Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Overview of Immune System
3.1. Immune System in Health
3.2. Immune Response
3.2.1. Innate (Natural or Native) Immunity
3.2.2. Adaptive (Specific or Acquired) Immunity
4. Cancer Disease and Tumour Microenvironment
4.1. Cancer Pathophysiology—Hallmarks of Cancer
4.2. Tumour Microenvironment
5. Cancer Immunoediting—From Immunosurveillance to Immune Evasion/Tumour Escape
6. Dual Effect of Immune Cells in Cancer
6.1. Immune Innate Response
6.1.1. Granulocytes (Neutrophils, Eosinophils and Basophils)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.1.2. Mast Cells
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.1.3. Macrophages
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.1.4. Dendritic Cells
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.1.5. Innate Lymphoid Cells (NK Cells and Helper ILC1, ILC2 and ILC3)
ILC Group 1 (NK and ILC1s Cells)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
ILC Group 2 (ILC2s)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
ILC Group 3 (ILC3s and Lymphoid-Tissue Inducer Cells)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.2. Adaptive Immune Response
6.2.1. B Cells—Humoral Immunity
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
6.2.2. T Cells—Cellular Immunity
CD4+ T Cells (T Helper Cells)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
Regulatory T Cells
CD8+ T Cells (Cytotoxic T Lymphocytes, or CTLs)
- ▪
- Anti-tumour immunity
- ▪
- Pro-tumour immunity
7. Concluding Remarks and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukaemia |
| APCs | antigen-presenting cells |
| BCR | B-cell receptor |
| Bregs | regulatory B cells |
| CAF | cancer-associated fibroblasts |
| CRC | colorectal cancer |
| CSCs | cancer stem cells |
| CTLs | cytotoxic T lymphocytes/CD8+ T lymphocytes/CD8+ T cells |
| DAMPs | damage-associated molecular patterns |
| DCs | dendritic cells |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| ICI | immune checkpoint inhibitor |
| IDO | indoleamine 2,3-dioxygenase |
| IFN | Interferon |
| IIC | infiltrating immune cells |
| IL | Interleukin |
| ILCs | innate lymphoid cells |
| IR | immune response |
| IS | immune system |
| LTis | lymphoid-tissue inducer cells |
| MCs | mast cells |
| MDSCs | myeloid-derived suppressor cells |
| MHC-I/-II | major histocompatibility complex-I/-II |
| NK cells | natural killer cells |
| NSCLC | non-small-cell lung cancer |
| PAMPs | pathogen-associated molecular patterns |
| PDAC | pancreatic ductal adenocarcinoma |
| PD-1 | programmed cell death protein 1 |
| PGE2 | prostaglandin E2 |
| ROS/RNS | reactive oxygen species/reactive nitrogen species |
| SCC | squamous cell carcinoma |
| TCR | T-cell receptor |
| TGF | transforming growth factor |
| Th | T helper/CD4+ T lymphocytes/CD4+ T cells |
| TIL B cells | tumour-infiltrating B cells |
| TLRs | toll-like receptors |
| TME | tumour microenvironment |
| TNF | tumour necrosis factor |
| Treg | regulatory T cells |
| VEGF | endothelial growth factor |
References
- Janeway, C.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001; pp. 1–5. [Google Scholar]
- Abbas, A.; Lichtman, A.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 1–37. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Pathogens, Infection, and Innate Immunity. In Molecular Biology of the Cell, 4th ed.; Theriot, J., Baldwin, D., Falkow, S., Levinson, W., Lowe, S., Noble, S., Portnoy, D., Sarnow, P., Eds.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26846/ (accessed on 6 January 2022).
- Ehrlich, P. Ueber den jetzigen Stand der Karzinomforschung. Ned. Tijdschr. Geneeskd. 1909, 5, 273–290. [Google Scholar]
- Burnet, M. Cancer: A biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957, 1, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L. Delayed hypersensitivity in health and disease. In Cellular and Humoral Aspects of the Hypersensitive States; Lawrence, H., Ed.; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–532. [Google Scholar]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumour escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumour Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, M.T.; Möller, A.; Smyth, M.J. Inflammation and immune surveillance in cancer. Semin. Cancer Biol. 2012, 22, 23–32. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Claus, M.; Dychus, N.; Ebel, M.; Damaschke, J.; Maydych, V.; Wolf, O.T.; Kleinsorge, T.; Watzl, C. Measuring the immune system: A comprehensive approach for the analysis of immune functions in humans. Arch. Toxicol. 2016, 90, 2481–2495. [Google Scholar] [CrossRef]
- Ito, T.; Connett, J.M.; Kunkel, S.L.; Matsukawa, A. The linkage of innate and adaptive immune response during granulomatous development. Front. Immunol. 2013, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, K.L. Overview of the immune system. Handb. Clin. Neurol. 2016, 133, 61–76. [Google Scholar] [PubMed]
- Iglesias, M.; Iglesias, A. Las células dendríticas y su papel de centinelas del sistema inmune. Acta Méd. Colomb. 2003, 28, 99–100. [Google Scholar]
- Tosi, M.F. Innate immune responses to infection. J. Allergy Clin. Immunol. 2005, 116, 241–250. [Google Scholar] [CrossRef]
- Metchnikoff, E. Sur la lutta des cellules de l’organismes centre l’invasion des microbes. Ann. Inst. Pasteur 1887, 1, 321. [Google Scholar]
- Danilova, N. The evolution of adaptive immuninty. Adv. Exp. Med. Biol. 2012, 738, 218–235. [Google Scholar]
- Medzhitov, R.; Janeway, C., Jr. Innate immune recognition: Mechanisms and pathways. Immunol. Rev. 2000, 173, 89–97. [Google Scholar] [CrossRef]
- Mackay, I.R. Tolerance and autoimmunity. West. J. Med. 2001, 174, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.F.; Braza, F.; Brouard, S.; Tak, P.P.; Baeten, D. The role of B lymphocytes in the progression from autoimmunity to autoimmune disease. Clin. Immunol. 2013, 146, 34–45. [Google Scholar] [CrossRef]
- Romo, M.R.; Pérez-Martínez, D.; Ferrer, C.C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef]
- Kaur, B.P.; Secord, E. Innate Immunity. Pediatr. Clin. N. Am. 2019, 66, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langerhans, P. Ueber die Nerven der menschlichen Haut. Arch. Pathol. Anat. 1868, 44, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells-programmed by the epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [Green Version]
- West, H.C.; Bennett, C.L. Redefining the role of Langerhans cells as immune regulators within the skin. Front. Immunol. 2018, 8, 1941. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Delves, P.J.; Roitt, I.M. The immune system. First of two parts. N. Engl. J. Med. 2008, 343, 37–49. [Google Scholar] [CrossRef]
- British Society for Immunology. Available online: https://www.immunology.org/public-information/bitesized-immunology/systems-and-processes/complement-system (accessed on 9 February 2022).
- Totsch, S.K.; Sorge, R.E. Immune system involvement in specific pain conditions. Mol. Pain 2017, 13, 1744806917724559. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, M.B.; Sureda, M.; Rebollo, J. Células dendríticas I: Aspectos básicos de su biología y funciones. Immunology 2011, 31, 21–30. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados, D.; Delgado, G. Células Dendríticas (CDs) diferenciadas a partir de monocitos humanos como herramienta para el estudio de agentes antileishmaniales. Nova 2008, 6, 162–169. [Google Scholar]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and Adaptive Immune Memory: An Evolutionary Continuum in the Host’s Response to Pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Ashton-Rickardt, P.G.; Opferman, J.T. Memory T lymphocytes. Cell. Mol. Life Sci. 1999, 56, 69–77. [Google Scholar] [CrossRef]
- Chi, X.; Li, Y.; Qiu, X. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: Mechanism and regulation. Immunology 2020, 160, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Goodnow, C.C. Balancing immunity and tolerance: Deleting and tuning lymphocyte repertoires. Proc. Natl. Acad. Sci. USA 1996, 93, 2264–2271. [Google Scholar] [CrossRef] [Green Version]
- Lebien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Connors, T.; Farber, D.L. Human T cell development, localization, and function throughout life. Immunity 2019, 48, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH. National Cancer Institute. NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/tumour-microenvironment (accessed on 9 February 2022).
- Weinberg, R. The Biology of Cancer, 2nd ed.; Garland Science: New York, NY, USA, 2014; pp. 32–50. [Google Scholar]
- Tian, T.; Olson, S.; Whitacre, J.M.; Harding, A. The origins of cancer robustness and evolvability. Integr. Biol. 2011, 3, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.H. Tumour progression and the nature of cancer. Br. J. Cancer 1991, 64, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Patel, A. Benign vs. Malignant Tumours. JAMA Oncol. 2020, 6, 1488. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased Cell Division as a Cause of Human Cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- WHO (World Health Organization). An overview of the evidence on environmental and occupational determinants of Cancer. In Proceedings of the International Conference on Environmental and Occupational Determinants of Cancer: Interventions for Primary Prevention, Asturias, Spain, 17–18 March 2011. [Google Scholar]
- Mohla, S.; Witz, I.P. The 5th International Conference on Tumour Microenvironment: Progression, Therapy and Prevention, Versailles, France, 20–24 October 2009; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; pp. 1–5. [Google Scholar]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- El-Sayes, N.; Vito, A.; Mossman, K. Tumour heterogeneity: A great barrier in the age of cancer immunotherapy. Cancers 2021, 13, 806. [Google Scholar] [CrossRef]
- Clemente, C.G.; Mihm, M.C.; Bufalino, R.; Zurrida, S.; Collini, P.; Cascinelli, N. Prognostic value of tumour infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996, 77, 1303–1310. [Google Scholar] [CrossRef]
- Krüger, J.M.; Wemmert, C.; Sternberger, L.; Bonnas, C.; Dietmann, G.; Gançarski, P.; Feuerhake, F. Combat or surveillance? Evaluation of the heterogeneous inflammatory breast cancer microenvironment. J. Pathol. 2013, 229, 569–578. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Cancer. In Molecular Biology of the Cell, 6th ed.; Berns, A., Bishop, J., Bivona, T., Bunz, F., Edwards, P., Mellman, I., Sousa, C., Shuman, M., Stratton, M., Tomlinson, I., Eds.; Garland Science: New York, NY, USA, 2008; pp. 1091–1143. [Google Scholar]
- Little, S.E.; Popov, S.; Jury, A.; Bax, D.A.; Doey, L.; Al-Sarraj, S.; Jurgensmeier, J.M.; Jones, C. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumour heterogeneity. Cancer Res. 2012, 72, 1614–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational implications of tumour heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowell, P.C. The clonal evolution of tumour cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Biological Hallmarks of Cancer. In Holland-Frei Cancer Medicine, 9th ed.; Bast, R., Jr., Croce, C., Hait, W., Hong, W., Kufe, D., Piccart-Gebhart, M., Pollock, R., Weichselbaum, R., Wang, H., Holland, J., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; Volume 1, pp. 1–10. [Google Scholar]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumour Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Witz, I.P. The tumour microenvironment: The making of a paradigm. Cancer Microenviron. 2009, 2 (Suppl. S1), 9–17. [Google Scholar] [CrossRef] [Green Version]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 1, 571–573. [Google Scholar] [CrossRef] [Green Version]
- Onuigbo, W.I. Human model for studying seed-soil factors in blood-borne metastasis. Arch. Pathol. 1975, 99, 342–343. [Google Scholar]
- Hart, I.R. “Seed and soil” revisited: Mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982, 1, 5–16. [Google Scholar] [CrossRef]
- Nicolson, G.L. Organ specificity of tumour metastasis: Role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988, 7, 143–188. [Google Scholar] [CrossRef] [PubMed]
