Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Hypoxia Is Caused by Imbalance between Oxygen Delivery and Oxygen Consumption Rate
- hypoxia,
- perfusion,
- metabolism and oxygen consumption rate and
- necrosis.
3. Challenges to Relating Temperatures Achieved during HT with Physiologic Response
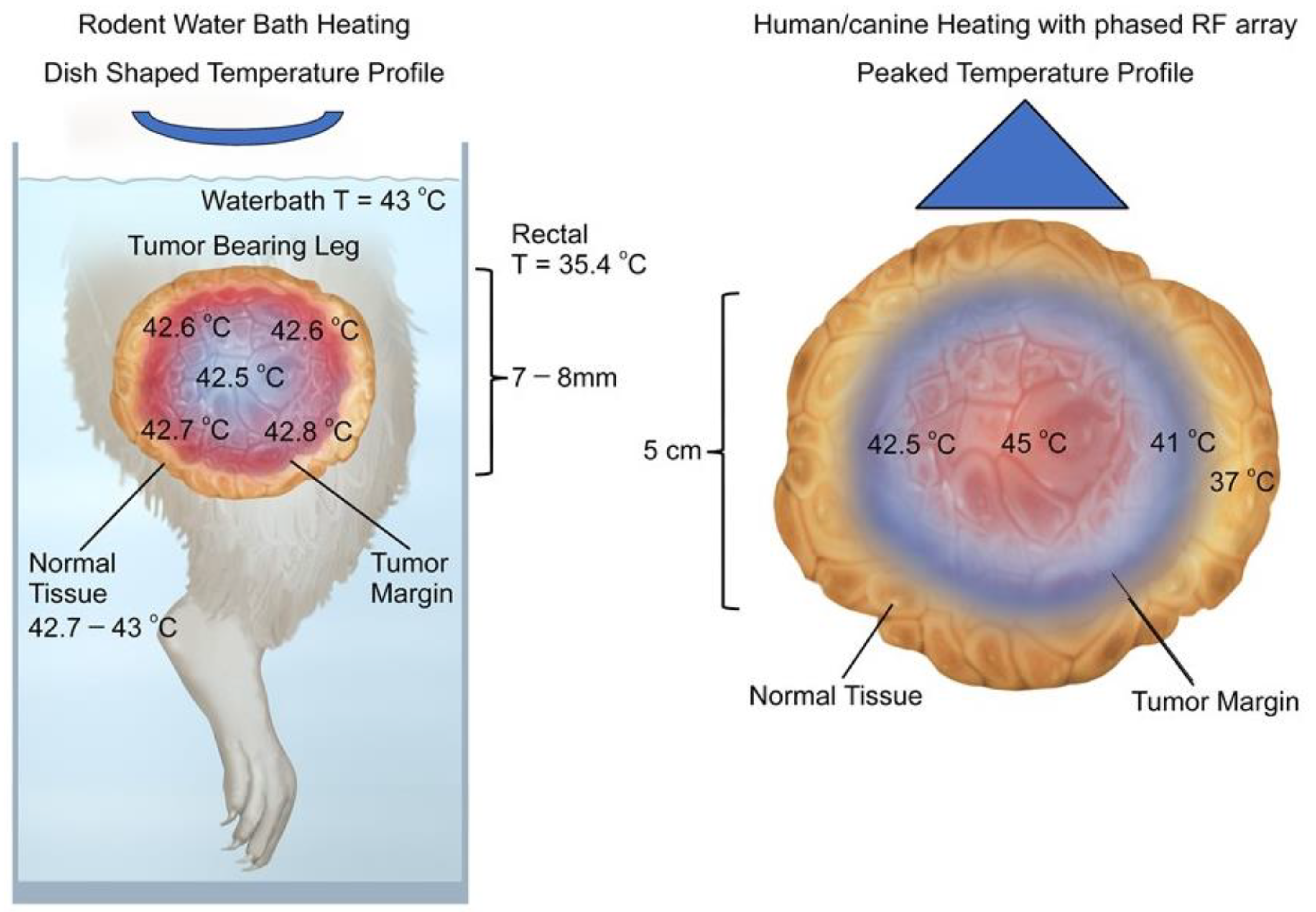
3.1. Difference in Temperature Distributions between Rodent and Human Tumors
3.2. Thermometry in Human Tumors Is Mainly Acquired from Implanted Thermal Probes
4. Effects of Hyperthermia on Tumor Metabolism
