Estrogens, Cancer and Immunity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Estrogens: Overview
- -
- Direct genomic pathway binding to ERs in cytoplasm;
- -
- Indirect genomic signaling;
- -
3. Estrogens and Cancer
3.1. Breast Cancer
3.2. Ovarian Cancer
3.3. Prostate Cancer
3.4. Colorectal Carcinoma
3.5. Lung Cancer
3.6. Other Malignancies: Examples
4. Sex Hormones and Immunity
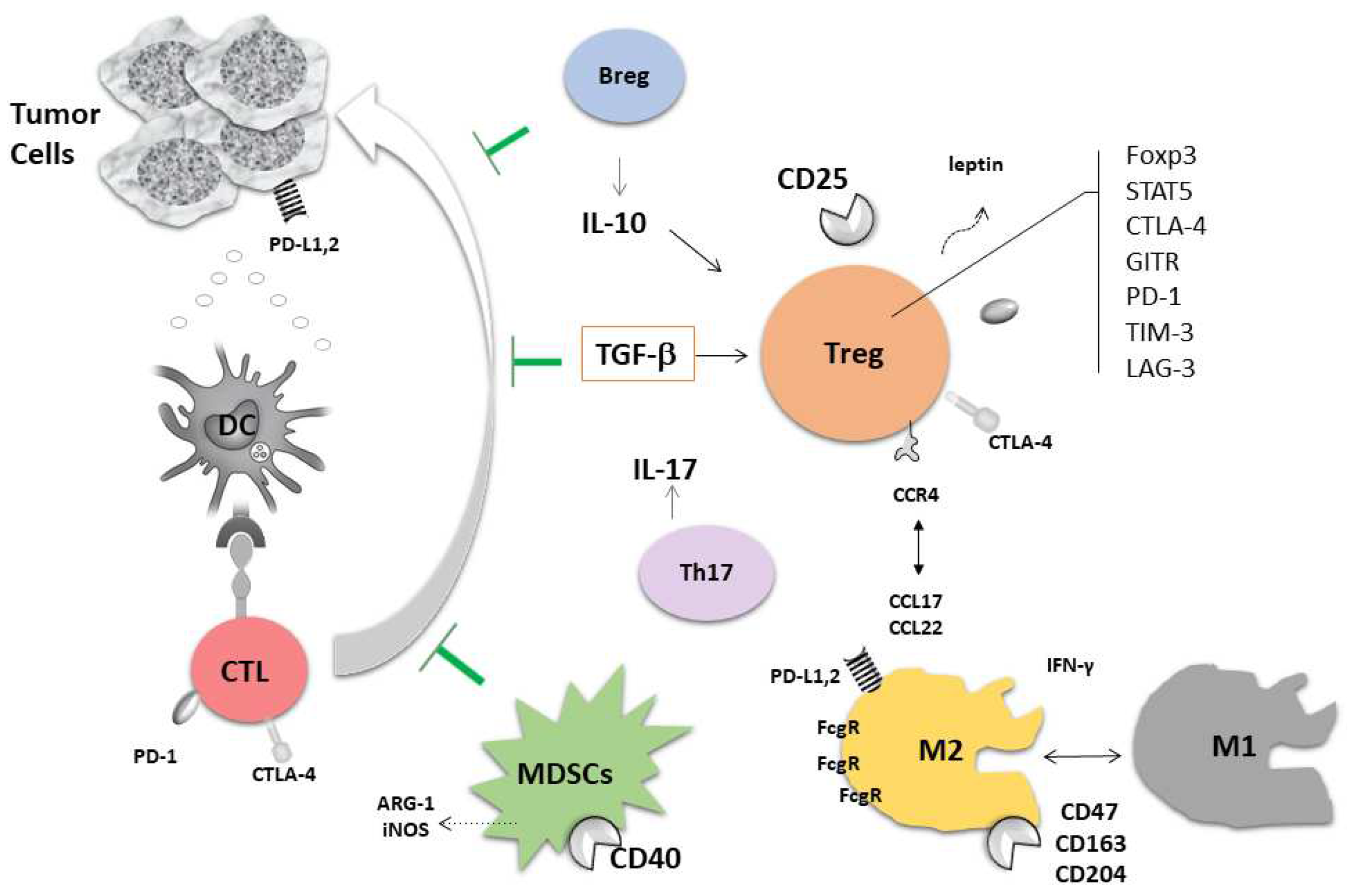
5. Cancer Immunity
6. Sex Hormones and Cancer Immunity: Therapeutic Implications
6.1. Estrogens and TME
- A natural response in women;
- Fluctuation of hormonal balance during life;
- Immunoaging;
- Direct impact of estrogens on immune cells and their progenitors;
- Influence of hormones given in HRT;
- Interplay of cancer immune response and natural hormonal status.