- Runa, F.; Hamalian, S.; Meade, K.; Shisgal, P.; Gray, P.C.; Kelber, J.A. Tumour microenvironment heterogeneity: Challenges and opportunities. Curr. Mol. Biol. Rep. 2017, 3, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, D.; Bogenrieder, T.; Elder, D.; Herlyn, M. Melanoma-stroma interactions: Structural and functional aspects. Lancet Oncol. 2002, 3, 35–43. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Houghton, J.M. Tumour microenvironment: The role of the tumour stroma in cancer. J. Cell. Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumour stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A tumour-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Park, C.C.; Bissell, M.J.; Barcellos-Hoff, M.H. The influence of the microenvironment on the malignant phenotype. Mol. Med. Today 2000, 6, 324–329. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Marusyk, A.; Tabassum, D.P.; Altrock, P.M.; Almendro, V.; Michor, F.; Polyak, K. Non-cell autonomous tumour-growth driving supports sub-clonal heterogeneity. Nature 2014, 514, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.J.; Werner, B.; Barnes, C.P.; Graham, T.A.; Sottoriva, A. Identification of neutral tumour evolution across cancer types. Nat. Genet. 2016, 48, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeClerck, Y.A.; Pienta, K.J.; Woodhouse, E.C.; Singer, D.S.; Mohla, S. The tumour microenvironment at a turning point knowledge gained over the last decade, and challenges and opportunities ahead: A white paper from the NCI TME network. Cancer Res. 2017, 77, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Metabolism within the tumour microenvironment and its implication on cancer progression: An ongoing therapeutic target. Med. Res. Rev. 2019, 39, 70–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, F.V.; Douglass, S.M.; Fane, M.E.; Weeraratna, A.T. Bad Company: Microenvironmentally Mediated Resistance to Targeted Therapy in Melanoma. Pigment Cell Melanoma Res. 2019, 32, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef] [Green Version]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumour Microenvironment: A Milieu Hindering and Obstructing Antitumour Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef]
- Martinet, L.; Poupot, R.; Fournié, J.J. Pitfalls on the roadmap to gammadelta T cell-based cancer immunotherapies. Immunol. Lett. 2009, 124, 1–8. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [Green Version]
- Baginska, J.; Viry, E.; Paggetti, J.; Medves, S.; Berchem, G.; Moussay, E.; Janji, B. The critical role of the tumour microenvironment in shaping natural killer cell-mediated anti-tumour immunity. Front. Immunol. 2013, 4, 490. [Google Scholar] [CrossRef] [Green Version]
- Schiavoni, G.; Gabriele, L.; Mattei, F. The tumour microenvironment: A pitch for multiple players. Front. Oncol. 2013, 3, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischmann, A.; Schlomm, T.; Köllermann, J.; Sekulic, N.; Huland, H.; Mirlacher, M.; Sauter, G.; Simon, R.; Erbersdobler, A. Immunological Microenvironment in Prostate Cancer: High Mast Cell Densities are Associated with Favorable Tumour Characteristics and Good Prognosis. Prostate 2009, 69, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikström, P.; et al. Mast Cells Are Novel Independent Prognostic Markers in Prostate Cancer and Represent a Target for Therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Imada, A.; Shijubo, N.; Kojima, H.; Abe, S. Mast cells correlate angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur. Respir. J. 2000, 15, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the tumour microenvironment. Trends Immunol. 2017, 37, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Birbrair, A. Tumour Microenvironment: Hematopoietic Cells—Part B, 1st ed.; Springer: New York, NY, USA, 2020; pp. 1–159. [Google Scholar]
- Pienta, K.J.; McGregor, N.; Axelrod, R.; Axelrod, D.E. Ecological therapy for cancer: Defining tumours using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl. Oncol. 2008, 1, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Dabiri, S.; Huntsman, D.; Makretsov, N.; Cheang, M.; Gilks, B.; Badjik, C.; Gelmon, K.; Chia, S.; Hayes, M. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod. Pathol. 2004, 17, 690–695. [Google Scholar] [CrossRef]
- Sang, J.; Yi, D.; Tang, X.; Zhang, Y.; Huang, T. The associations between mast cell infiltration, clinical features and molecular types of invasive breast cancer. Oncotarget 2016, 7, 81661–81669. [Google Scholar] [CrossRef] [Green Version]
- Glajcar, A.; Szpor, J.; Pacek, A.; Tyrak, K.E.; Chan, F.; Streb, J.; Hodorowicz-Zaniewska, D.; Oko, K. The relationship between breast cancer molecular subtypes and mast cell populations in tumour microenvironment. Virchows Arch. 2017, 470, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumour Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.M.; Grizzi, F. Cancer immunoediting and beyond in 2021. Int. J. Mol. Sci. 2021, 22, 13275. [Google Scholar] [CrossRef] [PubMed]
- Regoes, R.R.; Yates, A.; Antia, R. Mathematical models of cytotoxic T-lymphocyte killing. Immunol. Cell Biol. 2007, 85, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganusov, V.V.; De Boer, R.J. Estimating In Vivo Death Rates of Targets due to CD8 T-Cell-Mediated Killing. J. Virol. 2008, 82, 11749–11757. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Kunimasa, K.; Goto, T. Immunosurveillance and Immunoediting of Lung Cancer: Current Perspectives and Challenges. Int. J. Mol. Sci. 2020, 21, 597. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, A.; Fujii, N.; Murakami, T.; Kiura, K.; Aoe, K.; Yamane, H.; Masuda, K.; Maeda, T.; Sugi, K.; Darzynkiewicz, Z.; et al. High frequency of allele-specific rown-regulation of HLA class I expression in lung cancer cell lines. Anticancer Res. 2004, 24, 1525–1528. [Google Scholar]
- Campoli, M.; Ferrone, S. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene 2008, 27, 5869–5885. [Google Scholar] [CrossRef] [Green Version]
- Hicklin, D.J.; Marincola, F.M.; Ferrone, S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol. Med. Today 1999, 5, 178–186. [Google Scholar] [CrossRef]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumour microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumour immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Araya, R.E.; Goldszmid, R.S. IFNAR1 Degradation: A New Mechanism for Tumour Immune Evasion? Cancer Cell 2017, 31, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Han, X.; Shang, C.; Wang, Y.; Xu, B.; Jiang, S.; Mo, Y.; Wang, D.; Ke, Y.; Zeng, X. The downregulation of type I IFN signaling in G-MDSCs under tumour conditions promotes their development towards an immunosuppressive phenotype. Cell Death Dis. 2022, 13, 36. [Google Scholar] [CrossRef]
- Kinker, G.S.; Vitiello, G.A.F.; Ferreira, W.A.S.; Chaves, A.S.; de Lima, V.C.C.; Medina, T. B Cell Orchestration of Anti-tumour Immune Responses: A Matter of Cell Localization and Communication. Front. Cell Dev. Biol. 2021, 9, 678127. [Google Scholar] [CrossRef]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Martin, T.D.; Patel, R.S.; Cook, D.R.; Choi, M.Y.; Patil, A.; Liang, A.C.; Li, M.Z.; Haigis, K.M.; Elledge, S.J. The adaptive immune system is a major driver of selection for tumour suppressor gene inactivation. Science 2021, 373, 1327–1335. [Google Scholar] [CrossRef]
- Anichini, A.; Perotti, V.E.; Sgambelluri, F.; Mortarini, R. Immune escape mechanisms in non small cell lung cancer. Cancers 2020, 12, 3605. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katlinski, K.V.; Gui, J.; Katlinskaya, Y.V.; Ortiz, A.; Chakraborty, R.; Bhattacharya, S.; Carbone, C.J.; Beiting, D.P.; Girondo, M.A.; Peck, A.R.; et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumour Microenvironment. Cancer Cell 2017, 31, 194–207. [Google Scholar] [CrossRef] [Green Version]
- León-Letelier, R.A.; Bonifaz, L.C.; Fuentes-Pananá, E.M. OMIC signatures to understand cancer immunosurveillance and immunoediting: Melanoma and immune cells interplay in immunotherapy. J. Leukoc. Biol. 2019, 105, 915–933. [Google Scholar] [CrossRef]
- Del Alcazar, C.R.G.; Alečković, M.; Polyak, K. Immune Escape during Breast Tumour Progression. Cancer Immunol. Res. 2020, 8, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Ager, C.R.; Obradovic, A.Z.; Arriaga, J.M.; Chaimowitz, M.G.; Califano, A.; Abate-Shen, C.; Drake, C.G. Longitudinal Immune Profiling Reveals Unique Myeloid and T-cell Phenotypes Associated with Spontaneous Immunoediting in a Prostate Tumour Model. Cancer Immunol. Res. 2021, 9, 529–541. [Google Scholar] [CrossRef]
- Nakamura, K.; Smyth, M.J.; Martinet, L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood 2020, 136, 2731–2740. [Google Scholar] [CrossRef]
- Hirata, E.; Sahai, E. Tumour microenvironment and differential responses to therapy. Cold Spring Harb. Perspect. Med. 2017, 7, a026781. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; Von Der Maase, H. Presence of intratumoural neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 2009, 27, 4709–4717. [Google Scholar] [CrossRef]
- Rao, H.L.; Chen, J.W.; Li, M.; Xiao, Y.B.; Fu, J.; Zeng, Y.X.; Cai, M.Y.; Xie, D. Increased intratumoural neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar]
- Reid, M.D.; Basturk, O.; Thirabanjasak, D.; Hruban, R.H.; Klimstra, D.S.; Bagci, P.; Altinel, D.; Adsay, V. Tumour-infiltrating neutrophils in pancreatic neoplasia. Mod. Pathol. 2011, 24, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil plasticity in the tumour microenvironment. Blood 2019, 133, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Piccard, H.; Muschel, R.J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumour development and progression. Crit. Rev. Oncol. Hematol. 2012, 82, 296–309. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumour-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-beta regulate tumour angiogenesis and growth in a mouse tumour model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumour-associated neutrophils in cancer: Going pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef] [Green Version]
- Stoppacciaro, A.; Melani, C.; Parenza, M.; Mastracchio, A.; Bassi, C.; Baroni, C.; Parmiani, G.; Colombo, M.P. Regression of an established tumour genetically modified to release granulocyte colony-stimulatlng factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J. Exp. Med. 1993, 178, 151–161. [Google Scholar] [CrossRef]
- Seung, L.P.; Rowley, D.A.; Dubey, P.; Schreiber, H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumour rejection. Proc. Natl. Acad. Sci. USA 1995, 92, 6254–6258. [Google Scholar] [CrossRef] [Green Version]
- Pekarek, L.A.; Starr, B.A.; Toledano, A.Y.; Schreiber, H. Inhibition o f Tumour Growth by Elimination o f Granulocytes. J. Exp. Med. 1995, 181, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, A.; Crequer, A.; Columbus, D.; Daikoku, T.; Mittal, K.; Dey, S.K.; Erlebacher, A. Neutrophils Oppose Uterine Epithelial Carcinogenesis via Debridement of Hypoxic Tumour Cells. Cancer Cell 2015, 28, 785–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumour Associated Neutrophils. Their Role in Tumourigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Santoriello, C.; Mione, M.; Hurlstone, A.; Martin, P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: Parallels between tumour initiation and wound inflammation. PLoS Biol. 2010, 8, e1000562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, P.M. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin. Hematol. 1997, 34, 311–318. [Google Scholar]
- Zhou, S.L.; Dai, Z.; Zhou, Z.J.; Wang, X.Y.; Yang, G.H.; Wang, Z.; Huang, X.W.; Fan, J.; Zhou, J. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 2012, 56, 2242–2254. [Google Scholar] [CrossRef]
- Moore, R.J.; Owens, D.M.; Stamp, G.; Arnott, C.; Burke, F.; East, N.; Holdsworth, H.; Turner, L.; Rollins, B.; Pasparakis, M.; et al. Mice deficient in tumour necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999, 5, 828–831. [Google Scholar] [CrossRef]