4.1. Switch to Anaerobic Metabolism after Hyperthermia Treatment
4.2. Direct Cytotoxicity of HT
5. Effects of Hyperthermia on Tumor Perfusion and Hypoxia
5.1. Physiologic Effects during or Immediately after Heating
5.2. Physiologic Effects Occurring after Heating
5.3. Human Studies of Reoxygenation Post HT
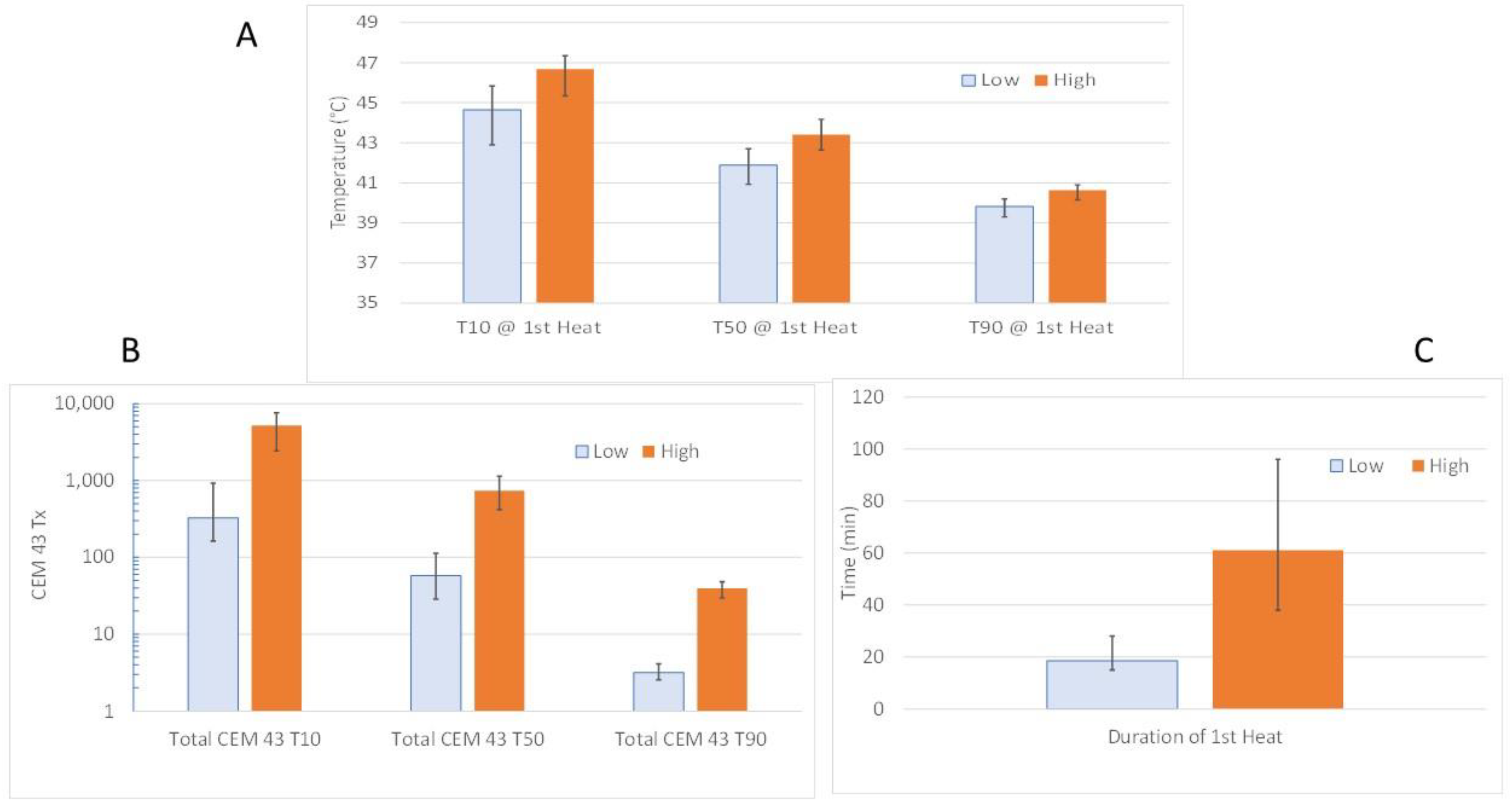
5.4. Canine Studies of Reoxygenation Post HT
- Higher CEM43T10 was associated with an improvement in average pO2 (p = 0.0214) and reduction in HF (% points < 10 mmHg; p = 0.0451), 24 h after the first HT.