6.2. Breast Cancer and Immunity
6.3. Estrogens and Immunotherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
| Abbreviation | Meaning |
| 17β | hydroxysteroid dehydrogenase type 1 |
| 3β | hydroxysteroid dehydrogenase |
| ARs | androgen receptors |
| ARO | aromatase |
| BALF | bronchoalveolar lavage fluid |
| BMDCs | bone marrow-derived dendritic cells |
| CSCs | cancer stem cells |
| CXCR4 C-X-C chemokine receptor type 4 | |
| CYP17 | 17aα-hydroxylase |
| CYP19A1 | aromatase |
| DCs | dendritic cells |
| DHEA | dehydroepiandrosterone |
| DHEAS | dehydroepiandrosterone sulfate |
| DHT | 5α-dihydrotestosterone |
| E1 | estrone |
| E2 | 17β-estradiol |
| E3 | estriol |
| EGFR | epidermal growth factor receptor |
| EGFR-TKIs | epidermal growth factor receptor-tyrosine kinase inhibitors |
| EMT | epithelial-mesenchymal transition |
| ERs | estrogen receptors |
| ERα, ER1 | estrogen receptor α |
| ERβ, ER2 | estrogen receptor β |
| ESR1 | estrogen receptor alpha gene |
| ESR2 | estrogen receptor beta gene |
| EVs | extracellular vesicles |
| G-CSF | granulocyte colony-stimulating factor |
| GPER1 | G protein-coupled estrogen receptor 1 |
| HDACi | histone deacetylase inhibitors |
| HER2 | human epidermal growth factor receptor 2 |
| HGPIN | high-grade prostatic intraepithelial neoplasia |
| HR+ | hormone-positive |
| HRT | hormonal replacement therapy |
| ICIs | immune checkpoint inhibitors |
| IL | interleukin |
| LAG-3 | lymphocyte activation gene 3 |
| LNs | lymph nodes |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MDSCs | myeloid-derived suppressor cells |
| MMP-2 | matrix metalloproteinase-2 |
| MMP-9 | matrix metalloproteinase-9 |
| MPs | microparticles |
| NO | nitric oxide |
| NSCLC | non-small cell lung carcinoma |
| OS | overall survival |
| P450scc | cytochrome P450 side-chain cleavage enzyme |
| PBMC | peripheral blood mononuclear cell |
| PD-L1 | programmed death ligand 1 |
| SASP | senescence-associated secretory phenotype |
| SCLC | small cell lung carcinoma |
| SHBG | sex hormone-binding protein |
| SNPs | single-nucleotide polymorphism |
| STS | steroid sulfatase |
| TAMs | tumor-associated macrophages |
| TES | testosterone |
| TEXs | tumor-derived exosomes |
| TILs | tumor-infiltrating lymphocytes |
| TIM3 | T-cell immunoglobulin and mucin domain-containing molecule 3 |
| TKIs | tyrosine kinase inhibitors |
| TMB | tumor mutational burden |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TNFα | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
| VISTA | V-domain Ig suppressor of T-cell activation |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J.; Trojnar, A. Lung cancer in women in 21th century. J. Thorac. Dis. 2020, 12, 4398–4410. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.-A. Estrogen Receptors: Their Actions and Functional Roles in Health and Disease; Springer: Dordrecht, The Netherlands, 2010; pp. 4398–4410. [Google Scholar]
- Reyes-Garcia, J.; Montano, L.M.; Carbajal-Garcia, A.; Wang, Y.X. Sex Hormones and Lung Inflammation. Adv. Exp. Med. Biol. 2021, 1304, 259–321. [Google Scholar] [PubMed]
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The necessary evil for human health, and ways to tame it. Biomed. Pharm. 2018, 102, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Maitra, R.; Malik, P.; Mukherjee, T.K. Targeting Estrogens and Various Estrogen-Related Receptors against Non-Small Cell Lung Cancers: A Perspective. Cancers 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Zaucha, R.; Kuban-Jankowska, A.; Konieczna, L.; Belka, M.; Marino Gammazza, A.; Baczek, T.; Cappello, F.; Wozniak, M.; Gorska-Ponikowska, M. Plausible Role of Estrogens in Pathogenesis, Progression and Therapy of Lung Cancer. Int. J. Environ. Res. Public Health 2021, 18, 648. [Google Scholar] [CrossRef] [PubMed]
- Yialamas, M.A.; Hayes, F.J. Androgens and the ageing male and female. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 223–236. [Google Scholar] [CrossRef]
- Bozovic, A.; Mandusic, V.; Todorovic, L.; Krajnovic, M. Estrogen Receptor Beta: The Promising Biomarker and Potential Target in Metastases. Int. J. Mol. Sci. 2021, 22, 1656. [Google Scholar] [CrossRef]
- Herynk, M.H.; Fuqua, S.A. Estrogen receptor mutations in human disease. Endocr. Rev. 2004, 25, 869–898. [Google Scholar] [CrossRef] [Green Version]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Estrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Folkerd, E.J.; Dowsett, M. Influence of sex hormones on cancer progression. J. Clin. Oncol. 2010, 28, 4038–4044. [Google Scholar] [CrossRef]
- Lønning, P.E.; Helle, H.; Duong, N.K.; Ekse, D.; Aas, T.; Geisler, J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J. Steroid. Biochem. Mol. Biol. 2009, 117, 31–41. [Google Scholar] [CrossRef]