- Benevides, L.; da Fonseca, D.M.; Donate, P.B.; Tiezzi, D.G.; De Carvalho, D.D.; de Andrade, J.M.; Martins, G.A.; Silva, J.S. IL17 Promotes Mammary Tumour Progression by Changing the Behavior of Tumour Cells and Eliciting Tumourigenic Neutrophils Recruitment. Cancer Res. 2015, 75, 3788–3799. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; De Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumour-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human Eosinophils Exert TNF-α and Granzyme A-Mediated Tumouricidal Activity toward Colon Carcinoma Cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefèvre, G.; Capron, M. IL-18 Is Involved in Eosinophil-Mediated Tumouricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005, 7, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Allen, F.; Bobanga, I.D.; Rauhe, P.; Barkauskas, D.; Teich, N.; Tong, C.; Myers, J.; Huang, A.Y. CCL3 augments tumour rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFN-γ. Oncoimmunology 2017, 7, e1393598. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.Y.; Lin, Y.C.; Mahalingam, J.; Huang, C.T.; Chen, T.W.; Kang, C.W.; Peng, H.M.; Chu, Y.Y.; Chiang, J.M.; Dutta, A.; et al. Tumour-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8+ T cells in colon cancer by T-regulatory cells. Cancer Res. 2012, 72, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Della Rovere, F.; Granata, A.; Familiari, D.; D’Arrigo, G.; Mondello, B.; Basile, G. Mast cells in invasive ductal breast cancer: Different behavior in high and minimum hormone-receptive cancers. Anticancer Res. 2007, 27, 2465–2471. [Google Scholar]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased intratumoural mast cells foster immune suppression and gastric cancer progression through TNF-alfa-PD-L1 pathway. J. Immunother. Cancer 2019, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Chintala, N.K.; Vadrevu, S.K.; Patel, J.; Karbowniczek, M.; Markiewski, M.M. Pulmonary Alveolar Macrophages Contribute to the Premetastatic Niche by Suppressing Antitumour T Cell Responses in the Lungs. J. Immunol. 2015, 194, 5529–5538. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.J.; Hogan, A.E.; Marry, J.; Tosetto, M.; Cox, F.; Hyland, J.M.; Sheahan, K.D.; O’Donoghue, D.P.; Mulcahy, H.E.; Ryan, E.J.; et al. Tumour tissue microenvironment can inhibit dendritic cell maturation in colorectal cancer. PLoS ONE 2011, 6, e27944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laoui, D.; Keirsse, J.; Morias, Y.; Van Overmeire, E.; Geeraerts, X.; Elkrim, Y.; Kiss, M.; Bolli, E.; Lahmar, Q.; Sichien, D.; et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat. Commun. 2016, 7, 13720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluth, M.J.; Zaba, L.C.; Moussai, D.; Suárez-Fariñas, M.; Kaporis, H.; Fan, L.; Pierson, K.C.; White, T.R.; Pitts-Kiefer, A.; Fuentes-Duculan, J.; et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Investig. Dermatol. 2009, 129, 2451–2462. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Chang, J.S.M.; Syu, S.H.; Wong, T.S.; Chan, J.Y.W.; Tang, Y.C.; Yang, Z.P.; Yang, W.C.; Chen, C.T.; Lu, S.C.; et al. IL-1β promotes malignant transformation and tumour aggressiveness in oral cancer. J. Cell. Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef]
- Kärre, K.; Klein, G.O.; Kiessling, R.; Klein, G. Genetic control of in vitro NK-activity and in vivo resistance to tumours. Tokai J. Exp. Clin. Med. 1983, 8, 429–448. [Google Scholar]
- Li, T.; Zhang, Q.; Jiang, Y.; Yu, J.; Hu, Y.; Mou, T.; Chen, G.; Li, G. Gastric cancer cells inhibit natural killer cell proliferation and induce apoptosis via prostaglandin E2. Oncoimmunology 2015, 5, e1069936. [Google Scholar] [CrossRef] [Green Version]
- Szudy-Szczyrek, A.; Ahern, S.; Kozioł, M.; Majowicz, D.; Szczyrek, M.; Krawczyk, J.; Hus, M. Therapeutic Potential of Innate Lymphoid Cells for Multiple Myeloma Therapy. Cancers 2021, 13, 4806. [Google Scholar] [CrossRef]
- Yuan, X.; Rasul, F.; Sun, C. Innate lymphoid cells and cancer: Role in tumour. Eur. J. Immunol. 2021, 51, 2188–2205. [Google Scholar] [CrossRef]
- Saranchova, I.; Han, J.; Huang, H.; Fenninger, F.; Choi, K.B.; Munro, L.; Pfeifer, C.; Welch, I.; Wyatt, A.W.; Fazli, L.; et al. Discovery of a Metastatic Immune Escape Mechanism Initiated by the Loss of Expression of the Tumour Biomarker Interleukin-33. Sci. Rep. 2016, 6, 30555. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.S.; et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumour immunotherapy. Mol. Aspects Med. 2021, 80, 100870. [Google Scholar] [CrossRef] [PubMed]
- Reinbach, G. Ueber das Verhalten der Leukocyten bei malignen Tumouren. Arch. Klin. Chir. 1893, 46, 486–562. [Google Scholar]
- Caruso, R.A.; Ieni, A.; Fedele, F.; Zuccalà, V.; Riccardo, M.; Parisi, E.; Parisi, A. Degranulation patterns of eosinophils in advanced gastric carcinoma: An electron microscopic study. Ultrastruct. Pathol. 2005, 29, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2017, 7, e1393134. [Google Scholar] [CrossRef] [Green Version]
- Van Driel, W.J.; Hogendoorn, P.C.W.; Jansen, F.W.; Zwinderman, A.H.; Trimbos, J.B.; Fleuren, G.J. Tumour-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum. Pathol. 1996, 27, 904–911. [Google Scholar] [CrossRef]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef] [Green Version]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumour growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef]
- Sakkal, S.; Miller, S.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016, 23, 650–666. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Hammad, H.; Lambrecht, B.N. Professional and “Amateur” Antigen Presenting Cells In Type 2 Immunity. Trends Immunol. 2019, 40, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Anthony, H.M. Blood basophils in lung cancer. Br. J. Cancer 1982, 45, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, L.M.; Oyesola, O.O.; Früh, S.P.; Kamynina, E.; Still, K.M.; Patel, R.K.; Peng, S.A.; Cubitt, R.L.; Grimson, A.; Grenier, J.K.; et al. The Notch signaling pathway promotes basophil responses during helminth-induced type 2 inflammation. J. Exp. Med. 2019, 216, 1268–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, M.M.; Leis, H.P., Jr. Cellular responses to autologous breast cancer tissue. Sequential observations. Cancer 1973, 32, 384–389. [Google Scholar] [CrossRef]
- De Monte, L.; Wörmann, S.; Brunetto, E.; Heltai, S.; Magliacane, G.; Reni, M.; Paganoni, A.M.; Recalde, H.; Mondino, A.; Falconi, M.; et al. Basophil recruitment into tumour-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res. 2016, 76, 1792–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Cao, Y.; Gu, Y.; Fang, H.; Wang, J.; Liu, X.; Lv, K.; Yu, K.; Fei, Y.; Lin, C.; et al. Clinical Outcomes and Immune Metrics in Intratumoural Basophil-Enriched Gastric Cancer Patients. Ann. Surg. Oncol. 2021, 28, 6439–6450. [Google Scholar] [CrossRef]
- Costamagna, D.; Duelen, R.; Penna, F.; Neumann, D.; Costelli, P.; Sampaolesi, M. Interleukin-4 administration improves muscle function, adult myogenesis, and lifespan of colon carcinoma-bearing mice. J. Cachexia Sarcopenia Muscle 2020, 11, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.L.; Whitters, M.J.; Jacobson, B.A.; Donaldson, D.D.; Collins, M.; Dunussi-Joannopoulos, K. Tumour cells secreting IL-13 but not IL-13Rα2 fusion protein have reduced tumourigenicity in vivo. Int. Immunol. 2004, 16, 1009–1017. [Google Scholar] [CrossRef]
- Williford, J.M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.M.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103+ dendritic cells via tumour-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 2019, 5, eaay1357. [Google Scholar] [CrossRef] [Green Version]
- Dvorak, H.F. Tumours: Wounds that do not heal. Similarities between tumour stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Antonio, N.; Bønnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.A.; Kulbe, H.; Soper, R.; Escorcio-Correia, M.; Lawrence, T.; Schultheis, A.; Chakravarty, P.; Thompson, R.G.; Kollias, G.; Smyth, J.F.; et al. The tumour-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Investig. 2009, 119, 3011–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitru, C.A.; Lang, S.; Brandau, S. Modulation of neutrophil granulocytes in the tumour microenvironment: Mechanisms and consequences for tumour progression. Semin. Cancer Biol. 2013, 23, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Di Mitri, D.; Toso, A.; Chen, J.J.; Sarti, M.; Pinton, S.; Jost, T.R.; D’Antuono, R.; Montani, E.; Garcia-Escudero, R.; Guccini, I.; et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 2014, 515, 134–137. [Google Scholar] [CrossRef]
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kühn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front. Immunol. 2013, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, E.; Wang, F.; Cui, H. CCDC25: Precise navigator for neutrophil extracellular traps on the prometastatic road. Signal Transduct. Target. Ther. 2020, 5, 162. [Google Scholar] [CrossRef]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumour growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Aceto, N.; Toner, M.; Maheswaran, S.; Haber, D.A. En Route to Metastasis: Circulating Tumour Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends Cancer 2015, 1, 44–52. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumour cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.G.; Chen, J.; Liu, J.S.; Zhang, J.Y.; Wang, T.T.; Teng, Y.S.; Mao, F.Y.; Cheng, P.; Zou, Q.M.; Zhou, W.Y.; et al. Activated neutrophils polarize protumourigenic interleukin-17A-producing T helper subsets through TNF-α-B7-H2-dependent pathway in human gastric cancer. Clin. Transl. Med. 2021, 11, e484. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumour Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Liu, L.-B.; Shang, W.-Q.; Chang, K.-K.; Meng, Y.-H.; Mei, J.; Yu, J.-J.; Li, D.-J.; Li, M.-Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, C.-Y.; Chang, K.-K.; Yu, J.-J.; Zhou, W.-J.; Yang, H.-L.; Shao, J.; Yu, J.-J.; Li, M.-Q.; Xie, F. TSLP promotes angiogenesis of human umbilical vein endothelial cells by strengthening the crosstalk between cervical cancer cells and eosinophils. Oncol. Lett. 2017, 14, 7483–7488. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, S.; Mei, R.; Kang, Y.; Duan, J.; Wei, R.; Xiang, C.; Wu, Y.; Lu, X.; Cai, Z.; et al. miR-29a suppresses IL-13-induced cell invasion by inhibiting YY1 in the AKT pathway in lung adenocarcinoma A549 cells. Oncol. Rep. 2018, 39, 2613–2623. [Google Scholar] [CrossRef] [Green Version]
- Lien, M.-Y.; Tsai, H.-C.; Chang, A.-C.; Tsai, M.-H.; Hua, C.-H.; Wang, S.-W.; Tang, C.-H. Chemokine CCL4 induces vascular endothelial growth factor C expression and lymphangiogenesis by miR-195-3p in oral squamous cell carcinoma. Front. Immunol. 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qin, J.; Zhong, L.; Gong, L.; Zhang, B.; Zhang, Y.; Gao, W.Q. CCL5-Mediated Th2 Immune Polarization Promotes Metastasis in Luminal Breast Cancer. Cancer Res. 2015, 75, 4312–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Jia, S.; Shao, C.; Shi, Y. Irradiation induces cancer lung metastasis through activation of the cGAS–STING–CCL5 pathway in mesenchymal stromal cells. Cell Death Dis. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Walens, A.; Dimarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumours. Elife 2019, 8, e43653. [Google Scholar] [CrossRef] [PubMed]
- Chimal-Ramírez, G.K.; Espinoza-Sánchez, N.A.; Fuentes-Pananá, E.M. Protumour activities of the immune response: Insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J. Oncol. 2013, 2013, 835956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are mast cells MASTers in cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zou, Y.; He, X.; Yuan, R.; Chen, Y.; Lan, N.; Lian, L.; Wang, F.; Fan, X.; Zeng, Y.; et al. Tumour-infiltrating mast cells in colorectal cancer as a poor prognostic factor. Int. J. Surg. Pathol. 2013, 21, 111–120. [Google Scholar] [CrossRef]
- Mao, Y.; Feng, Q.; Zheng, P.; Yang, L.; Zhu, D.; Chang, W.; Ji, M.; He, G.; Xu, J. Low tumour infiltrating mast cell density confers prognostic benefit and reflects immunoactivation in colorectal cancer. Int. J. Cancer 2018, 143, 2271–2280. [Google Scholar] [CrossRef] [Green Version]
- Pittoni, P.; Tripodo, C.; Piconese, S.; Mauri, G.; Parenza, M.; Rigoni, A.; Sangaletti, S.; Colombo, M.P. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011, 71, 5987–5997. [Google Scholar] [CrossRef] [Green Version]