- There was a significant positive correlation between CEM43T90 and perfusion at 24 h post first hyperthermia fraction.
- Increases in average pO2 and perfusion at 24 h after the first HT were correlated with tumor volume reduction at the end of treatment.
- Higher Total CEM43T10 and Total CEM43T50 were associated with change in ADC at the end of treatment (p = 0.007 and p = 0.0007, respectively), but the trends were different for the 5Fx HT vs. 20Fx HT groups. Reduction in ADC is associated with lower diffusion coefficient of water, which can be interpreted as a relative decrease in water mobility. It has been reported that early onset of apoptosis or apoptosis mixed with necrosis is associated with increased ADC [113,114]. However, in situations where there is necrosis in the absence of apoptosis, chronic necrosis or fibrosis, ADC tends to decrease [115,116]. The increase in ADC associated with relatively high CEM43T10 and -T50 in the 20Fx HT group is consistent with the notion that higher cumulative thermal doses cause cell killing and increased edema. Extensive cell death could reduce oxygen consumption rate across a tumor, thereby contributing to improved oxygenation.
- Higher CEM43T10 and -T50 were significantly negatively correlated with greater tumor volume reduction at the end of therapy.

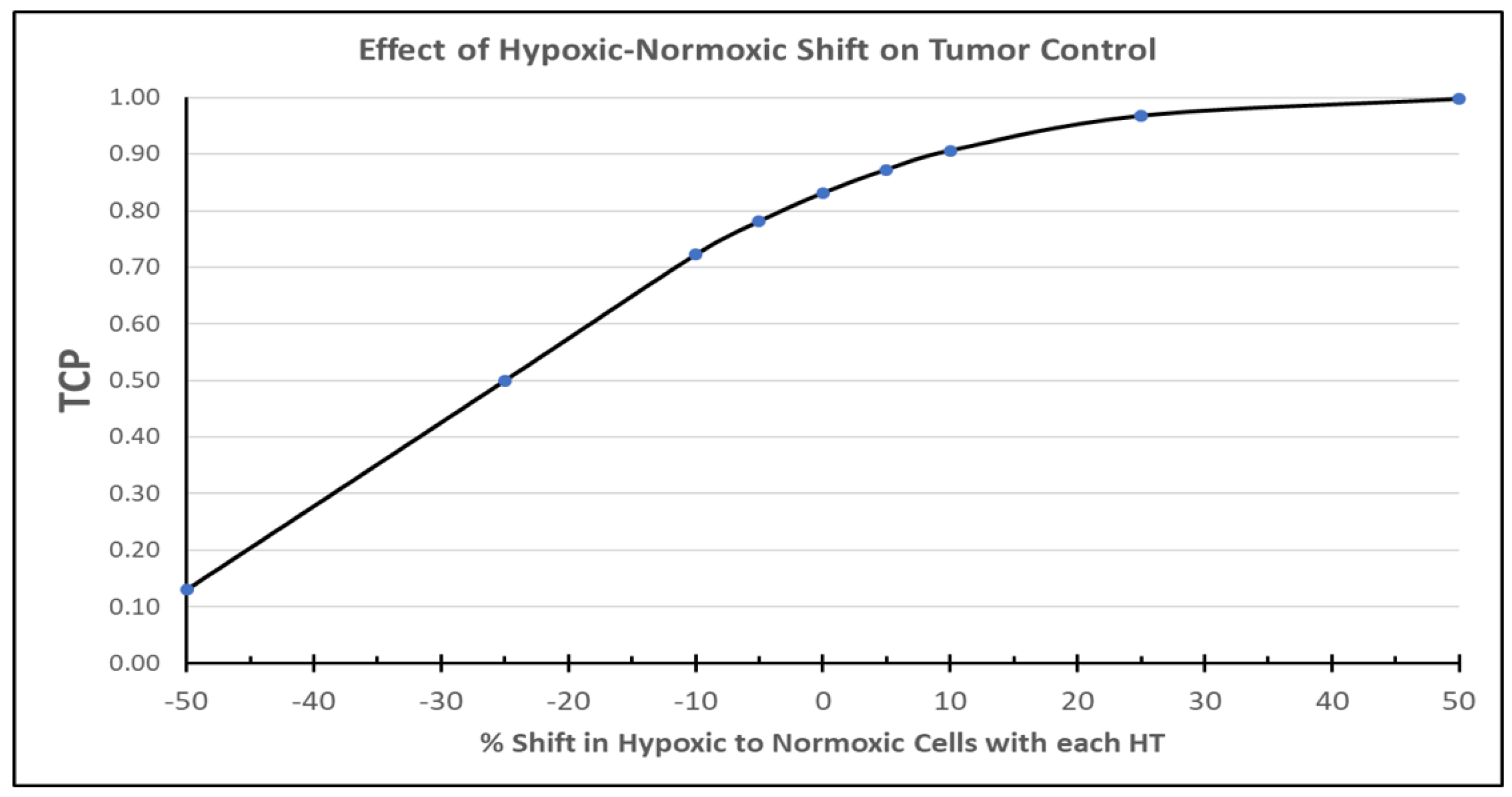
6. A Look Backward and Future Directions
7. Returning Back to Differences in Temperature Distributions between Rodent and Human Tumors
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, N.R.; Bodis, S. Hyperthermia with radiotherapy reduces tumour alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone. Radiother. Oncol. 2019, 138, 1–8. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. 2015, 94, 1073–1087. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.-C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.-M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Perez, C.A.; Pajak, T.; Emami, B.; Hornback, N.B.; Tupchong, L.; Rubin, P. Randomized Phase III Study Comparing Irradiation and Hyperthermia with Irradiation Alone in Superficial Measurable Tumors. Am. J. Clin. Oncol. 1991, 14, 133–141. [Google Scholar] [CrossRef]
- Harima, Y.; Ohguri, T.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int. J. Hyperth. 2016, 32, 801–808. [Google Scholar] [CrossRef]
