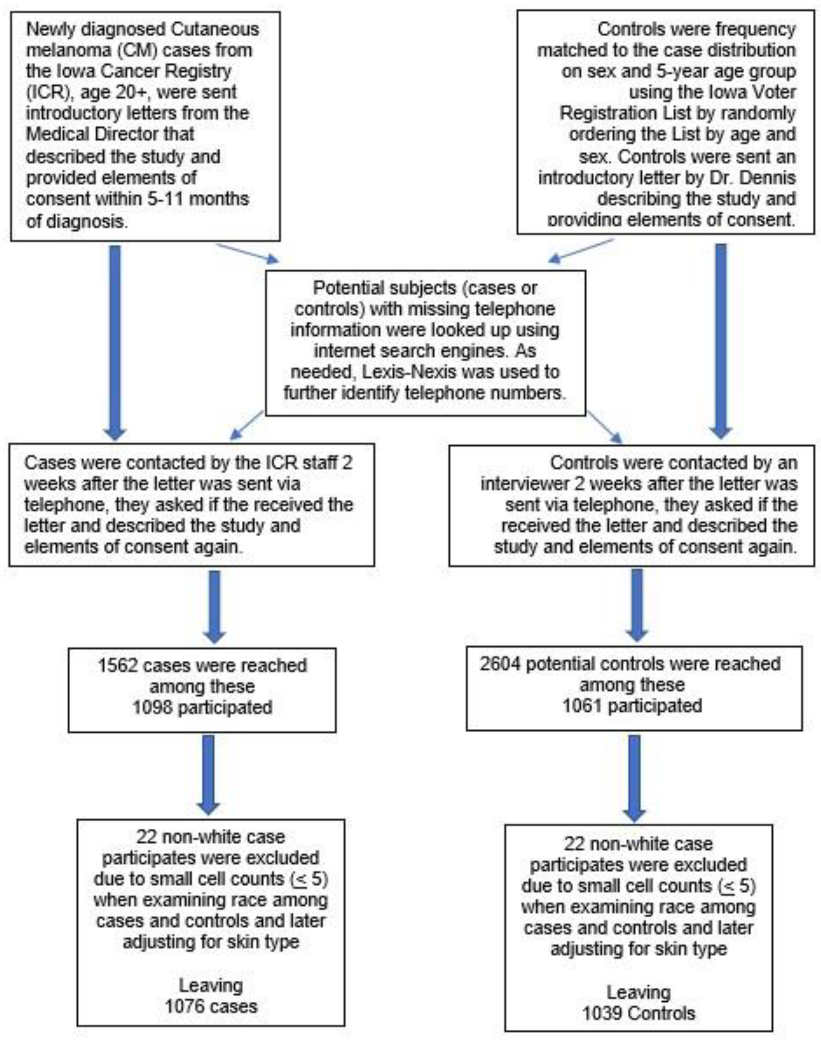
2. Materials and Methods
3. Results
3.1. Population Characteristics and Sun Sensitivity
3.2. Hours of Sun Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer; Reports of the Surgeon General; Department of Health and Human Services, Office of the Surgeon General: Washington, DC, USA, 2014.
- Matthews, N.H.; Li, W.Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Eds.; Codon Publications: Brisbane, AU, USA, 2017. [Google Scholar]
- Lens, M.B.; Dawes, M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br. J. Dermatol. 2004, 150, 179–185. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.; Coebergh, J.W. Cutaneous malignant melanoma in Europe. Eur. J. Cancer 2004, 40, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Lasithiotakis, K.; Leiter, U.; Kruger-Krasagakis, S.; Tosca, A.; Garbe, C. Comparative analysis of incidence and clinical features of cutaneous malignant melanoma in Crete (Greece) and southern Germany (central Baden-Wurttemberg). Br. J. Dermatol. 2006, 154, 1123–1127. [Google Scholar] [CrossRef]
- Howlader, N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/csr/1975_2017/ (accessed on 13 February 2022).