- Komi, D.E.A.; Rambasek, T.; Wöhrl, S. Mastocytosis: From a Molecular Point of View. Clin. Rev. Allergy Immunol. 2018, 54, 397–411. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.; Derks, Y.; Kumari, S.; Garssen, J.; Redegeld, F. Human mast cells promote colon cancer growth via bidirectional crosstalk: Studies in 2D and 3D coculture models. Oncoimmunology 2018, 7, e1504729. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta—Mol. Basis Dis. 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, T.; Aritake, K.; Matsumoto, S.; Kamauchi, S.; Nakagawa, T.; Hori, M.; Momotani, E.; Urade, Y.; Ozaki, H. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 19802–19807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikotra, A.; Ohri, C.M.; Green, R.H.; Waller, D.A.; Bradding, P. Mast cell phenotype, TNFα expression and degranulation status in non-small cell lung cancer. Sci. Rep. 2016, 6, 38352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.-P.; Peng, L.-S.; Wang, Q.-H.; Chen, N.; Teng, Y.-S.; Wang, T.-T.; Mao, F.-Y.; Zhang, J.-Y.; Cheng, P.; Liu, Y.-G.; et al. Degranulation of mast cells induced by gastric cancer-derived adrenomedullin prompts gastric cancer progression. Cell Death Dis. 2018, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef] [Green Version]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β signaling activation by mast cells contribute to resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Fakhrjou, A.; Naghavi-Behzad, M.; Montazeri, V.; Karkon-Shayan, F.; Norouzi-Panahi, L.; Piri, R. The relationship between histologic grades of invasive carcinoma of breast ducts and mast cell infiltration. South Asian J. Cancer 2016, 5, 5–7. [Google Scholar]
- Mu, L.; Ding, K.; Tu, R.; Yang, W. Identification of 4 immune cells and a 5-lncRNA risk signature with prognosis for early-stage lung adenocarcinoma. J. Transl. Med. 2021, 19, 127. [Google Scholar] [CrossRef]
- Nonomura, N.; Takayama, H.; Nishimura, K.; Oka, D.; Nakai, Y.; Shiba, M.; Tsujimura, A.; Nakayama, M.; Aozasa, K.; Okuyama, A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br. J. Cancer 2007, 97, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumour-Associated Macrophages in Tumour Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Gangi, L.; Paul, S.; Schioppa, T.; Saccani, A.; Sironi, M.; Bottazzi, B.; Doni, A.; Vincenzo, B.; Pasqualini, F.; et al. A distinct and unique transcriptional program expressed by tumour-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood 2006, 107, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnardel, J.; Guilliams, M. Developmental control of macrophage function. Curr. Opin. Immunol. 2018, 50, 64–74. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumour myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.E.; Terres, G.; Macatonia, S.E.; Hsieh, C.S.; Mattson, J.; Lanier, L.; Wysocka, M.; Trinchieri, G.; Murphy, K.; O’garra, A. B7 and interleukin 12 cooperate for proliferation and interferon γ production by mouse T helper clones that are unresponsive to B7 costimulation. J. Exp. Med. 1994, 180, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Watkins, S.K.; Egilmez, N.K.; Suttles, J.; Stout, R.D. IL-12 Rapidly Alters the Functional Profile of Tumour-Associated and Tumour-Infiltrating Macrophages In Vitro and In Vivo. J. Immunol. 2007, 178, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Rengaraju, M.; Valiante, N.M.; Chehimij, J.; Kubin, M.; Aste, M.; Chan, S.H.; Kobayashi, M.; Young, D.; Nickbarg, E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992, 176, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.; Singh, S.; Khar, A. Antitumour immune responses. Expert Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, V.; Gilmore, A.C.; Basudhar, D.; Palmieri, E.M.; Scheiblin, D.A.; Heinz, W.F.; Cheng, R.Y.S.; Ridnour, L.A.; Altan-Bonnet, G.; Lockett, S.J.; et al. Inducible nitric oxide synthase-derived extracellular nitric oxide flux regulates proinflammatory responses at the single cell level. Redox Biol. 2020, 28, 101354. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Daff, S. NO synthase: Structures and mechanisms. Nitric Oxide 2010, 23, 1–11. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Xu, J.; Yang, M.; Chen, P.; Xu, W.; Zhao, J.; Geng, L.; Gong, S. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumour-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol. Cancer 2018, 17, 13. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumour-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, J.L. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol. Med. 2018, 24, 472–489. [Google Scholar] [CrossRef]
- Bottazzi, B.; Polentarutti, N.; Acero, R.; Balsari, A.; Boraschi, D.; Ghezzi, P.; Salmona, M.; Mantovani, A. Regulation of the macrophage content of neoplasms by chemoattractants. Science 1983, 220, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Sun, L.; Xu, F.; Liu, L.; Hu, F.; Song, D.; Hou, Z.; Wu, W.; Luo, X.; Wang, J.; et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019, 79, 146–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Aydin, E.; Faehling, S.; Saleh, M.; Cid, L.L.; Seiffert, M.; Roessner, P.M. Phosphoinositide 3-Kinase Signaling in the Tumour Microenvironment: What Do We Need to Consider When Treating Chronic Lymphocytic Leukemia with PI3K Inhibitors? Front. Immunol. 2021, 11, 595818. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, B.; Wang, L.; Yang, H.; Zhang, H.; Chen, Y.; Wang, F.; Liu, C.; He, H. Macrophage-mediated vascular permeability via VLA4/VCAM1 pathway dictates ascites development in ovarian cancer. J. Clin. Investig. 2021, 131, e140315. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumour-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef]
- Dan, H.; Liu, S.; Liu, J.; Liu, D.; Yin, F.; Wei, Z.; Wang, J.; Zhou, Y.; Jiang, L.; Ji, N.; et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol. Oncol. 2020, 14, 795–807. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoural and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer 2019, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Binnemars-Postma, K.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumour-associated macrophages reduces tumour growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef] [Green Version]
- Tariq, M.; Zhang, J.Q.; Liang, G.K.; He, Q.J.; Ding, L.; Yang, B. Gefitinib inhibits M2-like polarization of tumour-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1501–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, N.B.; Lü, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumour microenvironments and the progression of tumours. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, N.; Nishio, A.; Sato, T.; Sugiyama, A.; Miyagawa, S.I. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am. J. Gastroenterol. 2004, 99, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Nishie, A.; Ono, M.; Shono, T.; Fukushi, J.; Otsubo, M.; Onoue, H.; Ito, Y.; Inamura, T.; Ikezaki, K.; Fukui, M.; et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin. Cancer Res. 1999, 5, 1107–1113. [Google Scholar]
- Hanada, T.; Nakagawa, M.; Emoto, A.; Nomura, T.; Nasu, N.; Nomura, Y. Prognostic value of tumour-associated macrophage count in human bladder cancer. Int. J. Urol. 2000, 7, 263–269. [Google Scholar] [CrossRef]
- Leek, R.D.; Lewis, C.E.; Whitehouse, R.; Greenall, M.; Clarke, J.; Harris, A.L. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996, 56, 4625–4629. [Google Scholar]
- Lissbrant, I.F.; Stattin, P.; Wikstrom, P.; Damber, J.E.; Egevad, L.; Bergh, A. Tumour associated macrophages in human prostate cancer: Relation to clinicopathological variables and survival. Int. J. Oncol. 2000, 17, 445–451. [Google Scholar]
- Nosaka, T.; Baba, T.; Tanabe, Y.; Sasaki, S.; Nishimura, T.; Imamura, Y.; Yurino, H.; Hashimoto, S.; Arita, M.; Nakamoto, Y.; et al. Alveolar Macrophages Drive Hepatocellular Carcinoma Lung Metastasis by Generating Leukotriene B4. J. Immunol. 2018, 200, 1839–1852. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, S.; Yasuda, K.; Suzuki, K.; Tahara, K.; Higashi, H.; Era, S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol. Rep. 2005, 14, 425–431. [Google Scholar] [CrossRef]
- Wang, F.Q.; So, J.; Reierstad, S.; Fishman, D.A. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int. J. Cancer 2005, 114, 19–31. [Google Scholar] [CrossRef]
- Hamada, I.; Kato, M.; Yamasaki, T.; Iwabuchi, K.; Watanabe, T.; Yamada, T.; Itoyama, S.; Ito, H.; Okada, K. Clinical effects of tumour-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res. 2002, 22, 4281–4284. [Google Scholar] [PubMed]
- Brüne, B.; Courtial, N.; Dehne, N.; Syed, S.N.; Weigert, A. Macrophage NOS2 in Tumour Leukocytes. Antioxid. Redox Signal. 2017, 26, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilliams, M.; Dutertre, C.A.; Scott, C.L.; McGovern, N.; Sichien, D.; Chakarov, S.; Van Gassen, S.; Chen, J.; Poidinger, M.; De Prijck, S.; et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity 2016, 45, 669–684. [Google Scholar] [CrossRef] [Green Version]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumour Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.; Andersen, M.H. The role of dendritic cells in cancer. Semin. Immunopathol. 2017, 39, 307–316. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Banchereau, J.; Bell, D.; Burkeholder, S.; Kraus, E.T.; Davoust, J.; Palucka, K.A. Dendritic Cells Capture Killed Tumour Cells and Present Their Antigens to Elicit Tumour-Specific Immune Responses. J. Immunol. 2000, 165, 3797–3803. [Google Scholar] [CrossRef]
- Arina, A.; Tirapu, I.; Alfaro, C.; Rodríguez-Calvillo, M.; Mazzolini, G.; Inogés, S.; López, A.; Feijoo, E.; Bendandi, M.; Melero, I. Clinical implications of antigen transfer mechanisms from malignant to dendritic cells: Exploiting cross-priming. Exp. Hematol. 2002, 30, 1355–1364. [Google Scholar] [CrossRef]
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019, 348, 69–121. [Google Scholar]
- Papatriantafyllou, M. Trafficking: Tracking immune cells on the lymph node map. Nat. Rev. Immunol. 2011, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurice, N.J.; McElrath, M.J.; Andersen-Nissen, E.; Frahm, N.; Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat. Commun. 2019, 10, 4987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, A.K.; Lake, R.A.; Marzo, A.L.; Scott, B.; Heath, W.R.; Collins, E.J.; Frelinger, J.A.; Robinson, B.W.S. Induction of Tumour Cell Apoptosis In Vivo Increases Tumour Antigen Cross-Presentation, Cross-Priming Rather than Cross-Tolerizing Host Tumour-Specific CD8 T Cells. J. Immunol. 2003, 170, 4905–4913. [Google Scholar] [CrossRef] [Green Version]
- Theisen, D.J.; Davidson, J.T.; Briseño, C.G.; Gargaro, M.; Lauron, J.; Wang, Q.; Desai, P.; Durai, V.; Bagadia, P.; Joshua, R.; et al. WDFY4 is required for cross-presentation in response to viral and tumour antigens. Science 2018, 362, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef]
- de Mingo Pulido, Á.; Gardner, A.; Hiebler, S.; Soliman, H.; Rugo, H.S.; Krummel, M.F.; Coussens, L.M.; Ruffell, B. TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018, 33, 60–74.e6. [Google Scholar] [CrossRef] [Green Version]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumour microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic Cells and Their Role in Immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumour Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoural dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e7. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.; Vijayan, D.; Putz, E.M.; Aguilera, A.R.; Markey, K.A.; Straube, J.; Kazakoff, S.; Nutt, S.L.; Takeda, K.; Hill, G.R.; et al. Interleukin-12 from CD103+ Batf3-dependent dendritic cells required for NK-cell suppression of metastasis. Cancer Immunol. Res. 2017, 5, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumour Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maier, B.; Leader, A.M.; Chen, S.T.; Tung, N.; Chang, C.; LeBerichel, J.; Chudnovskiy, A.; Maskey, S.; Walker, L.; Finnigan, J.P.; et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature 2020, 580, 257–262. [Google Scholar] [CrossRef]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Gordon, C.L.; Miron, M.; Kumar, B.; Griesemer, A.; Ho, H.; Lerner, H.; et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Binnewies, M.; Mujal, A.M.; Pollack, J.L.; Combes, A.J.; Hardison, E.A.; Barry, K.C.; Tsui, J.; Ruhland, M.K.; Kersten, K.; Abushawish, M.A.; et al. Unleashing Type-2 Dendritic Cells to Drive Protective Antitumour CD4+ T Cell Immunity. Cell 2019, 177, 556–571.e16. [Google Scholar] [CrossRef]