- Vasanthan, A.; Mitsumori, M.; Park, J.H.; Zhi-Fan, Z.; Yu-Bin, Z.; Oliynychenko, P.; Tatsuzaki, H.; Tanaka, Y.; Hiraoka, M. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: A multi-institutional prospective randomized trial of the international atomic energy agency. Int. J. Radiat. Oncol. 2005, 61, 145–153. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Mendez, F.; Sandigursky, M.; Franklin, W.A.; Kenny, M.K.; Kureekattil, R.; Bases, R. Heat-Shock Proteins Associated with Base Excision Repair Enzymes in HeLa Cells. Radiat. Res. 2000, 153, 186–195. [Google Scholar] [CrossRef]
- Takahashi, A.; Yamakawa, N.; Mori, E.; Ohnishi, K.; Yokota, S.-I.; Sugo, N.; Aratani, Y.; Koyama, H.; Ohnishi, T. Development of thermotolerance requires interaction between polymerase-β and heat shock proteins. Cancer Sci. 2008, 99, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Raaphorst, G.P.; Yang, D.P.; Bussey, A.; Ng, C.E. Cell killing, DNA polymerase inactivation and radiosensitization to low dose rate irradiation by mild hyperthermia in four human cell lines. Int. J. Hyperth. 1995, 11, 841–854. [Google Scholar] [CrossRef] [PubMed]
- Stege, G.; Kampinga, H.; Konings, A. Heat-induced Intranuclear Protein Aggregation and Thermal Radiosensitization. Int. J. Radiat. Biol. 1995, 67, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.M.; Oei, A.L.; Chin, K.W.T.K.; Crezee, J.; Bel, A.; Westermann, A.M.; Buist, M.R.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. A short time interval between radiotherapy and hyperthermia reduces in-field recurrence and mortality in women with advanced cervical cancer. Radiat. Oncol. 2017, 12, 75. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; Van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; Van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. The Effect of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in 400 Locally Advanced Cervical Carcinoma Patients. Front. Oncol. 2019, 9, 134. [Google Scholar] [CrossRef]
- Kroesen, M.; Mulder, H.T.; Van Rhoon, G.C.; Franckena, M. Commentary: The Impact of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in Patients With Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 1387. [Google Scholar] [CrossRef] [Green Version]
- Crezee, J.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. Response: Commentary: The Impact of the Time Interval Between Radiation and Hyperthermia on Clinical Outcome in Patients With Locally Advanced Cervical Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Ademaj, A.; Veltsista, D.P.; Ghadjar, P.; Marder, D.; Oberacker, E.; Ott, O.J.; Wust, P.; Puric, E.; Hälg, R.A.; Rogers, S.; et al. Clinical Evidence for Thermometric Parameters to Guide Hyperthermia Treatment. Cancers 2022, 14, 625. [Google Scholar] [CrossRef]