- Dunbier, A.K.; Anderson, H.; Ghazoui, Z.; Folkerd, E.J.; A’Hern, R.; Crowder, R.J.; Hoog, J.; Smith, I.E.; Osin, P.; Nerurkar, A.; et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor-positive breast cancer in postmenopausal women. J. Clin. Oncol. 2010, 28, 1161–1167. [Google Scholar] [CrossRef]
- Kim, H.; Abd Elmageed, Z.Y.; Ju, J.; Naura, A.S.; Abdel-Mageed, A.B.; Varughese, S.; Paul, D.; Alahari, S.; Catling, A.; Kim, J.G.; et al. PDZK1 is a novel factor in breast cancer that is indirectly regulated by estrogen through IGF-1R and promotes estrogen-mediated growth. Mol. Med. 2013, 19, 253–262. [Google Scholar] [CrossRef]
- Ghali, R.M.; Al-Mutawa, M.A.; Ebrahim, B.H.; Jrah, H.H.; Zaied, S.; Bhiri, H.; Hmila, F.; Mahjoub, T.; Almawi, W.Y. Progesterone Receptor (PGR) Gene Variants Associated with Breast Cancer and Associated Features: A Case-Control Study. Pathol. Oncol. Res. 2020, 26, 141–147. [Google Scholar] [CrossRef]
- Yi, J.; Ren, L.; Li, D.; Wu, J.; Li, W.; Du, G.; Wang, J. Trefoil factor 1 (TFF1) is a potential prognostic biomarker with functional significance in breast cancers. Biomed. Pharm. 2020, 124, 109827. [Google Scholar] [CrossRef]
- Rae, J.M.; Johnson, M.D.; Scheys, J.O.; Cordero, K.E.; Larios, J.M.; Lippman, M.E. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res. Treat. 2005, 92, 141–149. [Google Scholar] [CrossRef]
- Dunnwald, L.K.; Rossing, M.A.; Li, C.I. Hormone receptor status, tumor characteristics, and prognosis: A prospective cohort of breast cancer patients. Breast Cancer Res. 2007, 9, R6. [Google Scholar] [CrossRef]
- Grann, V.R.; Troxel, A.B.; Zojwalla, N.J.; Jacobson, J.S.; Hershman, D.; Neugut, A.I. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 2005, 103, 2241–2251. [Google Scholar] [CrossRef]
- Hwang, N.M.; Stabile, L.P. Estrogen Receptor ß in Cancer: To ß(e) or not to ß(e)? Endocrinology 2021, 162, bqab162. [Google Scholar] [CrossRef]
- Song, P.; Li, Y.; Dong, Y.; Liang, Y.; Qu, H.; Qi, D.; Lu, Y.; Jin, X.; Guo, Y.; Jia, Y.; et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Tripathy, D. Co-targeting estrogen receptor and HER2 pathways in breast cancer. Breast 2014, 23, 2–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, Q.; Chen, R. Breast Cancer Incidence and Mortality in Relation to Hormone Replacement Therapy Use Among Postmenopausal Women: Results From a Prospective Cohort Study. Clin. Breast Cancer 2022, 22, e206–e213. [Google Scholar] [CrossRef]
- Fournier, A.; Berrino, F.; Clavel-Chapelon, F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008, 107, 103–111. [Google Scholar] [CrossRef]
- Beral, V.; Collaborators, M.W.S. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Dotto, G.P. Sex Hormones and Anticancer Immunity. Clin. Cancer Res. 2019, 25, 4603–4610. [Google Scholar] [CrossRef] [Green Version]
- Azam, S.; Lange, T.; Huynh, S.; Aro, A.R.; von Euler-Chelpin, M.; Vejborg, I.; Tjønneland, A.; Lynge, E.; Andersen, Z.J. Hormone replacement therapy, mammographic density, and breast cancer risk: A cohort study. Cancer Causes Control. 2018, 29, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Lyons, T.G. Targeted Therapies for Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2019, 20, 82. [Google Scholar] [CrossRef]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, S.M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003, 1, 73. [Google Scholar] [CrossRef] [Green Version]
- Galtier-Dereure, F.; Capony, F.; Maudelonde, T.; Rochefort, H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J. Clin. Endocrinol. Metab. 1992, 75, 1497–1502. [Google Scholar]
- Langdon, S.P.; Hirst, G.L.; Miller, E.P.; Hawkins, R.A.; Tesdale, A.L.; Smyth, J.F.; Miller, W.R. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J. Steroid. Biochem. Mol. Biol. 1994, 50, 131–135. [Google Scholar] [CrossRef]
- Brandenberger, A.W.; Tee, M.K.; Jaffe, R.B. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: Down-regulation of ER-beta in neoplastic tissues. J. Clin. Endocrinol. Metab. 1998, 83, 1025–1028. [Google Scholar]
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Muñoz, A.; Mor, G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar]
- Trabert, B.; Coburn, S.B.; Falk, R.T.; Manson, J.E.; Brinton, L.A.; Gass, M.L.; Kuller, L.H.; Rohan, T.E.; Pfeiffer, R.M.; Qi, L.; et al. Circulating estrogens and postmenopausal ovarian and endometrial cancer risk among current hormone users in the Women’s Health Initiative Observational Study. Cancer Causes Control. 2019, 30, 1201–1211. [Google Scholar] [CrossRef]
- Tanha, K.; Mottaghi, A.; Nojomi, M.; Moradi, M.; Rajabzadeh, R.; Lotfi, S.; Janani, L. Investigation on factors associated with ovarian cancer: An umbrella review of systematic review and meta-analyses. J. Ovarian Res. 2021, 14, 153. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.A.; Hasan, M.N.; Akhter, H.; Noor, P.; Islam, R.; Shin, Y.; Rahman, M.D.H.; Gazi, M.S.; Huda, M.N.; et al. Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells 2022, 11, 650. [Google Scholar] [CrossRef]