- Sittig, S.P.; van Beek, J.J.P.; Flórez-Grau, G.; Weiden, J.; Buschow, S.I.; van der Net, M.C.; van Slooten, R.; Verbeek, M.M.; Geurtz, P.B.H.; Textor, J.; et al. Human type 1 and type 2 conventional dendritic cells express indoleamine 2,3-dioxygenase 1 with functional effects on T cell priming. Eur. J. Immunol. 2021, 51, 1494–1504. [Google Scholar] [CrossRef]
- Hatscher, L.; Lehmann, C.; Purbojo, A.; Onderka, C.; Liang, C.; Hartmann, A.; Cesnjevar, R.; Bruns, H.; Gross, O.; Nimmerjahn, F.; et al. Select hyperactivating NLRP3 ligands enhance the TH1- and TH17-inducing potential of human type 2 conventional dendritic cells. Sci. Signal. 2021, 14, eabe1757. [Google Scholar] [CrossRef]
- Rogers, G.L.; Shirley, J.L.; Zolotukhin, I.; Kumar, S.R.P.; Sherman, A.; Perrin, G.Q.; Hoffman, B.E.; Srivastava, A.; Basner-Tschakarjan, E.; Wallet, M.A.; et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood 2017, 129, 3184–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewitz, A.; Eickhoff, S.; Quast, T.; Bedoui, S.; Kroczek, R.A.; Kurts, C.; Garbi, N.; Barchet, W.; Iannacone, M.; Klauschen, F.; et al. CD8+ T cells orchestrate pDC—XCR1+ dendritic cell spatial and functional cooperativity to optimize priming. Immunity 2017, 46, 205–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumours. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Hyde, E.J.; Yang, J.; Rich, F.J.; Harper, J.L.; Kirman, J.R.; Ronchese, F. Increased Numbers of Monocyte-Derived Dendritic Cells during Successful Tumour Immunotherapy with Immune-Activating Agents. J. Immunol. 2013, 191, 1984–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, S.; Yang, J.; Ronchese, F. Monocyte-derived dendritic cells are essential for CD8+ T cell activation and antitumour responses after local immunotherapy. Front. Immunol. 2015, 6, 584. [Google Scholar] [CrossRef] [Green Version]
- Tsujitani, S.; Furukawa, T.; Tamada, R.; Okamura, T.; Yasumoto, K.; Sugimachi, K. Langerhans cells and prognosis in patients with gastric carcinoma. Cancer 1987, 59, 501–505. [Google Scholar] [CrossRef]
- Herber, D.L.; Cao, W.; Nefedova, Y.; Novitskiy, S.V.; Nagaraj, S.; Tyurin, V.A.; Corzo, A.; Cho, H., II; Celis, E.; Lennox, B.; et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 2010, 16, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, R.; Tuyrin, V.A.; Veglia, F.; Condamine, T.; Amoscato, A.; Mohammadyani, D.; Johnson, J.J.; Zhang, L.M.; Klein-Seetharaman, J.; Celis, E.; et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 2014, 192, 2920–2931. [Google Scholar]
- Bharadwaj, U.; Li, M.; Zhang, R.; Chen, C.; Yao, Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007, 67, 5479–5488. [Google Scholar] [CrossRef] [Green Version]
- Alshamsan, A. Induction of tolerogenic dendritic cells by IL-6-secreting CT26 colon carcinoma. Immunopharmacol. Immunotoxicol. 2012, 34, 465–469. [Google Scholar] [CrossRef]
- Pahne-Zeppenfeld, J.; Schröer, N.; Walch-Rückheim, B.; Oldak, M.; Gorter, A.; Hegde, S.; Smola, S. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int. J. Cancer 2014, 134, 2061–2073. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumour microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Barrat, F.J.; Castro, A.G.; Vicari, A.; Hawrylowicz, C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008, 223, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kroemer, G. CD103+ Dendritic Cells Producing Interleukin-12 in Anticancer Immunosurveillance. Cancer Cell 2014, 26, 591–593. [Google Scholar] [CrossRef] [Green Version]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I.; et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008, 112, 1822–1831. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [Green Version]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumour-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef] [Green Version]
- Faget, J.; Bendriss-Vermare, N.; Gobert, M.; Durand, I.; Olive, D.; Biota, C.; Bachelot, T.; Treilleux, I.; Goddard-Leon, S.; Lavergne, E.; et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012, 72, 6130–6141. [Google Scholar] [CrossRef] [Green Version]
- Curiel, T.J.; Cheng, P.; Mottram, P.; Alvarez, X.; Moons, L.; Evdemon-Hogan, M.; Wei, S.; Zou, L.; Kryczek, I.; Hoyle, G.; et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004, 64, 5535–5538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakdash, G.; Buschow, S.I.; Gorris, M.A.J.; Halilovic, A.; Hato, S.V.; Sköld, A.E.; Schreibelt, G.; Sittig, S.P.; Torensma, R.; Duiveman-De Boer, T.; et al. Expansion of a BDCA1+ CD14+ myeloid cell population in melanoma patients may attenuate the efficacy of dendritic cell vaccines. Cancer Res. 2016, 76, 4332–4346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, A.B.; Kulkarni, A.B.; Tennenbaum, T.; Hennings, H.; Flanders, K.C.; O’Reilly, M.; Sporn, M.B.; Karlsson, S.; Yuspa, S.H. Loss of expression of transforming growth factor β in skin and skin tumours is associated with hyperproliferation and a high risk for malignant conversion. Proc. Natl. Acad. Sci. USA 1993, 90, 6076–6080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Investigating the role of Langerhans cells in primary cutaneous melanoma. J. Dermatol. Sci. 2017, 86, 209. [Google Scholar] [CrossRef]
- Tugues, S.; Ducimetiere, L.; Friebel, E.; Becher, B. Innate lymphoid cells as regulators of the tumour microenvironment. Semin. Immunol. 2019, 41, 101270. [Google Scholar] [CrossRef]
- Ljunggren, H.-G.; Malmberg, K.-J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 2007, 7, 329–339. [Google Scholar] [CrossRef]
- Palmieri, G.; Gismondi, A.; Galandrini, R.; Milella, M.; Serra, A.; De Maria, R.; Santoni, A. Interaction of natural killer cells with extracellular matrix induces early intracellular signalling events and enhances cytotoxic functions. Nat. Immun. 1996, 15, 147–153. [Google Scholar]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Thia, K.Y.; Street, S.E.; MacGregor, D.; Godfrey, D.I.; Trapani, J.A. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 2000, 192, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Payer, Á.R.; Gonzalez, S.; López-Soto, A. Mechanisms of apoptosis resistance to NK cell-mediated cytotoxicity in cancer. Int. J. Mol. Sci. 2020, 21, 3726. [Google Scholar] [CrossRef]
- Watzl, C.; Long, E.O. Signal transduction during activation and inhibition of natural killer cells. Curr. Protoc. Immunol. 2010, 11, 11.9B.1–11.9B.17. [Google Scholar]
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The Prognostic Significance of Natural Killer Cytotoxicity in Patients with Colorectal Cancer. Arch. Surg. 1987, 122, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Schantz, S.P.; Savage, H.E.; Racz, T.; Taylor, D.L.; Sacks, P.G. Natural killer cells and metastases from pharyngeal carcinoma. Am. J. Surg. 1989, 158, 361–366. [Google Scholar] [CrossRef]
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376. [Google Scholar] [PubMed]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Natural Killer Cell Immunotherapy Targeting Cancer Stem Cells. Expert Opin. Biol. Ther. 2017, 17, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Tallerico, R.; Todaro, M.; Di Franco, S.; Maccalli, C.; Garofalo, C.; Sottile, R.; Palmieri, C.; Tirinato, L.; Pangigadde, P.N.; La Rocca, R.; et al. Human NK Cells Selective Targeting of Colon Cancer–Initiating Cells: A Role for Natural Cytotoxicity Receptors and MHC Class I Molecules. J. Immunol. 2013, 190, 2381–2390. [Google Scholar] [CrossRef] [Green Version]
- Leong, J.W.; Chase, J.M.; Romee, R.; Schneider, S.E.; Ryan, P.; Cooper, M.A.; Fehniger, T.A. Pre-activation with IL-12, IL-15, and IL-18 induces CD25 and a functional high affinity IL-2 receptor on human cytokine-induced memory-like NK cells. Biol. Blood Marrow Transplant. 2014, 20, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef]
- Delahaye, N.F.; Rusakiewicz, S.; Martins, I.; Ménard, C.; Roux, S.; Lyonnet, L.; Paul, P.; Sarabi, M.; Chaput, N.; Semeraro, M.; et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumours. Nat. Med. 2011, 17, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C.; Gravis, G.; Guerin, M.; Granjeaud, S.; Thomassin-Piana, J.; Rocchi, P.; Paciencia-Gros, M.; Poizat, F.; Bentobji, M.; Azario-Cheillan, F.; et al. Inherent and tumour-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumour activity. Cancer Res. 2016, 76, 2153–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habif, G.; Crinier, A.; André, P.; Vivier, E.; Narni-Mancinelli, E. Targeting natural killer cells in solid tumours. Cell. Mol. Immunol. 2019, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front. Immunol. 2018, 9, 2324. [Google Scholar] [CrossRef]
- Remark, R.; Alifano, M.; Cremer, I.; Lupo, A.; Dieu-Nosjean, M.C.; Riquet, M.; Crozet, L.; Ouakrim, H.; Goc, J.; Cazes, A.; et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: Influence of tumour origin. Clin. Cancer Res. 2013, 19, 4079–4091. [Google Scholar] [CrossRef] [Green Version]
- Versluis, M.A.C.; Marchal, S.; Plat, A.; de Bock, G.H.; van Hall, T.; de Bruyn, M.; Hollema, H.; Nijman, H.W. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur. J. Cancer 2017, 86, 285–295. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the Tumour Microenvironment on NK Cell Function in Solid Tumours. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Hangai, S.; Ao, T.; Kimura, Y.; Matsuki, K.; Kawamura, T.; Negishi, H.; Nishio, J.; Kodama, T.; Taniguchi, T.; Yanai, H. PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proc. Natl. Acad. Sci. USA 2016, 113, 3844–3849. [Google Scholar] [CrossRef] [Green Version]
- Linnemeyer, P.A.; Pollack, S.B. Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. J. Immunol. 1993, 150, 3747–3754. [Google Scholar] [PubMed]
- Joshi, P.C.; Zhou, X.; Cuchens, M.; Jones, Q. Prostaglandin E2 Suppressed IL-15-Mediated Human NK Cell Function through Down-Regulation of Common γ-Chain. J. Immunol. 2001, 166, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinet, L.; Jean, C.; Dietrich, G.; Fournie, J.J.; Poupot, R. PGE2 inhibits natural killer and γδ T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem. Pharmacol. 2010, 80, 838–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Holt, D.; Kundu, N.; Reader, J.; Goloubeva, O.; Take, Y.; Fulton, A.M. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2013, 2, e22647. [Google Scholar] [CrossRef] [Green Version]
- Spits, H.; Bernink, J.H.; Lanier, L. NK cells and type 1 innate lymphoid cells: Partners in host defense. Nat. Immunol. 2016, 17, 758–764. [Google Scholar] [CrossRef]
- Scanlon, S.T.; Mckenzie, A.N.J. Type 2 innate lymphoid cells: New players in asthma and allergy. Curr. Opin. Immunol. 2012, 24, 707–712. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Moon, U.J.; Kim, H.J.; Choi, H.J.; Sin, J.I.; Park, N.H.; Cho, H.R.; Kwon, B. Intratumourally Establishing Type 2 Innate Lymphoid Cells Blocks Tumour Growth. J. Immunol. 2016, 196, 2410–2423. [Google Scholar] [CrossRef] [Green Version]
- Ikutani, M.; Yanagibashi, T.; Ogasawara, M.; Tsuneyama, K.; Yamamoto, S.; Hattori, Y.; Kouro, T.; Itakura, A.; Nagai, Y.; Takaki, S.; et al. Identification of Innate IL-5—Producing Cells and Their Role in Lung Eosinophil Regulation and Antitumour Immunity. J. Immunol. 2012, 188, 703–713. [Google Scholar] [CrossRef] [Green Version]
- Trabanelli, S.; Chevalier, M.F.; Martinez-Usatorre, A.; Gomez-Cadena, A.; Salomé, B.; Lecciso, M.; Salvestrini, V.; Verdeil, G.; Racle, J.; Papayannidis, C.; et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 2017, 8, 593. [Google Scholar] [CrossRef] [Green Version]
- Eisenring, M.; vorn Berg, J.; Kristiansen, G.; Saller, E.; Becher, B. IL-12 initiates tumour rejection via lymphoid tissue—Inducer cells bearing the natural cytotoxicity receptor NKp46. Nat. Immunol. 2010, 11, 1030–1038. [Google Scholar] [CrossRef]