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunol. Res. 2013, 1, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Song, C.W. Physiological mechanisms underlying heat-induced radiosensitization. Int. J. Hyperth. 2004, 20, 163–174. [Google Scholar] [CrossRef]
- Vaupel, P. Tumor Hypoxia: Causative Factors, Compensatory Mechanisms, and Cellular Response. Oncol. 2004, 9, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef]
- Overgaard, J.; Horsman, M.R. Horsman Modification of Hypoxia-Induced Radioresistance in Tumors by the Use of Oxygen and Sensitizers. In Seminars in radiation oncology; WB Saunders: Philadelphia, PA, USA, 1996; Volume 6, pp. 10–21. [Google Scholar] [CrossRef]
- Overgaard, J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – A systematic review and meta-analysis. Radiother. Oncol. 2011, 100, 22–32. [Google Scholar] [CrossRef]
- Minassian, L.M.; Cotechini, T.; Huitema, E.; Graham, C.H. Hypoxia-Induced Resistance to Chemotherapy in Cancer. In Hypoxia and Cancer Metastasis; Gilkes, D.M., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1136, pp. 123–139. [Google Scholar]
- Zhang, X.; Ashcraft, K.A.; Warner, A.B.; Nair, S.K.; Dewhirst, M.W. Can Exercise-Induced Modulation of the Tumor Physiologic Microenvironment Improve Antitumor Immunity? Cancer Res. 2019, 79, 2447–2456. [Google Scholar] [CrossRef] [Green Version]
- Brizel, D.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Bean, J.M.; Prosnitz, L.R.; Dewhirst, M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996, 56. [Google Scholar]
- Chan, D.A.; Giaccia, A.J. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007, 26, 333–339. [Google Scholar] [CrossRef]
- Hockel, M.; Schlenger, K.; Aral, B.; Mitze, M.; Schaffer, U.; Vaupel, P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996, 56. [Google Scholar]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar]
- Rich, L.; Damasco, J.; Bulmahn, J.; Kutscher, H.; Prasad, P.; Seshadri, M. Photoacoustic and Magnetic Resonance Imaging of Hybrid Manganese Dioxide-Coated Ultra-small NaGdF4 Nanoparticles for Spatiotemporal Modulation of Hypoxia in Head and Neck Cancer. Cancers 2020, 12, 3294. [Google Scholar] [CrossRef]
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers 2020, 13, 23. [Google Scholar] [CrossRef]