- Fujimura, M.; Hidaka, T.; Kataoka, K.; Yamakawa, Y.; Akada, S.; Teranishi, A.; Saito, S. Absence of estrogen receptor-alpha expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. Am. J. Surg. Pathol. 2001, 25, 667–672. [Google Scholar] [CrossRef]
- Signoretti, S.; Loda, M. Estrogen receptor beta in prostate cancer: Brake pedal or accelerator? Am. J. Pathol. 2001, 159, 13–16. [Google Scholar] [CrossRef]
- Nelson, A.W.; Tilley, W.D.; Neal, D.E.; Carroll, J.S. Estrogen receptor beta in prostate cancer: Friend or foe? Endocr. Relat. Cancer 2014, 21, T219–T234. [Google Scholar] [CrossRef] [Green Version]
- Bosland, M.C. The role of estrogens in prostate carcinogenesis: A rationale for chemoprevention. Rev. Urol. 2005, 7 (Suppl. S3), S4–S10. [Google Scholar]
- Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Fröhlich, T.; Mai, C.; Bogautdinov, R.P.; Morozkina, S.N.; Shavva, A.G.; Friedrich, O.; Gilbert, D.F.; Tsogoeva, S.B. Synthesis of Tamoxifen-Artemisinin and Estrogen-Artemisinin Hybrids Highly Potent Against Breast and Prostate Cancer. ChemMedChem 2020, 15, 1473–1479. [Google Scholar] [CrossRef]
- Qu, L.G.; Wardan, H.; Davis, I.D.; Pezaro, C.; Sluka, P. Effects of estrogen receptor signaling on prostate cancer carcinogenesis. Transl. Res. 2020, 222, 56–66. [Google Scholar] [CrossRef]
- Lafront, C.; Germain, L.; Weidmann, C.; Audet-Walsh, É. ASystematic Study of the Impact of Estrogens and Selective Estrogen Receptor Modulators on Prostate Cancer Cell Proliferation. Sci. Rep. 2020, 10, 4024. [Google Scholar] [CrossRef] [Green Version]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Tong, D. Selective estrogen receptor modulators contribute to prostate cancer treatment by regulating the tumor immune microenvironment. J. Immunother. Cancer 2022, 10, e002944. [Google Scholar] [CrossRef]
- Maingi, J.W.; Tang, S.; Liu, S.; Ngenya, W.; Bao, E. Targeting estrogen receptors in colorectal cancer. Mol. Biol. Rep. 2020, 47, 4087–4091. [Google Scholar] [CrossRef]
- Looijer-van Langen, M.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G621–G626. [Google Scholar] [CrossRef]
- Pierdominici, M.; Maselli, A.; Varano, B.; Barbati, C.; Cesaro, P.; Spada, C.; Zullo, A.; Lorenzetti, R.; Rosati, M.; Rainaldi, G.; et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 2015, 6, 40443–40451. [Google Scholar] [CrossRef]
- Rudolph, A.; Toth, C.; Hoffmeister, M.; Roth, W.; Herpel, E.; Jansen, L.; Marx, A.; Brenner, H.; Chang-Claude, J. Expression of oestrogen receptor β and prognosis of colorectal cancer. Br. J. Cancer 2012, 107, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Sareddy, G.R.; Vadlamudi, R.K. Cancer therapy using natural ligands that target estrogen receptor beta. Chin. J. Nat. Med. 2015, 13, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Domagala-Kulawik, J. New Frontiers for Molecular Pathology. Front. Med. 2019, 6, 284. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, S.M.; Mazhawidza, W.; Bohn, A.R.; Robinson, K.A.; Mattingly, K.A.; Blankenship, K.A.; Huff, M.O.; McGregor, W.G.; Klinge, C.M. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr. Relat. Cancer 2006, 13, 113–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niikawa, H.; Suzuki, T.; Miki, Y.; Suzuki, S.; Nagasaki, S.; Akahira, J.; Honma, S.; Evans, D.B.; Hayashi, S.; Kondo, T.; et al. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin. Cancer Res. 2008, 14, 4417–4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, W.; Liao, Y.; Chen, J.; Wang, Y.; Meng, Y.; Li, K.; Xiao, H. Upregulation of estrogen receptor beta protein but not mRNA predicts poor prognosis and may be associated with enhanced translation in non-small cell lung cancer: A systematic review and meta-analysis. J. Thorac. Dis. 2021, 13, 4281–4300. [Google Scholar] [CrossRef]
- Stabile, L.P.; Dacic, S.; Land, S.R.; Lenzner, D.E.; Dhir, R.; Acquafondata, M.; Landreneau, R.J.; Grandis, J.R.; Siegfried, J.M. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin. Cancer Res. 2011, 17, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.H.; Chu, N.M.; Kao, S.H. Estrogen, Estrogen Receptor and Lung Cancer. Int. J. Mol. Sci. 2017, 18, 1713. [Google Scholar] [CrossRef]
- Zhang, G.; Yanamala, N.; Lathrop, K.L.; Zhang, L.; Klein-Seetharaman, J.; Srinivas, H. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol. Endocrinol. 2010, 24, 1737–1747. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Xie, Q.; Li, P.; Zhong, X.; Chen, Y. Mitochondrial estrogen receptor β inhibits cell apoptosis via interaction with Bad in a ligand-independent manner. Mol. Cell Biochem. 2015, 401, 71–86. [Google Scholar] [CrossRef]
- Liao, T.L.; Tzeng, C.R.; Yu, C.L.; Wang, Y.P.; Kao, S.H. Estrogen receptor-β in mitochondria: Implications for mitochondrial bioenergetics and tumorigenesis. Ann. N. Y. Acad. Sci. 2015, 1350, 52–60. [Google Scholar] [CrossRef]
- Liu, S.; Hu, C.; Li, M.; An, J.; Zhou, W.; Guo, J.; Xiao, Y. Estrogen receptor beta promotes lung cancer invasion via increasing CXCR4 expression. Cell Death Dis. 2022, 13, 70. [Google Scholar] [CrossRef]