- Nussbaum, K.; Burkhard, S.H.; Ohs, I.; Mair, F.; Klose, C.S.N.; Arnold, S.J.; Diefenbach, A.; Tugues, S.; Becher, B. Tissue microenvironment dictates the fate and tumour- suppressive function of type 3 ILCs. J. Exp. Med. 2017, 214, 2331–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchberger, S.; Royston, D.J.; Boulard, O.; Thornton, E.; Franchini, F.; Szabady, R.L.; Harrison, O.; Powrie, F. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 2013, 210, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate lymphoid cells drive IL-23 dependent innate intestinal pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Hu, X.; Zhang, H.; Hu, H. Tertiary Lymphoid Organs in Cancer Immunology: Mechanisms and the New Strategy for Immunotherapy. Front. Immunol. 2019, 10, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumours that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irshad, S.; Flores-Borja, F.; Lawler, K.; Monypenny, J.; Evans, R.; Male, V.; Gordon, P.; Cheung, A.; Gazinska, P.; Noor, F.; et al. RORγt+ Innate Lymphoid Cells Promote Lymph Node Metastasis of Breast Cancers. Cancer Res. 2017, 77, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Feng, Z.; Luo, J.; He, Z.; Liu, J.; Wu, J. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumour Immunity and Potential Therapeutic Induction Strategies. Front. Immunol. 2021, 12, 689270. [Google Scholar] [CrossRef]
- Fridman, W.H.; Petitprez, F.; Meylan, M.; Chen, T.W.; Sun, C.M.; Roumenina, L.T. Sautès-Fridman, C. B cells and cancer: To B or not to B? J. Exp. Med. 2021, 218, e20200851. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, C.; Wu, F.; Tao, L.; Zhang, C.; Zhao, D.; Yang, S.; Jiang, D.; Wang, J.; Sun, Y.; et al. T Follicular Helper-Like Cells Are Involved in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 944. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. T follicular helper cell biology: A decade of discovery and diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Brodt, P.; Gordon, J. Anti-tumour immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumour. J. Immunol. 1978, 121, 359–362. [Google Scholar] [PubMed]
- Wouters, M.C.A.; Nelson, B.H. Prognostic Significance of Tumour-Infiltrating B Cells and Plasma Cells in Human Cancer. Clin. Cancer Res. 2018, 24, 6125–6136. [Google Scholar] [CrossRef] [Green Version]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shain, K.H.; Dalton, W.S.; Tao, J. The tumour microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene 2015, 34, 4673–4682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Pimenta, E.M.; Barnes, B.J. Role of Tertiary Lymphoid Structures (TLS) in Anti-Tumour Immunity: Potential Tumour-Induced Cytokines/Chemokines that Regulate TLS Formation in Epithelial-Derived Cancers. Cancers 2014, 6, 969–997. [Google Scholar] [CrossRef] [Green Version]
- Bergomas, F.; Grizzi, F.; Doni, A.; Pesce, S.; Laghi, L.; Allavena, P.; Mantovani, A.; Marchesi, F. Tertiary Intratumour Lymphoid Tissue in Colo-Rectal Cancer. Cancers 2011, 4, 1–10. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B Cells in Tertiary Lymphoid Structures Is Associated with a Protective Immunity in Patients with Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Dizier, B.; Callegaro, A.; Debois, M.; Dreno, B.; Hersey, P.; Gogas, H.J.; Kirkwood, J.M.; Vansteenkiste, J.F.; Sequist, L.V.; Atanackovic, D.; et al. A Th1/IFN-γ Gene Signature Is Prognostic in the Adjuvant Setting of Resectable High-Risk Melanoma but Not in Non—Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1725–1735. [Google Scholar] [CrossRef] [Green Version]
- Kachler, K.; Holzinger, C.; Trufa, D.I.; Sirbu, H.; Finotto, S. The role of Foxp3 and Tbet co-expressing Treg cells in lung carcinoma. Oncoimmunology 2018, 7, e1456612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stearns, M.E.; Rhim, J.; Wang, M. Interleukin 10 (IL-10) Inhibition of Primary Human Prostate Cell-induced Angiogenesis: IL-10 Stimulation of Tissue Inhibitor of Metalloproteinase-1 and Inhibition of Matrix Metalloproteinase (MMP)-2/MMP-9 Secretion. Clin. Cancer Res. 1999, 5, 189–196. [Google Scholar] [PubMed]
- Chen, J.; Gong, C.; Mao, H.; Li, Z.; Fang, Z.; Chen, Q.; Lin, M.I.N.; Jiang, X.; Hu, Y.; Wang, W.E.I.; et al. E2F1/SP3/STAT6 axis is required for IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int. J. Oncol. 2018, 53, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, B.; Yang, T.; Zhang, L.; Jin, K. The outstanding antitumour capacity of CD4+ T helper lymphocytes. Biochim. Biophys. Acta. Rev. Cancer 2020, 1874, 188439. [Google Scholar] [CrossRef]
- Salazar, Y.; Zheng, X.; Brunn, D.; Raifer, H.; Picard, F.; Zhang, Y.; Winter, H.; Guenther, S.; Weigert, A.; Weigmann, B.; et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J. Clin. Investig. 2020, 130, 3560–3575. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; De Mare, V.; Rocchi, C.; Stolfi, C.; Colantoni, A.; Neurath, M.F.; Macdonald, T.T.; Pallone, F.; Monteleone, G.; Fantini, M.C. Smad7 induces plasticity in tumour- infiltrating Th17 cells and enables TNF-alpha-mediated killing of colorectal cancer cells. Carcinogenesis 2014, 35, 1536–1546. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Duan, Y.; Cheng, X.; Chen, X.; Xie, W.; Long, H.; Lin, Z.; Zhu, B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem. Biophys. Res. Commun. 2011, 407, 348–354. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Pasetto, A.; Jia, L.; Deniger, D.C.; Stevanović, S.; Robbins, P.F.; Rosenberg, S.A. Tumour-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumour and neoantigen reactivity. Sci. Immunol. 2019, 4, eaao4310. [Google Scholar] [CrossRef]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumour infiltrating lymphocytes is associated with tumour recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. 2015, 64, 419–427. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3+ CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.S.; Ohashi, P.S. The Roles of CD8+ T Cell Subsets in Antitumour Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumour antigen—Specific CD8 T cells infiltrating the tumour express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Hu, G. Prognostic role of intratumoural IL-17A expression immunohistochemistry in solid tumours: A meta-analysis. Oncotarget 2017, 8, 66382–66391. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-H.; Chang, J.T.-C.; Liao, C.-T.; Chen, Y.-S.; Kuo, M.-L.; Shen, C.-R. Interleukin 17 and peripheral IL-17-expressing T cells are negatively correlated with the overall survival of head and neck cancer patients. Oncotarget 2018, 9, 9825–9837. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Peng, L.S.; Zhao, Y.L.; Shi, Y.; Mao, X.H.; Chen, W.; Pang, K.C.; Liu, X.F.; Liu, T.; Zhang, J.Y.; et al. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012, 143, 951–962.e8. [Google Scholar] [CrossRef]
- Zhang, S.; Fujita, H.; Mitsui, H.; Yanofsky, V.R.; Fuentes-Duculan, J.; Pettersen, J.S.; Suárez-Fariñas, M.; Gonzalez, J.; Wang, C.Q.F.; Krueger, J.G.; et al. Increased Tc22 and Treg/CD8 Ratio Contribute to Aggressive Growth of Transplant Associated Squamous Cell Carcinoma. PLoS ONE 2013, 8, e62154. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, G.; Zhang, J.; Wu, X.; Chen, X. The Dual Roles of Human γδ T Cells: Anti-Tumour or Tumour-Promoting. Front. Immunol. 2021, 11, 619954. [Google Scholar] [CrossRef]
- Patil, R.S.; Shah, S.U.; Shrikhande, S.V.; Goel, M.; Dikshit, R.P.; Chiplunkar, S.V. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 2016, 139, 869–881. [Google Scholar] [CrossRef]
- Hoff, S.T.; Salman, A.M.; Ruhwald, M.; Ravn, P.; Brock, I.; Elsheikh, N.; Andersen, P.; Agger, E.M. Human B cells produce chemokine CXCL10 in the presence of Mycobacterium tuberculosis specific T cells. Tuberculosis 2015, 95, 40–47. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Menard, L.C.; Minns, L.A.; Darche, S.; Mielcarz, D.W.; Foureau, D.M.; Roos, D.; Dzierszinski, F.; Kasper, L.H.; Buzoni-Gatel, D. B Cells Amplify IFN-gamma Production by T Cells via a TNF-α-Mediated Mechanism. J. Immunol. 2007, 179, 4857–4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaniel, C.; Pardali, E.; Sallusto, F.; Speletas, M.; Ruedl, C.; Shimizu, T.; Seidl, T.; Andersson, J.; Melchers, F.; Rolink, A.G.; et al. Activated Murine B Lymphocytes and Dendritic Cells Produce a Novel CC Chemokine which Acts Selectively on Activated T Cells. J. Exp. Med. 1998, 188, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deola, S.; Panelli, M.C.; Maric, D.; Selleri, S.; Dmitrieva, N.I.; Voss, C.Y.; Klein, H.; Stroncek, D.; Wang, E.; Marincola, F.M. Helper B Cells Promote Cytotoxic T Cell Survival and Proliferation Independently of Antigen Presentation through CD27/CD70 Interactions. J. Immunol. 2008, 180, 1362–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Cancer Genome Atlas Program. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 9 February 2022).
- Macdonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Tsay, J.C.J.; Li, J.; Yie, T.A.; Munger, J.S.; Pass, H.; Rom, W.N.; Zhang, Y.; Tan, E.M.; Zhang, J.Y. Autoantibodies against tumour-associated antigens in the early detection of lung cancer. Lung Cancer 2016, 99, 172–179. [Google Scholar] [CrossRef]
- Fremd, C.; Stefanovic, S.; Beckhove, P.; Pritsch, M.; Lim, H.; Wallwiener, M.; Heil, J.; Golatta, M.; Rom, J.; Sohn, C.; et al. Mucin 1-specific B cell immune responses and their impact on overall survival in breast cancer patients. Oncoimmunology 2015, 5, e1057387. [Google Scholar] [CrossRef] [Green Version]
- Garaud, S.; Zayakin, P.; Buisseret, L.; Rulle, U.; Silina, K.; de Wind, A.; Van den Eyden, G.; Larsimont, D.; Willard-Gallo, K.; Line, A. Antigen specificity and clinical significance of igG and igA autoantibodies produced in situ by tumour-infiltrating B cells in breast cancer. Front. Immunol. 2018, 9, 2660. [Google Scholar] [CrossRef]
- Rossetti, R.A.M.; Lorenzi, N.P.C.; Yokochi, K.; Sartor de Faria Rosa, M.B.; Benevides, L.; Margarido, P.F.R.; Baracat, E.C.; Caravalho, J.P.; Villa, L.L.; Lepique, A.P. B lymphocytes can be activated to act as antigen presenting cells to promote anti-tumour responses. PLoS ONE 2018, 13, e0199034. [Google Scholar] [CrossRef]
- Rodríguez-Pinto, D. B cells as antigen presenting cells. Cell. Immunol. 2005, 238, 67–75. [Google Scholar] [CrossRef]
- Selitsky, S.R.; Mose, L.E.; Smith, C.C.; Chai, S.; Hoadley, K.A.; Dittmer, D.P.; Moschos, S.J.; Parker, J.S.; Vincent, B.G. Prognostic value of B cells in cutaneous melanoma. Genome Med. 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoural Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, S.M.A.; Lee, A.H.S.; Paish, E.C.; Macmillan, R.D.; Ellis, I.O.; Green, A.R. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 2012, 132, 545–553. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-in fi ltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef] [Green Version]
- van Herpen, C.M.; van der Voort, R.; van der Laak, J.A.; Klasen, I.S.; de Graaf, A.O.; van Kempen, L.C.; de Vries, I.J.; Boer, T.D.; Dolstra, H.; Torensma, R.; et al. Intratumoural rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int. J. Cancer 2008, 123, 2354–2361. [Google Scholar] [CrossRef]
- Edin, S.; Kaprio, T.; Hagström, J.; Larsson, P.; Mustonen, H.; Böckelman, C.; Strigård, K.; Gunnarsson, U.; Haglund, C.; Palmqvist, R. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci. Rep. 2019, 9, 19997. [Google Scholar] [CrossRef]
- Bodogai, M.; Chang, C.L.; Wejksza, K.; Lai, J.; Merino, M.; Wersto, R.P.; Gress, R.E.; Chan, A.C.; Hesdorffer, C.; Biragyn, A. Anti-CD20 antibody promotes cancer escape via enrichment of tumour-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013, 73, 2127–2138. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B cell regulation in cancer and anti-tumour immunity. Cell. Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tai, Y.-T.; Ho, M.; Xing, L.; Chauhan, D.; Gang, A.; Qiu, L.; Anderson, K.C. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J. 2017, 7, e547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodogai, M.; Moritoh, K.; Chang, C.L.; Hollander, C.M.; Sherman-Baust, C.A.; Wersto, R.P.; Araki, Y.; Miyoshi, I.; Yang, L.; Trinchieri, G.; et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumour-Associated B Cells. Cancer Res. 2015, 75, 3456–3465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammirante, M.; Luo, J.; Grivennikov, S.; Nedospasov, S. B cell–derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010, 464, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Gallastegui, N.; Rosenblatt, J.D. Regulatory B cells in anti-tumour immunity. Int. Immunol. 2015, 27, 521–530. [Google Scholar] [CrossRef]