- Armstrong, B.K.; Cust, A.E. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma: A perspective on Fears et al. Mathematical Models of Age and Ultraviolet Effects on the Incidence of Skin Cancer among Whites in the United States. American Journal of Epidemiology 1977; 105: 420–427. Cancer Epidemiol. 2017, 48, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A.; English, D.R. Sun exposure and skin cancer. Australas. J. Dermatol. 1997, 38 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- Dennis, L.K.; Vanbeek, M.J.; Beane Freeman, L.E.; Smith, B.J.; Dawson, D.V.; Coughlin, J.A. Sunburns and risk of cutaneous melanoma: Does age matter? A comprehensive meta-analysis. Ann. Epidemiol. 2008, 18, 614–627. [Google Scholar] [CrossRef] [Green Version]
- Elwood, J.M.; Jopson, J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer 1997, 73, 198–203. [Google Scholar] [CrossRef]
- Espinosa Arranz, J.; Sanchez Hernandez, J.J.; Bravo Fernandez, P.; Gonzalez-Baron, M.; Zamora Aunon, P.; Espinosa Arranz, E.; Jalon Lopez, J.I.; Ordonez Gallego, A. Cutaneous malignant melanoma and sun exposure in Spain. Melanoma Res. 1999, 9, 199–205. [Google Scholar] [CrossRef]
- Hakansson, N.; Floderus, B.; Gustavsson, P.; Feychting, M.; Hallin, N. Occupational sunlight exposure and cancer incidence among Swedish construction workers. Epidemiology 2001, 12, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K. Epidemiology of malignant melanoma: Intermittent or total accumulated exposure to the sun? J. Dermatol. Surg. Oncol. 1988, 14, 835–849. [Google Scholar] [CrossRef]
- Green, A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control 1992, 3, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, D.C.; Parsons, P.G.; Green, A.C. p53 expression and risk factors for cutaneous melanoma: A case-control study. Int. J. Cancer 1998, 77, 843–848. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Watt, P.; Purdie, D.M.; Hughes, M.C.; Hayward, N.K.; Green, A.C. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J. Natl. Cancer Inst. 2003, 95, 806–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodenas, J.M.; Delgado-Rodriguez, M.; Herranz, M.T.; Tercedor, J.; Serrano, S. Sun exposure, pigmentary traits, and risk of cutaneous malignant melanoma: A case-control study in a Mediterranean population. Cancer Causes Control 1996, 7, 275–283. [Google Scholar] [CrossRef]
- Holman, C.D.; Armstrong, B.K.; Heenan, P.J.; Blackwell, J.B.; Cumming, F.J.; English, D.R.; Holland, S.; Kelsall, G.R.; Matz, L.R.; Rouse, I.L.; et al. The causes of malignant melanoma: Results from the West Australian Lions Melanoma Research Project. Recent Results Cancer Res. 1986, 102, 18–37. [Google Scholar] [CrossRef]
- Watts, C.G.; Drummond, M.; Goumas, C.; Schmid, H.; Armstrong, B.K.; Aitken, J.F.; Jenkins, M.A.; Giles, G.G.; Hopper, J.L.; Mann, G.J.; et al. Sunscreen Use and Melanoma Risk Among Young Australian Adults. JAMA Dermatol. 2018, 154, 1001–1009. [Google Scholar] [CrossRef]
- Nikolaou, V.A.; Sypsa, V.; Stefanaki, I.; Gogas, H.; Papadopoulos, O.; Polydorou, D.; Plaka, M.; Tsoutsos, D.; Dimou, A.; Mourtzoukou, E.; et al. Risk associations of melanoma in a Southern European population: Results of a case/control study. Cancer Causes Control 2008, 19, 671–679. [Google Scholar] [CrossRef]