- Fan, S.; Liao, Y.; Liu, C.; Huang, Q.; Liang, H.; Ai, B.; Fu, S.; Zhou, S. Estrogen promotes tumor metastasis via estrogen receptor beta-mediated regulation of matrix-metalloproteinase-2 in non-small cell lung cancer. Oncotarget 2017, 8, 56443–56459. [Google Scholar] [CrossRef]
- Hsu, L.H.; Liu, K.J.; Tsai, M.F.; Wu, C.R.; Feng, A.C.; Chu, N.M.; Kao, S.H. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci. 2015, 106, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Skjefstad, K.; Grindstad, T.; Khanehkenari, M.R.; Richardsen, E.; Donnem, T.; Kilvaer, T.; Andersen, S.; Bremnes, R.M.; Busund, L.T.; Al-Saad, S. Prognostic relevance of estrogen receptor α, β and aromatase expression in non-small cell lung cancer. Steroids 2016, 113, 5–13. [Google Scholar] [CrossRef]
- Mah, V.; Seligson, D.B.; Li, A.; Márquez, D.C.; Wistuba, I.I.; Elshimali, Y.; Fishbein, M.C.; Chia, D.; Pietras, R.J.; Goodglick, L. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007, 67, 10484–10490. [Google Scholar] [CrossRef] [Green Version]
- Mah, V.; Marquez, D.; Alavi, M.; Maresh, E.L.; Zhang, L.; Yoon, N.; Horvath, S.; Bagryanova, L.; Fishbein, M.C.; Chia, D.; et al. Expression levels of estrogen receptor beta in conjunction with aromatase predict survival in non-small cell lung cancer. Lung Cancer 2011, 74, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.S.; Mok, T.S.; Rebbeck, T.R. Cancer Genomics: Diversity and Disparity Across Ethnicity and Geography. J. Clin. Oncol. 2016, 34, 91–101. [Google Scholar] [CrossRef]
- Rodriguez-Lara, V.; Hernandez-Martinez, J.M.; Arrieta, O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J. Thorac. Dis. 2018, 10, 482–497. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, H.; Miki, Y.; Iwabuchi, E.; Saito, R.; Ono, K.; Sato, I.; Okada, Y.; Sasano, H. Estrogen Receptor β Is Involved in Acquired Resistance to EGFR-tyrosine Kinase Inhibitors in Lung Cancer. Anticancer Res. 2021, 41, 2371–2381. [Google Scholar] [CrossRef]
- Schabath, M.B.; Wu, X.; Vassilopoulou-Sellin, R.; Vaporciyan, A.A.; Spitz, M.R. Hormone replacement therapy and lung cancer risk: A case-control analysis. Clin. Cancer Res. 2004, 10, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Titan, A.L.; He, H.; Lui, N.; Liou, D.; Berry, M.; Shrager, J.B.; Backhus, L.M. The influence of hormone replacement therapy on lung cancer incidence and mortality. J. Thorac. Cardiovasc. Surg. 2020, 159, 1546–1556.e4. [Google Scholar] [CrossRef] [Green Version]
- Slatore, C.G.; Chien, J.W.; Au, D.H.; Satia, J.A.; White, E. Lung cancer and hormone replacement therapy: Association in the vitamins and lifestyle study. J. Clin. Oncol. 2010, 28, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Schwartz, A.G.; Wakelee, H.; Anderson, G.L.; Stefanick, M.L.; Manson, J.E.; Rodabough, R.J.; Chien, J.W.; Wactawski-Wende, J.; Gass, M.; et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): A post-hoc analysis of a randomised controlled trial. Lancet 2009, 374, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.M.; Garcia-Lopez, P. Estrogen Receptors as Molecular Targets of Endocrine Therapy for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 2404. [Google Scholar] [CrossRef]
- Yakimchuk, K.; Iravani, M.; Hasni, M.S.; Rhönnstad, P.; Nilsson, S.; Jondal, M.; Okret, S. Effect of ligand-activated estrogen receptor β on lymphoma growth in vitro and in vivo. Leukemia 2011, 25, 1103–1110. [Google Scholar] [CrossRef]
- Yakimchuk, K.; Norin, S.; Kimby, E.; Hägglund, H.; Warner, M.; Gustafsson, J. Up-regulated estrogen receptor β2 in chronic lymphocytic leukemia. Leuk. Lymphoma 2012, 53, 139–144. [Google Scholar] [CrossRef]
- Yakimchuk, K.; Hasni, M.S.; Guan, J.; Chao, M.P.; Sander, B.; Okret, S. Inhibition of lymphoma vascularization and dissemination by estrogen receptor β agonists. Blood 2014, 123, 2054–2061. [Google Scholar] [CrossRef] [Green Version]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol. 2014, 35, 97–104. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Griner, L.M.; Keller, J.M.; Hu, X.; Southall, N.; Marugan, J.; David, J.M.; Ferrer, M.; Palena, C. Targeting Estrogen Receptor Signaling with Fulvestrant Enhances Immune and Chemotherapy-Mediated Cytotoxicity of Human Lung Cancer. Clin. Cancer Res. 2016, 22, 6204–6216. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Bupp, M.R.G.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Porter, V.R.; Greendale, G.A.; Schocken, M.; Zhu, X.; Effros, R.B. Immune effects of hormone replacement therapy in post-menopausal women. Exp. Gerontol. 2001, 36, 311–326. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ’Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Mourlanette, J.; Gouin, S.; Dormoy, I.; Favre, G.; et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Inoue, Y.; Karayama, M.; Tsuchiya, K.; Mori, K.; Suzuki, Y.; Iwashita, Y.; Kahyo, T.; Kawase, A.; Tanahashi, M.; et al. Heterogeneity analysis of PD-L1 expression and copy number status in EBUS-TBNA biopsy specimens of non-small cell lung cancer: Comparative assessment of primary and metastatic sites. Lung Cancer 2019, 134, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.G.; Hegmans, J.P. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013, 73, 2381–2388. [Google Scholar] [CrossRef] [Green Version]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef] [Green Version]