- Horii, M.; Matsushita, T. Regulatory B cells and T cell regulation in cancer. J. Mol. Biol. 2021, 433, 166685. [Google Scholar] [CrossRef]
- Wang, R. The role of MHC class II-restricted tumour antigens and CD4+ T cells in antitumour immunity. Trends Immunol. 2001, 22, 269–276. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy -new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Guisier, F.; Barros-Filho, M.; Rock, L.; Strachan-Whaley, M.; Marshall, E.; Dellaire, G.; Lam, W. Janus or Hydra: The many faces of T helper cells in the human tumour microenvironment. In Tumour Microenvironment: Hematopoietic Cells—Part A, 1st ed.; Birbrair, A., Ed.; Springer: New York, NY, USA, 2020; pp. 35–53. [Google Scholar]
- Nishimura, T.; Iwakabe, K.; Sekimoto, M.; Ohmi, Y.; Yahata, T.; Nakui, M.; Sato, T.; Habu, S.; Tashiro, H.; Sato, M.; et al. Distinct Role of Antigen-specific T Helper Type 1 (Th1) and Th2 Cells in Tumour Eradication In Vivo. J. Exp. Med. 1999, 190, 617–627. [Google Scholar] [CrossRef]
- Tahara, H.; Zeh, H.J., 3rd; Storkus, W.J.; Pappo, I.; Watkins, S.C.; Gubler, U.; Wolf, S.F.; Robbins, P.D.; Lotze, M.T. Fibroblasts Genetically Engineered to Secrete Interleukin 12 Can Suppress Tumour Growth and Induce Antitumour Immunity to a Murine Melanoma in Vivo. Cancer Res. 1994, 54, 182–189. [Google Scholar]
- Ryffel, B. Interleukin-12: Role of interferon-gamma in IL-12 adverse effects. Clin. Immunol. Immunopathol. 1997, 83, 18–20. [Google Scholar] [CrossRef]
- Ellyard, J.I.; Simson, L.; Parish, C.R. Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens 2007, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Ohashi, Y.; Suzuki, T.; Miyazaki, S.; Moriya, T.; Satomi, S.; Sasano, H. Tumour-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006, 26, 1419–1424. [Google Scholar] [PubMed]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumour-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Schreiber, S.; Hammers, C.M.; Kaasch, A.J.; Schraven, B.; Dudeck, A.; Kahlfuss, S. Metabolic Interdependency of Th2 Cell-Mediated Type 2 Immunity and the Tumour Microenvironment. Front. Immunol. 2021, 12, 632581. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [CrossRef]
- Végran, F.; Berger, H.; Apetoh, L. Role of IL-17 and IL-17 Family Cytokines on Tumour Development. In IL-17, IL-22 and Their Producing Cells: Role in Inflammation and Autoimmunity, 2nd ed.; Quesniaux, V., Ryffel, B., Di Padova, F., Eds.; Springer: Basel, Switzerland, 2013; pp. 219–227. [Google Scholar]
- Kryczek, I.; Wei, S.; Szeliga, W.; Vatan, L.; Zou, W. Endogenous IL-17 contributes to reduced tumour growth and metastasis. Blood 2009, 114, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T cell activation in tumour immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Muranski, P.; Boni, A.; Antony, P.A.; Cassard, L.; Irvine, K.R.; Kaiser, A.; Paulos, C.M.; Palmer, D.C.; Touloukian, C.E.; Ptak, K.; et al. Tumour-specific Th17-polarized cells eradicate large established melanoma. Blood 2008, 112, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Punt, S.; Dronkers, E.A.C.; Welters, M.J.P.; Goedemans, R.; Koljenović, S.; Bloemena, E.; Snijders, P.J.; Gorter, A.; van der Burg, S.H.; de Jong, R.J.B.; et al. A beneficial tumour microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17+ cell frequency. Cancer Immunol. Immunother. 2016, 65, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Haghshenas, M.R.; Khademi, B.; Faghih, Z.; Ghaderi, A.; Erfani, N. Immune regulatory cells and IL17-producing lymphocytes in patients with benign and malignant salivary gland tumours. Immunol. Lett. 2015, 164, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumour environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.-J.; Zhou, Q.; Gu, Y.-Y.; Qin, S.-M.; Ma, W.-L.; Xin, J.-B.; Tao, X.-N.; Shi, H.-Z. Generation and Differentiation of IL-17–Producing CD4+ T Cells in Malignant Pleural Effusion. J. Immunol. 2010, 185, 6348–6354. [Google Scholar] [CrossRef] [Green Version]
- Muranski, P.; Borman, Z.A.; Kerkar, S.P.; Klebanoff, C.A.; Ji, Y.; Sanchez-Perez, L.; Sukumar, M.; Reger, R.N.; Yu, Z.; Kern, S.J.; et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011, 35, 972–985. [Google Scholar] [CrossRef] [Green Version]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Cai, Z.; Li, B.; Sun, L.; Shen, Y.; Wang, S.; Wang, Z.; Wang, Z.; Wang, Y.; et al. Th9 Cell Differentiation and Its Dual Effects in Tumour Development. Front. Immunol. 2020, 11, 1026. [Google Scholar] [CrossRef]
- Schmitt, E.; Klein, M.; Bopp, T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014, 35, 61–68. [Google Scholar] [CrossRef]
- Végran, F.; Berger, H.; Boidot, R.; Mignot, G.; Bruchard, M.; Dosset, M.; Chalmin, F.; Rébé, C.; Dérangère, V.; Ryffel, B.; et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol. 2014, 15, 758–766. [Google Scholar] [CrossRef]
- Kurata, I.; Matsumoto, I.; Sumida, T. T follicular helper cell subsets: A potential key player in autoimmunity. Immunol. Med. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Hetta, H.F.; Elkady, A.; Yahia, R.; Meshall, A.K.; Saad, M.M.; Mekky, M.A.; Al-Kadmy, I.M.S. T follicular helper and T follicular regulatory cells in colorectal cancer: A complex interplay. J. Immunol. Methods 2020, 480, 112753. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, C.G.; Linterman, M.A.; Yu, D.; Maclennan, I.C.M. Follicular Helper T Cells. Annu. Rev. Immunol. 2016, 34, 335–368. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liang, H.; Xu, Y.; Liu, L.; Ren, X.; Zhang, S.; Wei, S.; Xu, P. The role of circulating T follicular helper cells and regulatory cells in non-small cell lung cancer patients. Scand. J. Immunol. 2017, 86, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Linnemann, C.; van Buuren, M.M.; Bies, L.; Verdegaal, E.M.E.; Schotte, R.; Calis, J.J.A.; Behjati, S.; Velds, A.; Hilkmann, H.; Atmioui, D.E.; et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 2015, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Galaine, J.; Turco, C.; Vauchy, C.; Royer, B.; Mercier-Letondal, P.; Queiroz, L.; Loyon, R.; Mouget, V.; Boidot, R.; Laheurte, C.; et al. CD4 T cells target colorectal cancer antigens upregulated by oxaliplatin. Int. J. Cancer 2019, 145, 3112–3125. [Google Scholar] [CrossRef] [PubMed]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.S.; Rancan, C.; et al. Intratumoural CD4+ T Cells Mediate Anti-tumour Cytotoxicity in Human Bladder Cancer. Cell 2020, 181, 1612–1625.e13. [Google Scholar] [CrossRef]
- Beckermann, K.E.; Dudzinski, S.O.; Rathmell, J.C. Dysfunctional T cell metabolism in the tumour microenvironment. Cytokine Growth Factor Rev. 2017, 35, 7–14. [Google Scholar] [CrossRef]
- Clever, D.; Roychoudhuri, R.; Constantinides, M.G.; Askenase, M.H.; Sukumar, M.; Klebanoff, C.A.; Eil, R.L.; Hickman, H.D.; Yu, Z.; Pan, J.H.; et al. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell 2016, 166, 1117–1131.e14. [Google Scholar] [CrossRef] [Green Version]
- Aspord, C.; Pedroza-Gonzalez, A.; Gallegos, M.; Tindle, S.; Burton, E.C.; Su, D.; Marches, F.; Banchereau, J.; Palucka, A.K. Breast cancer instructs dendritic cells to prime interleukin 13—Secreting CD4+ T cells that facilitate tumour development. J. Exp. Med. 2007, 204, 1037–1047. [Google Scholar] [CrossRef] [Green Version]
- Prokopchuk, O.; Liu, Y.; Henne-Bruns, D.; Kornmann, M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: Evidence for autocrine and paracrine actions. Br. J. Cancer 2005, 92, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and Clinical Significance of Th17 Cells in the Tumour Microenvironment and Peripheral Blood of Pancreatic Cancer Patients. Int. J. Mol. Sci. 2011, 12, 7424–7437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Yan, J.; Xu, J.; Pang, X.H.; Chen, M.S.; Li, L.; Wu, C.; Li, S.P.; Zheng, L. Increased intratumoural IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009, 50, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Derhovanessian, E.; Adams, V.; Hähnel, K.; Groeger, A.; Pandha, H.; Ward, S.; Pawelec, G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer 2009, 125, 1372–1379. [Google Scholar] [CrossRef]
- Iida, T.; Iwahashi, M.; Katsuda, M.; Ishida, K.; Nakamori, M.; Nakamura, M.; Naka, T.; Ojima, T.; Ueda, K.; Hayata, K.; et al. Tumour-infiltrating CD4+ Th17 cells produce IL-17 in tumour microenvironment and promote tumour progression in human gastric cancer. Oncol. Rep. 2011, 25, 1271–1277. [Google Scholar]
- Chang, S.H.; Mirabolfathinejad, S.G.; Katta, H.; Cumpian, A.M.; Gong, L.; Caetano, M.S.; Moghaddam, S.J.; Dong, C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 5664–5669. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Mulcahy, L.A.; Mohammed, R.A.A.; Lee, A.H.S.; Franks, H.A.; Kilpatrick, L.; Yilmazer, A.; Paish, E.C.; Ellis, I.O.; Patel, P.M.; et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008, 10, R95. [Google Scholar] [CrossRef] [Green Version]
- Najafi, S.; Mirshafiey, A. The role of T helper 17 and regulatory T cells in tumour microenvironment. Immunopharmacol. Immunotoxicol. 2019, 41, 16–24. [Google Scholar] [CrossRef]
- Feng, L.L.; Gao, J.M.; Li, P.P.; Wang, X. IL-9 Contributes to Immunosuppression Mediated by Regulatory T Cells and Mast Cells in B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Immunol. 2011, 31, 1084–1094. [Google Scholar] [CrossRef]
- Qiu, L.; Lai, R.; Lin, Q.; Lau, E.; Thomazy, D.M.; Calame, D.; Ford, R.J.; Kwak, L.W.; Kirken, R.A.; Amin, H.M. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood 2006, 108, 2407–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, W.; Zhang, M.; Jiang, J.; Thomas, C.J.; Oh, U.; Bryant, B.R.; Chen, J.; Sato, N.; Tagaya, Y.; Morris, J.C.; et al. CP-690,550, a therapeutic agent, inhibits cytokine-mediated Jak3 activation and proliferation of T cells from patients with ATL and HAM/TSP. Blood 2011, 117, 1938–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagato, T.; Kobayashi, H.; Kishibe, K.; Takahara, M.; Ogino, T.; Ishii, H.; Oikawa, K.; Aoki, N.; Sato, K.; Kimura, S.; et al. Expression of Interleukin-9 in Nasal Natural Killer/T-Cell Lymphoma Cell Lines and Patients. Clin. Cancer Res. 2005, 11, 8250–8257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Lv, X.; Li, P.; Lu, K.; Wang, X. Role of high expression of IL-9 in prognosis of CLL. Int. J. Clin. Exp. Pathol. 2014, 7, 716–721. [Google Scholar] [PubMed]
- Kumar, S.; Dhamija, B.; Marathe, S.; Ghosh, S.; Dwivedi, A.; Karulkar, A.; Sharma, N.; Sengar, M.; Sridhar, E.; Bonda, A.; et al. The Th9 axis reduces the oxidative stress and promotes the survival of malignant T-cells in cutaneous T-cell lymphoma patients. Mol. Cancer Res. 2020, 18, 657–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Wang, S.; Zhao, L. A tumour-promoting role of Th9 cells in hepatocellular carcinoma through CCL20 and STAT3 pathways. Clin. Exp. Pharmacol. Physiol. 2017, 44, 213–221. [Google Scholar] [CrossRef]
- de Leval, L.; Gisselbrecht, C.; Gaulard, P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2010, 148, 673–689. [Google Scholar] [CrossRef]
- Ahearne, M.J.; Willimott, S.; Piñon, L.; Kennedy, D.B.; Miall, F.; Dyer, M.J.S.; Wagner, S.D. Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. Br. J. Haematol. 2013, 162, 360–370. [Google Scholar] [CrossRef]
- Amé-Thomas, P.; Le Priol, J.; Yssel, H.; Caron, G.; Pangault, C.; Jean, R.; Martin, N.; Marafioti, T.; Gaulard, P.; Lamy, T.; et al. Characterization of intratumoural follicular helper T cells in follicular lymphoma: Role in the survival of malignant B cells. Leukemia 2012, 26, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Savage, P.A.; Klawon, D.E.J.; Miller, C.H. Regulatory T Cell Development. Annu. Rev. Immunol. 2020, 38, 421–453. [Google Scholar] [CrossRef] [Green Version]