- Puntoni, R.; Ceppi, M.; Casella, C.; Ugolini, D.; Gennaro, V.; Puntoni, M.; Vercelli, M.; Merlo, D.F. Increased incidence of cutaneous malignant melanoma among longshoremen in Genoa, Italy: The role of sunlight and occupational exposure. Occup. Environ. Med. 2005, 62, 270–271. [Google Scholar] [CrossRef] [Green Version]
- Nagore, E.; Hueso, L.; Botella-Estrada, R.; Alfaro-Rubio, A.; Serna, I.; Guallar, J.; Gonzalez, I.; Ribes, I.; Guillen, C. Smoking, sun exposure, number of nevi and previous neoplasias are risk factors for melanoma in older patients (60 years and over). J. Eur. Acad. Dermatol. Venereol. 2010, 24, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kaskel, P.; Lange, U.; Sander, S.; Huber, M.A.; Utikal, J.; Leiter, U.; Krahn, G.; Meurer, M.; Kron, M. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 134–142. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Kirkpatrick, C.S.; Lee, J.A. Case-control study of malignant melanoma in Washington State. I. Constitutional factors and sun exposure. Am. J. Epidemiol. 1994, 139, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Marshall, J.; Haughey, B.; Stoll, H.; Zielezny, M.; Brasure, J.; West, D. An inquiry into the epidemiology of melanoma. Am. J. Epidemiol. 1985, 122, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Rosso, S.; Zanetti, R.; Pippione, M.; Sancho-Garnier, H. Parallel risk assessment of melanoma and basal cell carcinoma: Skin characteristics and sun exposure. Melanoma Res. 1998, 8, 573–583. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Boffetta, P.; Innocenzi, L.; Antonelli, G.; Giovinazzo, R.; Anzidei, P.; Melchi, F.; D’Atri, S.; Pasquini, P.; et al. Polymorphisms of GSTM1 and GSTT1, sun exposure and the risk of melanoma: A case-control study. Acta Derm. Venereol. 2011, 91, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Lazovich, D.; Vogel, R.I.; Berwick, M.; Weinstock, M.A.; Anderson, K.E.; Warshaw, E.M. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Newton-Bishop, J.A.; Chang, Y.M.; Elliott, F.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; Fitzgibbon, E.; Kukalizch, K.; Randerson-Moor, J.; et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur. J. Cancer 2011, 47, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Elwood, J.M.; Gallagher, R.P.; Hill, G.B.; Pearson, J.C. Cutaneous melanoma in relation to intermittent and constant sun exposure--the Western Canada Melanoma Study. Int. J. Cancer 1985, 35, 427–433. [Google Scholar] [CrossRef]
- Dennis, L.K.; Lowe, J.B.; Lynch, C.F.; Alavanja, M.C. Cutaneous melanoma and obesity in the Agricultural Health Study. Ann. Epidemiol. 2008, 18, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Loria, D.; Matos, E. Risk factors for cutaneous melanoma: A case-control study in Argentina. Int. J. Dermatol. 2001, 40, 108–114. [Google Scholar] [CrossRef]
- Bataille, V.; Winnett, A.; Sasieni, P.; Newton Bishop, J.A.; Cuzick, J. Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: A case-control study. Eur. J. Cancer 2004, 40, 429–435. [Google Scholar] [CrossRef]