- Domagala-Kulawik, J.; Osinska, I.; Hoser, G. Mechanisms of immune response regulation in lung cancer. Transl. Lung Cancer Res. 2014, 3, 15–22. [Google Scholar]
- Domagala-Kulawik, J. The relevance of bronchoalveolar lavage fluid analysis for lung cancer patients. Expert Rev. Respir. Med. 2020, 14, 329–337. [Google Scholar] [CrossRef]
- Raniszewska, A.; Vroman, H.; Dumoulin, D.; Cornelissen, R.; Aerts, J.; Domagala-Kulawik, J. PD-L1(+) lung cancer stem cells modify the metastatic lymph-node immunomicroenvironment in nsclc patients. Cancer Immunol. Immunother. 2021, 70, 453–461. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The "cancer immunogram". Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Chamoto, K.; Al-Habsi, M.; Honjo, T. Role of PD-1 in Immunity and Diseases. Curr. Top. Microbiol. Immunol. 2017, 410, 75–97. [Google Scholar]
- Whiteside, T.L. The Role of Tumor-Derived Exosomes (TEX) in Shaping Anti-Tumor Immune Competence. Cells 2021, 10, 3054. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Ock, C.Y.; Keam, B.; Kim, S.; Lee, J.S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef]
- Mazzarella, L.; Duso, B.A.; Trapani, D.; Belli, C.; D’Amico, P.; Ferraro, E.; Viale, G.; Curigliano, G. The evolving landscape of ’next-generation’ immune checkpoint inhibitors: A review. Eur. J. Cancer 2019, 117, 14–31. [Google Scholar] [CrossRef]
- Matarrese, P.; Mattia, G.; Pagano, M.T.; Pontecorvi, G.; Ortona, E.; Malorni, W.; Carè, A. The Sex-Related Interplay between TME and Cancer: On the Critical Role of Estrogen, MicroRNAs and Autophagy. Cancers 2021, 13, 3287. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef] [Green Version]
- Kwiecień, I.; Rutkowska, E.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Modulation of the immune response by heterogeneous monocytes and dendritic cells in lung cancer. World J. Clin. Oncol. 2021, 12, 966–982. [Google Scholar] [CrossRef]
- Laffont, S.; Seillet, C.; Guéry, J.C. Estrogen Receptor-Dependent Regulation of Dendritic Cell Development and Function. Front. Immunol. 2017, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3(+) Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Keselman, A.; Fang, X.; White, P.B.; Heller, N.M. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization during Asthma. J. Immunol. 2017, 199, 1573–1583. [Google Scholar] [CrossRef]
- He, M.; Yu, W.; Chang, C.; Miyamoto, H.; Liu, X.; Jiang, K.; Yeh, S. Estrogen receptor α promotes lung cancer cell invasion via increase of and cross-talk with infiltrated macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling pathways. Mol. Oncol. 2020, 14, 1779–1799. [Google Scholar] [CrossRef]
- Siegfried, J.M.; Stabile, L.P. Estrongenic steroid hormones in lung cancer. Semin. Oncol. 2014, 41, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Stabile, L.P.; Farooqui, M.; Kanterewicz, B.; Abberbock, S.; Kurland, B.F.; Diergaarde, B.; Siegfried, J.M. Preclinical Evidence for Combined Use of Aromatase Inhibitors and NSAIDs as Preventive Agents of Tobacco-Induced Lung Cancer. J. Thorac. Oncol. 2018, 13, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [Green Version]
- Smida, T.; Bruno, T.C.; Stabile, L.P. Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front. Oncol. 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hühn, D.; Martí-Rodrigo, P.; Mouron, S.; Hansel, C.; Tschapalda, K.; Porebski, B.; Häggblad, M.; Lidemalm, L.; Quintela-Fandino, M.; Carreras-Puigvert, J.; et al. Prolonged estrogen deprivation triggers a broad immunosuppressive phenotype in breast cancer cells. Mol. Oncol. 2022, 16, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, R.K.; Hahn, B.H.; Singh, R.P. PD-1, gender, and autoimmunity. Autoimmun. Rev. 2010, 9, 583–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.K.; Ye, F.; Wu, X.; An, H.X.; Wu, J.X. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: A meta-analysis. J. Thorac. Dis. 2015, 7, 462–470. [Google Scholar]
- Raniszewska, A.; Kwiecien, I.; Sokolowski, R.; Rutkowska, E.; Domagala-Kulawik, J. Immunomodulatory Molecules On Lung Cancer Stem Cells From Lymph Nodes Aspirates. Cancers 2020, 12, 838. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Cellular senescence: Putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, J.; Pastorello, R.G.; Vallius, T.; Davis, J.; Cui, Y.X.; Agudo, J.; Waks, A.G.; Keenan, T.; McAllister, S.S.; Tolaney, S.M.; et al. The Immunology of Hormone Receptor Positive Breast Cancer. Front. Immunol. 2021, 12, 674192. [Google Scholar] [CrossRef]