- Shitara, K.; Nishikawa, H. Regulatory T cells: A potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 2018, 1417, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.C.; Wong, L.Y.; Jang, T.; Shah, A.H.; Park, I.; Yang, X.; Zhang, Q.; Lonning, S.; Teicher, B.A.; Lee, C. Tumour Evasion of the Immune System by converting CD4+ CD25− T Cells into CD4+ CD25+ T Regulatory Cells: Role of Tumour-Derived TGF-β. J. Immunol. 2007, 178, 2883–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Chen, L.; Zhang, T.T.; Ma, Y.H.; Zhou, Y.L.; Zhao, Y.; Wang, W.W.; Dong, P.; Yu, L.; Zhang, Y.Y.; et al. Gastric cancer cells induce human CD4+ Foxp3+ regulatory T cells through the production of TGF-β1. World J. Gastroenterol. 2011, 17, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, J.U.N.; Li, H.; Li, W.E.I.; Wang, X.; Ma, J.; Tong, Q.; Wu, K.; Wang, G. Conversion of Intratumoural Regulatory T Cells by Human Gastric Cancer Cells is Dependent on Transforming Growth Factor-β1. Surg. Oncol. 2011, 104, 571–577. [Google Scholar] [CrossRef]
- Maimela, N.R.; Liu, S.; Zhang, Y. Fates of CD8+ T cells in Tumour Microenvironment. Comput. Struct. Biotechnol. J. 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Du, X.; Tang, F.; Liu, M.; Su, J.; Zhang, Y.; Wu, W.; Devenport, M.; Lazarski, C.A.; Zhang, P.; Wang, X.A.; et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018, 28, 416–432. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Kreuzaler, M.; Khan, T.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Overacre-Delgoffe, A.E.; Chikina, M.; Dadey, R.E.; Yano, H.; Brunazzi, E.A.; Shayan, G.; Horne, W.; Moskovitz, J.M.; Kolls, J.K.; Sander, C.; et al. Interferon-γ drives Treg fragility to promote anti-tumour immunity. Cell 2017, 169, 1130–1141.e11. [Google Scholar] [CrossRef] [Green Version]
- Delgoffe, G.M.; Woo, S.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D. Regulatory T cell stability is maintained by a neuropilin-1:semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Weigelin, B.; den Boer, A.T.; Wagena, E.; Broen, K.; Dolstra, H.; de Boer, R.J.; Figdor, C.G.; Textor, J.; Friedl, P. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun. 2021, 12, 5217. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Romero, R.; Watson, E.V.; Liang, A.C.; Burger, M.; Westcott, P.; Mercer, K.L.; Bronson, R.T.; Wooten, E.C.; Bhutkar, A.; et al. A GATA4-regulated secretory program suppresses tumours through recruitment of cytotoxic CD8 T cells. Nat. Commun. 2022, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ—Related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103+ Dendritic Cell Progenitors at the Tumour Site Enhances Tumour Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.R.; Milne, K.; Nelson, B.H. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 926–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.R.; Milne, K.; Watson, P.; Deleeuw, R.J.; Nelson, B.H. Tumour-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2014, 20, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Milne, K.; Derocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. CD103 and Intratumoural Immune Response in Breast Cancer. Clin. Cancer Res. 2016, 22, 6290–6297. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, A.-P.; Clarke, J.; Wood, O.; Garrido-Martin, E.M.; Chee, S.J.; Mellows, T.; Samaniego-Castruita, D.; Singh, D.; Seumois, G.; Alzetani, A.; et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017, 18, 940–950. [Google Scholar] [CrossRef]
- Reading, J.L.; Gálvez-Cancino, F.; Swanton, C.; Lladser, A.; Peggs, K.S.; Quezada, S.A. The function and dysfunction of memory CD8+ T cells in tumour immunity. Immunol. Rev. 2018, 283, 194–212. [Google Scholar] [CrossRef]
- Raverdeau, M.; Cunningham, S.P.; Harmon, C.; Lynch, L. γδ T cells in cancer: A small population of lymphocytes with big implications. Clin. Transl. Immunol. 2019, 8, e01080. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Scotet, E. Human Vgamma9Vdelta2 T cells: Promising new leads for immunotherapy of infections and tumours. Curr. Opin. Immunol. 2006, 18, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Caras, I.; Grigorescu, A.; Stavaru, C.; Radu, D.L.; Mogos, I.; Szegli, G.; Salageanu, A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol. Immunother. 2004, 53, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Fairchild, L.; Sun, L.; Horste, E.L.; Camara, S.; Shakiba, M.; Scott, A.C.; Viale, A.; Lauer, P.; Merghoub, T.; et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452–456. [Google Scholar] [CrossRef]
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hämmerling, G.J.; et al. Tumour-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumourigenesis. Immunity 2016, 45, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Ros-Martínez, S.; Navas-Carrillo, D.; Alonso-Romero, J.L.; Orenes-Piñero, E. Immunoscore: A novel prognostic tool. Association with clinical outcome, response to treatment and survival in several malignancies. Crit. Rev. Clin. Lab. Sci. 2020, 57, 432–443. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhu, Y.T.; Wang, J.J.; Zeng, D.X.; Mu, C.Y.; Lei, W.; Zhu, Y.H.; Huang, J.A. The prognostic value of interleukin-17 in lung cancer: A systematic review with meta-analysis based on Chinese patients. PLoS ONE 2017, 12, e0185168. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Wevers, E.; Schwartz, T.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; et al. Tumour-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J. Immunol. 2012, 189, 5029–5036. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.F. Tumour-Infiltrating γδ T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Shang, W.; Xu, R.; Wu, M.; Zhang, X.; Huang, P.; Wang, F.; Pan, S. Distribution and functions of γδ T cells infiltrated in the ovarian cancer microenvironment. J. Transl. Med. 2019, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Daley, D.; Zambirinis, C.P.; Seifert, L.; Akkad, N.; Mohan, N.; Werba, G.; Barilla, R.; Torres-Hernandez, A.; Hundeyin, M.; Mani, V.; et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell 2016, 166, 1485–1499.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, L.; Li, K.; Li, R.; Liu, H.M.; Sun, R.; Liu, X.Y. Analysis of tumour-infiltrating gamma delta T cells in rectal cancer. World J. Gastroenterol. 2016, 22, 3573–3580. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+ T Cells in the Tumour Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2021, 11, 622509. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shayan, G.; Avery, L.; Jie, H.B.; Gildener-Leapman, N.; Schmitt, N.; Lu, B.F.; Kane, L.P.; Ferris, R.L. Tumour-infiltrating Tim-3+ T cells proliferate avidly except when PD-1 is co-expressed: Evidence for intracellular cross talk. Oncoimmunology 2016, 5, e1200778. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Yang, L.; Yao, D.; Wu, X.; Li, J.; Liu, X.; Deng, L.; Huang, C.; Wang, Y.; Li, D.; et al. Tumour antigen-specific CD8+ T cells are negatively regulated by PD-1 and Tim-3 in human gastric cancer. Cell. Immunol. 2017, 313, 43–51. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumour infiltrating NY-ESO-1—Specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [Green Version]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hogg, G.D.; Denardo, D.G. Rethinking immune checkpoint blockade: ‘Beyond the T cell’. J. Immunother. Cancer 2021, 9, e001460. [Google Scholar] [CrossRef]

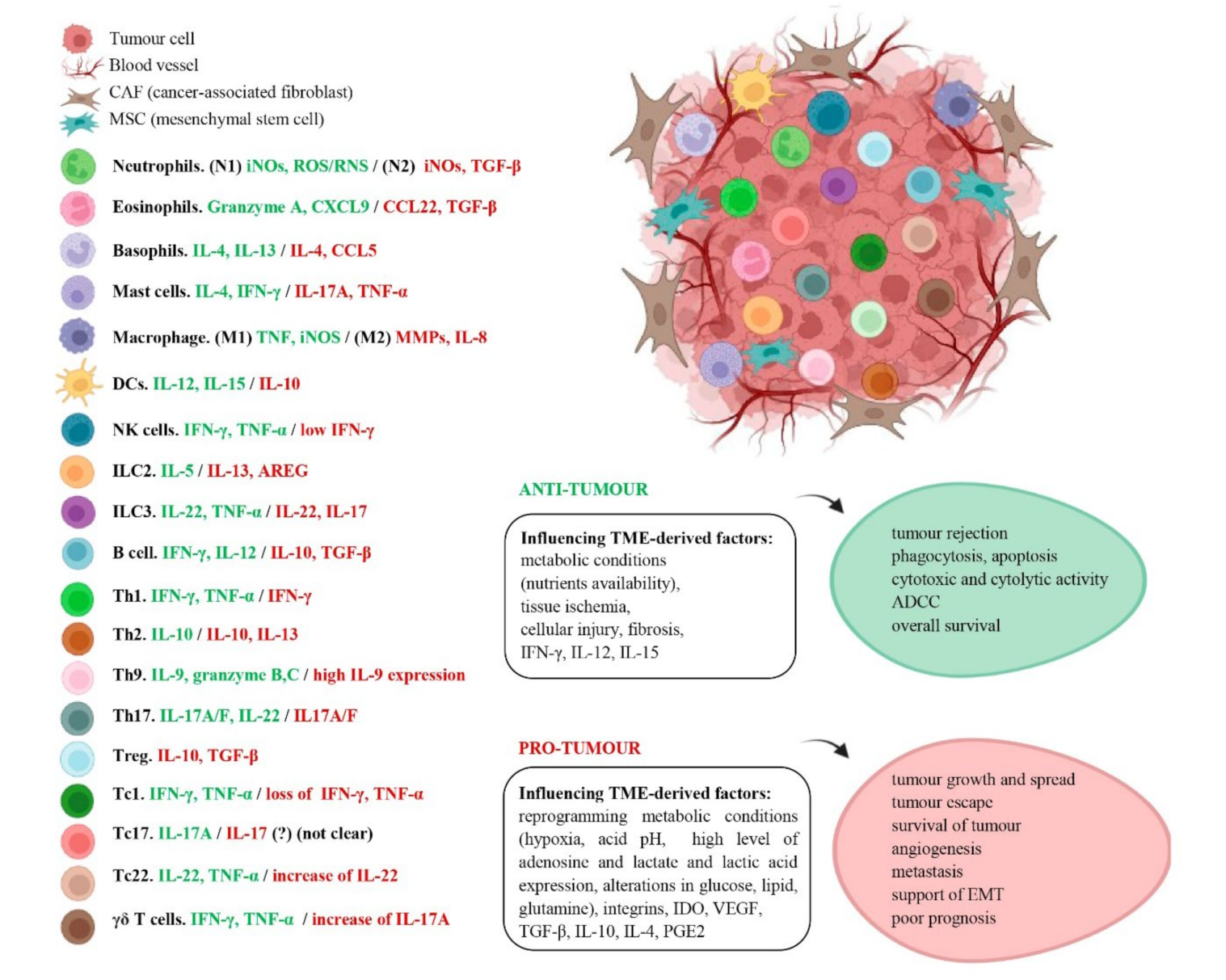

| Immune Cell Type | Anti-Tumor Immunity | Pro-Tumor/Immunosuppressive Immunity | References |
|---|---|---|---|
| Granulocytes | Neutrophils (N1): promote T cell cytotoxic function in human lung cancer Eosinophils: strong cytotoxic activity in human colon carcinoma cells Basophils: involved in CD8+ T cell infiltration in murine colon cancer | Neutrophils (N2): promote M2 phenotype macrophages in mouse lung cancer Eosinophils: inhibition of T and NK cell function in human NSCLC Basophils: reduction of CD8+ T cell infiltration both human and murine colon cancer | [153]/[97] [154,155]/[156] [157]/[158] |
| Mast cells | Cytolytic activity in breast cancer patients | Immunosuppression of T cells in human gastric cancer | [159]/[160] |
| Macrophages | (M1): Apoptosis in xenograft mice pancreatic cancer | (M2): Th1 responses suppression in mice breast tumor cells | [161]/[162] |
| Dendritic cells | CD103+cDC1s: cross-presentation, trafficking tumor antigen to lymph nodes and enhancement of effector CD8+ T cells in hepatocellular carcinoma patients moDCs: capacity to scavenge tumor antigen in lung and CRC patients Langerhans cells: APC function in primary lung adenocarcinoma patients | CD103+cDC1s: impairment of anti-tumor CD8+ T-cell responses in human CRC moDCs: impairment of T cell activation in human SCC Langerhans cells: dysregulation of DOK via IL-1β in human OSCC cells | [163]/[164] [165]/[166] [98]/[167] |
| ILC Group 1 | NK cells: elimination of tumor cells in leukaemia mouse model ILC1s cells: cytotoxicity through ILC1s-derived IFN-γ in early-stage of human multiple myeloma | NK cells: defect of cDC1 recruitment into the TME by NK cells in human gastric cancer cells ILC1s cells: inhibitors receptors expression at a late stage in CRC patients | [168]/[169] [170]/[171] |
| ILC Group 2 | ILC2s: indirect support by enhancement of DCs and effector T cells in primary and metastatic murine lung | ILC2s: involved in MDSCs immunosuppressive function in human bladder cancer | [172]/[173] |
| ILC Group 3 | ILC3s: contribute to tumor suppression by TLS, cytokines secretion and tumor cells recognition in human NSCLC | ILC3s: contribute to metastasis by secreting IL-17, IL-23 and IL-22 in human CRC | [174]/[174] |
| Immune Cell Type | Anti-Tumor Immunity | Pro-Tumor/Immunosuppressive Immunity | References |
|---|---|---|---|
| B cells | B cells: antibodies production in human lung cancer (anti-MUC1; anti-p53). | B cells: immune complexes in human OSCC Breg cells: disruption of Th1/Th2 balance in human gastric cancer | [371,376]/[370] [370] |
| CD4+ T cells | Th1: IFN-γ production in melanoma patients Th2: IL-10 production in primary human prostate cancer Th9: Granzyme production in human lung cancer Th17: induce apoptosis in human CRC Tfh: organisation of TLS in breast cancer patients Treg cells: only when subjected to conversion (to effector CD4+ T cells) as suggested in metastatic melanoma, gastrointestinal, and ovarian cancer patients | Th1: defective function in both murine and human lung carcinoma Th2: IL-4 production induces EMT in human colon cancer. Th9: tumor metastasis in human lung cancer Th17: angiogenesis in human CRC Tfh: IgA+ cells in mice hepatocellular carcinoma. Treg cells: inhibition of effector T cells in glioblastoma and CRC patients | [382]/[383] [384]/[385] [386]/[387] [388]/[389] [390]/[373] [391]/[392,393] |
| CD8+ T cells | Tc1: cytotoxic activity and high IFN-γ production in melanoma patients Tc17:IL-17A production in oesophageal SCC patients Tc22: through by IL-6 induction in human ovarian cancer γδ T cells: NKp30 cytotoxicity in human acute myeloid leukemia | Tc1: low of IFN-γ, TNF-α and high levels of PD-1, incapacity to control tumor progression in melanoma patients Tc17: angiogenic and immunosuppressive function in human cancers such as HNSCC and gastric cancer Tc22: increase of IL-22 related to tumor growth in transplant-associated SSC patients γδ T cells: angiogenesis by IL-17 production in human gallbladder cancer | [394]/[395] [396]/[397,398] [394]/[399] [400]/[401] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Romero, A.C.; Orenes-Piñero, E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers 2022, 14, 1681. https://doi.org/10.3390/cancers14071681
Peña-Romero AC, Orenes-Piñero E. Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers. 2022; 14(7):1681. https://doi.org/10.3390/cancers14071681
Chicago/Turabian StylePeña-Romero, Alicia Cristina, and Esteban Orenes-Piñero. 2022. "Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers" Cancers 14, no. 7: 1681. https://doi.org/10.3390/cancers14071681
APA StylePeña-Romero, A. C., & Orenes-Piñero, E. (2022). Dual Effect of Immune Cells within Tumour Microenvironment: Pro- and Anti-Tumour Effects and Their Triggers. Cancers, 14(7), 1681. https://doi.org/10.3390/cancers14071681