- Gaudy-Marqueste, C.; Madjlessi, N.; Guillot, B.; Avril, M.F.; Grob, J.J. Risk factors in elderly people for lentigo maligna compared with other melanomas: A double case-control study. Arch. Dermatol. 2009, 145, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Elwood, J.M.; Williamson, C.; Stapleton, P.J. Malignant melanoma in relation to moles, pigmentation, and exposure to fluorescent and other lighting sources. Br. J. Cancer 1986, 53, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Nelemans, P.J.; Groenendal, H.; Kiemeney, L.A.; Rampen, F.H.; Ruiter, D.J.; Verbeek, A.L. Effect of intermittent exposure to sunlight on melanoma risk among indoor workers and sun-sensitive individuals. Environ. Health Perspect. 1993, 101, 252–255. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Lopes, F.; Sleiman, M.G.; Sebastian, K.; Bogucka, R.; Jacobs, E.A.; Adamson, A.S. UV Exposure and the Risk of Cutaneous Melanoma in Skin of Color: A Systematic Review. JAMA Dermatol. 2021, 157, 213–219. [Google Scholar] [CrossRef]
- Dennis, L.K.; Lashway, S.G.; Langston, M.E. Sun Sensitivity and Sunburns as Related to Cutaneous Melanoma among Populations of Spanish Descent: A Meta-Analysis. J. Dermatol. Res. Ther. 2015, 1, 1–5. [Google Scholar]
| Host Factors | Cases 1 | % | Controls 1 | % | OR 2 | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age at interview | |||||||
| 20–39 | 145 | 13.5 | 167 | 16.1 | 1.01 | 0.76 | 1.34 |
| 40–49 | 144 | 13.4 | 162 | 15.6 | 1.03 | 0.77 | 1.37 |
| 50–59 | 223 | 20.7 | 258 | 24.8 | ref | ||
| 60–69 | 253 | 23.5 | 268 | 25.8 | 1.09 | 0.85 | 1.40 |
| 70–79 | 193 | 17.9 | 137 | 13.2 | 1.63 | 1.23 | 2.16 |
| 80+ | 118 | 11.0 | 47 | 4.5 | 2.91 | 1.98 | 4.26 |
| Sex | |||||||
| Female | 523 | 48.6 | 567 | 54.6 | ref | ||
| Male | 553 | 51.4 | 472 | 45.4 | 1.27 | 1.07 | 1.51 |
| Upper inner arm skin color | |||||||
| Fair | 844 | 78.5 | 710 | 68.4 | 1.69 | 1.39 | 2.05 |
| Medium/Dark | 231 | 21.5 | 328 | 31.6 | ref | ||
| Self-reported skin-type | |||||||
| Always burn/never tan | 101 | 9.3 | 60 | 5.9 | 2.44 | 1.71 | 3.47 |
| Usually burn, tan diff | 222 | 20.4 | 167 | 16.0 | 1.92 | 1.49 | 2.48 |
| Some burn, then tan | 473 | 44.3 | 409 | 39.3 | 1.67 | 1.37 | 2.05 |
| Rarely burn/tan easy | 275 | 26.0 | 398 | 38.8 | ref | ||
| Tendency to sunburn | |||||||
| A severe and painful sunburn | 72 | 6.8 | 58 | 5.7 | 1.24 | 0.84 | 1.82 |
| A moderate sunburn | 265 | 24.9 | 231 | 22.6 | 1.14 | 0.89 | 1.46 |
| A mild sunburn | 470 | 44.1 | 478 | 46.7 | 0.98 | 0.79 | 1.21 |
| No sunburn | 258 | 24.2 | 257 | 25.1 | ref | ||
| Ability to tan | |||||||
| Deeply tanned | 161 | 15.0 | 161 | 21.0 | ref | ||
| Moderately tanned | 448 | 41.7 | 448 | 46.5 | 1.26 | 0.99 | 1.60 |
| Mildly tanned | 347 | 32.3 | 347 | 26.4 | 1.72 | 1.33 | 2.23 |
| Have no tan | 118 | 11.0 | 118 | 6.1 | 2.54 | 1.76 | 3.66 |
| Eye color | |||||||
| Blue | 538 | 50.1 | 438 | 42.4 | 1.61 | 1.30 | 1.99 |
| Green | 310 | 28.8 | 298 | 28.9 | 1.36 | 1.08 | 1.72 |
| Brown or Black | 227 | 21.1 | 297 | 28.8 | ref | ||
| Natural hair color at age 20 | |||||||
| Red | 154 | 14.3 | 87 | 8.4 | 2.13 | 1.60 | 2.84 |
| Blond | 336 | 31.3 | 246 | 23.7 | 1.65 | 1.35 | 2.01 |
| Brown or Black | 585 | 54.4 | 705 | 67.9 | ref | ||
| Hair shade | |||||||
| Light | 345 | 32.1 | 254 | 24.5 | 1.98 | 1.57 | 2.50 |
| Medium | 497 | 46.3 | 444 | 42.9 | 1.63 | 1.32 | 2.01 |
| Dark | 232 | 21.6 | 338 | 32.6 | ref | ||
| Sun Exposure | Cases | % | Controls | % | OR 2 | 95% CI | |
|---|---|---|---|---|---|---|---|