- Terranova-Barberio, M.; Pawlowska, N.; Dhawan, M.; Moasser, M.; Chien, A.J.; Melisko, M.E.; Rugo, H.; Rahimi, R.; Deal, T.; Daud, A.; et al. Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat. Commun. 2020, 11, 3584. [Google Scholar] [CrossRef]
- Dannenfelser, R.; Nome, M.; Tahiri, A.; Ursini-Siegel, J.; Vollan, H.K.M.; Haakensen, V.D.; Helland, Å.; Naume, B.; Caldas, C.; Børresen-Dale, A.L.; et al. Data-driven analysis of immune infiltrate in a large cohort of breast cancer and its association with disease progression, ER activity, and genomic complexity. Oncotarget 2017, 8, 57121–57133. [Google Scholar] [CrossRef] [Green Version]
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452. [Google Scholar] [CrossRef]
- Terranova-Barberio, M.; Thomas, S.; Ali, N.; Pawlowska, N.; Park, J.; Krings, G.; Rosenblum, M.D.; Budillon, A.; Munster, P.N. HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget 2017, 8, 114156–114172. [Google Scholar] [CrossRef] [Green Version]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef]
- Emens, L.A.; Adams, S.; Cimino-Mathews, A.; Disis, M.L.; Gatti-Mays, M.E.; Ho, A.Y.; Kalinsky, K.; McArthur, H.L.; Mittendorf, E.A.; Nanda, R.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J. Immunother. Cancer 2021, 9, e002597. [Google Scholar] [CrossRef]
- Stovgaard, E.S.; Nielsen, D.; Hogdall, E.; Balslev, E. Triple negative breast cancer-prognostic role of immune-related factors: A systematic review. Acta Oncol. 2018, 57, 74–82. [Google Scholar] [CrossRef]
- Pinto, J.A.; Vallejos, C.S.; Raez, L.E.; Mas, L.A.; Ruiz, R.; Torres-Roman, J.S.; Morante, Z.; Araujo, J.M.; Gómez, H.L.; Aguilar, A.; et al. Gender and outcomes in non-small cell lung cancer: An old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018, 3, e000344. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [Green Version]
- Burcher, K.M.; Lantz, J.W.; Gavrila, E.; Abreu, A.; Burcher, J.T.; Faucheux, A.T.; Xie, A.; Jackson, C.; Song, A.H.; Hughes, R.T.; et al. Relationship between Tumor Mutational Burden, PD-L1, Patient Characteristics, and Response to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5733. [Google Scholar] [CrossRef]
- Li, W.; Tse, L.A.; Wang, F. Prognostic value of estrogen receptors mRNA expression in non-small cell lung cancer: A systematic review and meta-analysis. Steroids 2015, 104, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xin, B.; Pang, H.; Han, L.; Shen, W.; Zhao, Z.; Duan, L.; Cao, P.; Liu, L.; Zhang, H. Downregulation of estrogen receptor β inhibits lung adenocarcinoma cell growth. Oncol. Rep. 2019, 41, 2967–2974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Che, J.; Jiang, M.; Cui, M.; Feng, G.; Dong, J.; Zhang, S.; Lu, L.; Liu, W.; Fan, S. CLPTM1L induces estrogen receptor β signaling-mediated radioresistance in non-small cell lung cancer cells. Cell Commun. Signal. 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Dou, L.; Li, Y.; Deng, L.; Wei, X.; Guo, Y. Roles of ERα and ERβ in estrogen-induced DDP chemoresistance in non-small cell lung cancer. Genet. Mol. Res. 2016, 15, 8995. [Google Scholar] [CrossRef]
- Fu, S.; Liu, C.; Huang, Q.; Fan, S.; Tang, H.; Fu, X.; Ai, B.; Liao, Y.; Chu, Q. Estrogen receptor β1 activation accelerates resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in non-small cell lung cancer. Oncol. Rep. 2018, 39, 1313–1321. [Google Scholar] [CrossRef] [Green Version]
- Rades, D.; Setter, C.; Dahl, O.; Schild, S.E.; Noack, F. The prognostic impact of tumor cell expression of estrogen receptor-α, progesterone receptor, and androgen receptor in patients irradiated for nonsmall cell lung cancer. Cancer 2012, 118, 157–163. [Google Scholar] [CrossRef]
- Olivo-Marston, S.E.; Mechanic, L.E.; Mollerup, S.; Bowman, E.D.; Remaley, A.T.; Forman, M.R.; Skaug, V.; Zheng, Y.L.; Haugen, A.; Harris, C.C. Serum estrogen and tumor-positive estrogen receptor-alpha are strong prognostic classifiers of non-small-cell lung cancer survival in both men and women. Carcinogenesis 2010, 31, 1778–1786. [Google Scholar] [CrossRef] [Green Version]
- Lund-Iversen, M.; Scott, H.; Strøm, E.H.; Theiss, N.; Brustugun, O.T.; Grønberg, B.H. Expression of Estrogen Receptor-α and Survival in Advanced-stage Non-small Cell Lung Cancer. Anticancer. Res. 2018, 38, 2261–2269. [Google Scholar]
- Cheng, L.C.; Lin, C.J.; Chen, P.Y.; Li, L.A. ERα-dependent estrogen-TNFα signaling crosstalk increases cisplatin tolerance and migration of lung adenocarcinoma cells. Biochim. Biophys. Acta Gene Regul. Mech. 2021, 1864, 194715. [Google Scholar] [CrossRef]