| Hours of sun ages 0–21 | |||||||
| 0–15,200 h | 251 | 23.3 | 278 | 26.8 | ref | ||
| 15,201–23,500 h | 268 | 24.9 | 244 | 23.5 | 1.22 | 0.96 | 1.56 |
| 23,500–33,800 h | 284 | 26.4 | 255 | 24.5 | 1.28 | 1.00 | 1.63 |
| 33,801–78,900 h | 273 | 25.4 | 262 | 25.2 | 1.23 | 0.96 | 1.57 |
| Total | 1076 | 1039 | |||||
| Trend OR 3 | 1.22 | p = 0.097 | |||||
| Hours of sun ages 22–39 | |||||||
| 0–7350 h | 255 | 23.9 | 273 | 26.7 | ref | ||
| 7351–13,200 h | 256 | 24.0 | 238 | 23.3 | 1.19 | 0.93 | 1.52 |
| 13,201–22,050 h | 296 | 27.7 | 269 | 26.4 | 1.25 | 0.98 | 1.59 |
| 22,051–82,350 h | 262 | 24.5 | 241 | 23.6 | 1.27 | 0.99 | 1.62 |
| Total | 1069 | 1021 | |||||
| Trend OR 3 | 1.26 | p = 0.056 | |||||
| Hours of sun ages 40–59 | |||||||
| 0–5150 h | 223 | 24.1 | 236 | 27.2 | ref | ||
| 5151–11,750 h | 216 | 23.3 | 216 | 24.9 | 1.11 | 0.85 | 1.45 |
| 11,751–22,050 h | 233 | 25.2 | 224 | 25.8 | 1.22 | 0.94 | 1.59 |
| 22,050–93,100 h | 254 | 27.4 | 191 | 22.0 | 1.59 | 1.22 | 2.09 |
| Total | 926 | 867 | |||||
| Trend OR 3 | 1.56 | p = 0.0006 | |||||
| Sun Exposure | Fair Skin | Medium and Dark Skin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | OR 2 | 95% CI | Cases | Controls | OR 2 | 95% CI | |||
| Hours of sun ages 60+ | ||||||||||
| 0–1950 h | 83 | 86 | ref | 26 | 45 | ref | ||||
| 1951–5900 h | 114 | 70 | 1.73 | 1.12 | 2.67 | 24 | 57 | 0.81 | 0.41 | 1.62 |
| 5901–14,200 h | 103 | 76 | 1.59 | 1.03 | 2.47 | 36 | 31 | 1.94 | 0.97 | 3.89 |
| 14,201–79,400 h | 120 | 59 | 2.72 | 1.72 | 4.30 | 52 | 21 | 4.81 | 2.34 | 9.87 |
| Total | 420 | 291 | 138 | 154 | ||||||
| Trend OR 3 | 2.39 | p < 0.0001 | Trend OR 3 | 5.50 | p < 0.0001 | |||||
| Lifetime Hours | ||||||||||
| 0–31,500 h | 211 | 194 | ref | 28 | 85 | ref | ||||
| 31,501–60,200 h | 288 | 264 | 1.03 | 0.80 | 1.34 | 79 | 117 | 2.09 | 1.24 | 3.52 |
| 60,201–79,000 h | 126 | 94 | 1.31 | 0.94 | 1.83 | 48 | 41 | 3.71 | 2.03 | 6.79 |
| 79,001–249,000 h | 219 | 158 | 1.44 | 1.08 | 1.92 | 76 | 85 | 2.81 | 1.65 | 4.81 |
| Total | 844 | 710 | 231 | 328 | ||||||
| Trend OR 3 | 1.49 | p = 0.005 | Trend OR 3 | 2.52 | p = 0.001 | |||||
| Females | Males | |||||||||
| Lifetime Hours | ||||||||||
| 0–31,500 h | 179 | 197 | ref | 60 | 82 | ref | ||||
| 31,501–60,200 h | 216 | 234 | 1.07 | 0.81 | 1.41 | 151 | 148 | 1.41 | 0.94 | 2.12 |
| 60,201–79,000 h | 69 | 65 | 1.24 | 0.83 | 1.85 | 106 | 70 | 2.23 | 1.41 | 3.51 |
| 79,001–249,000 h | 59 | 71 | 0.99 | 0.66 | 1.50 | 236 | 172 | 2.08 | 1.40 | 3.09 |
| Total | 523 | 567 | 553 | 472 | ||||||
| Trend OR 3 | 1.08 | p = 0.681 | Trend OR 3 | 2.01 | p < 0.0001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, L.K. Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter? Cancers 2022, 14, 1008. https://doi.org/10.3390/cancers14041008
Dennis LK. Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter? Cancers. 2022; 14(4):1008. https://doi.org/10.3390/cancers14041008
Chicago/Turabian StyleDennis, Leslie K. 2022. "Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter?" Cancers 14, no. 4: 1008. https://doi.org/10.3390/cancers14041008
APA StyleDennis, L. K. (2022). Cumulative Sun Exposure and Melanoma in a Population-Based Case–Control Study: Does Sun Sensitivity Matter? Cancers, 14(4), 1008. https://doi.org/10.3390/cancers14041008