- Kadota, K.; Eguchi, T.; Villena-Vargas, J.; Woo, K.M.; Sima, C.S.; Jones, D.R.; Travis, W.D.; Adusumilli, P.S. Nuclear estrogen receptor-α expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 2015, 6, 27505–27518. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Vayalil, J.; Lee, G.; Wang, Y.; Peng, G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J. Immunother. Cancer 2021, 9, e003217. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes in Cancer: Another Mechanism of Tumor-Induced Immune Suppression. Adv. Exp. Med. Biol. 2017, 1036, 81–89. [Google Scholar]
- Xing, C.; Li, H.; Li, R.J.; Yin, L.; Zhang, H.F.; Huang, Z.N.; Cheng, Z.; Li, J.; Wang, Z.H.; Peng, H.L. The roles of exosomal immune checkpoint proteins in tumors. Mil. Med. Res. 2021, 8, 56. [Google Scholar] [CrossRef]
- Czystowska-Kuzmicz, M.; Sosnowska, A.; Nowis, D.; Ramji, K.; Szajnik, M.; Chlebowska-Tuz, J.; Wolinska, E.; Gaj, P.; Grazul, M.; Pilch, Z.; et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019, 10, 3000. [Google Scholar] [CrossRef]
- Garon, E.B.; Siegfried, J.M.; Stabile, L.P.; Young, P.A.; Marquez-Garban, D.C.; Park, D.J.; Patel, R.; Hu, E.H.; Sadeghi, S.; Parikh, R.J.; et al. Randomized phase II study of fulvestrant and erlotinib compared with erlotinib alone in patients with advanced or metastatic non-small cell lung cancer. Lung Cancer 2018, 123, 91–98. [Google Scholar] [CrossRef]




| Targeted Cells | Influence of Estrogens | Influence of Progesterone | Influence of Androgens | References |
|---|---|---|---|---|
| Neutrophils | Promote neutrophil infiltration: number, degranulation, elastase release Significantly higher expression of neutrophil-attracting chemokines (CCL20, CXCL5 AND CXCL2) in females | Not defined | Increase in numbers Lower function Initiation of neutrophil production via modulation of G-CSF Suppression of superoxide production and antimicrobial capacity of neutrophils Genetic ablation of ARO diminishes (90%) the proliferative activity of neutrophils and retards their maturation | [4,89] |
| Monocytes | Increase in the number of chemoattractants for monocytes | Not defined | Negative regulation of monocyte levels Lower monocyte infiltration Decrease in IL-6 and TNF-α production | [4] |
| Macrophages | Promote phagocytic activity Facilitate resolution phase of inflammation toward M2 phenotype dependent on IL-10 Contribution to tissue remodeling and shortening of proinflammatory state Enhance cytokine secretion Increase expression of TLR4, TLR7 and TLR9 | Inhibition of NO production and the release of MPs with proinflammatory and prothrombic properties | Decreased expression of TLR4 Reduced production of IL-1β, IL-6 and TNF-α increases the production of IL-10 TES can diminish NO production induced by the stimulus of LPS | [4,89] |
| Eosinophils | Lower numbers and mobilization | Progesterone treatment enhances recruitment of eosinophils and induces airway hyperresponsiveness | TES decreases human eosinophil viability and adhesion properties in vitro | [4] |
| Mast Cells | Serum levels of IgE fluctuate depending on the menstrual cycle phase Estrogen enhances IgE-induced mast cell degranulation and release of histamine | Progesterone diminishes the migration of mast cells and histamine secretion | TES interferes with the production of IL-6 and induces the expression of IL-33 | [4] |
| DCs | Promotes differentiation of DCs from bone marrow precursors and enhances their T-cell stimulatory capacity Activation of antigen presentation | Decrease in the production of proinflammatory cytokines TNF-α and IL-1β by BMDCs | Not defined In vitro, 5α-DHT decreases the production of IL-4, IL-10 and IL-13 | [86] |
| Lymphocytes | ||||
| B | Restrain B-cell lymphopoiesis Enhance activity and antibody production of mature B cells | Androgens negatively regulate B-cell development | [4] | |
| T | Elevated CD4:CD8 ratio High CD8 response Physiological concentrations of E2 promote proliferation of T lymphocytes and production of IFN-γ in vitro
| Th2 > Th1 Low CD8 response | Androgens negatively regulate T-cell development Low CD8 activity Direction to Th-17 differentiation In vitro: reduction in TNF-α secretion, stimulates production of IL-10 In rat models: androgen deficiency decreases levels of IL-2, IL-6, IL-10, IL-12 and IL-13, whereas TES supplementation restores those levels | [4,29,87,89] |
| NK | High INFγ and granzyme B production | Increased numbers, apoptosis | Not defined | [89] |
| Cytokines | ↑IL-4, IL-10, TGFβ ↓IL-17
| ↓TNF, IFNγ ↑IL-6 ↑IL-4, IL-5, TGFβ | ↓IL-4,TGFβ, IL-10 ↑IL-17 | [4,89] [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. https://doi.org/10.3390/cancers14092265
Orzołek I, Sobieraj J, Domagała-Kulawik J. Estrogens, Cancer and Immunity. Cancers. 2022; 14(9):2265. https://doi.org/10.3390/cancers14092265
Chicago/Turabian StyleOrzołek, Izabela, Jan Sobieraj, and Joanna Domagała-Kulawik. 2022. "Estrogens, Cancer and Immunity" Cancers 14, no. 9: 2265. https://doi.org/10.3390/cancers14092265
APA StyleOrzołek, I., Sobieraj, J., & Domagała-Kulawik, J. (2022). Estrogens, Cancer and Immunity. Cancers, 14(9), 2265. https://doi.org/10.3390/cancers14092265