- Frost, J.; Frost, M.; Batie, M.; Jiang, H.; Rocha, S. Roles of HIF and 2-Oxoglutarate-Dependent Dioxygenases in Controlling Gene Expression in Hypoxia. Cancers 2021, 13, 350. [Google Scholar] [CrossRef]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef]
- Benyahia, Z.; Blackman, M.; Hamelin, L.; Zampieri, L.; Capeloa, T.; Bedin, M.; Vazeille, T.; Schakman, O.; Sonveaux, P. In Vitro and In Vivo Characterization of MCT1 Inhibitor AZD3965 Confirms Preclinical Safety Compatible with Breast Cancer Treatment. Cancers 2021, 13, 569. [Google Scholar] [CrossRef]
- Cheung, S.; Jain, P.; So, J.; Shahidi, S.; Chung, S.; Koritzinsky, M. p38 MAPK Inhibition Mitigates Hypoxia-Induced AR Signaling in Castration-Resistant Prostate Cancer. Cancers 2021, 13, 831. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102. [Google Scholar] [CrossRef]
- Benej, M.; Wu, J.; Kreamer, M.; Kery, M.; Corrales-Guerrero, S.; Papandreou, I.; Williams, T.; Li, Z.; Graves, E.; Selmic, L.; et al. Pharmacological Regulation of Tumor Hypoxia in Model Murine Tumors and Spontaneous Canine Tumors. Cancers 2021, 13, 1696. [Google Scholar] [CrossRef]
- Xu, J.; Yu, T.; Zois, C.; Cheng, J.-X.; Tang, Y.; Harris, A.; Huang, W. Unveiling Cancer Metabolism through Spontaneous and Coherent Raman Spectroscopy and Stable Isotope Probing. Cancers 2021, 13, 1718. [Google Scholar] [CrossRef]
- Elming, P.; Wittenborn, T.; Busk, M.; Sørensen, B.; Thomsen, M.; Strandgaard, T.; Dyrskjøt, L.; Nielsen, S.; Horsman, M. Refinement of an Established Procedure and Its Application for Identification of Hypoxia in Prostate Cancer Xenografts. Cancers 2021, 13, 2602. [Google Scholar] [CrossRef]
- Uva, P.; Bosco, M.; Eva, A.; Conte, M.; Garaventa, A.; Amoroso, L.; Cangelosi, D. Connectivity Map Analysis Indicates PI3K/Akt/mTOR Inhibitors as Potential Anti-Hypoxia Drugs in Neuroblastoma. Cancers 2021, 13, 2809. [Google Scholar] [CrossRef]
- Zhang, Y.; Coleman, M.; Brekken, R. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Ancel, J.; Perotin, J.-M.; Dewolf, M.; Launois, C.; Mulette, P.; Nawrocki-Raby, B.; Dalstein, V.; Gilles, C.; Deslée, G.; Polette, M.; et al. Hypoxia in Lung Cancer Management: A Translational Approach. Cancers 2021, 13, 3421. [Google Scholar] [CrossRef]
- Birindelli, G.; Drobnjakovic, M.; Morath, V.; Steiger, K.; D’Alessandria, C.; Gourni, E.; Afshar-Oromieh, A.; Weber, W.; Rominger, A.; Eiber, M.; et al. Is Hypoxia a Factor Influencing PSMA-Directed Radioligand Therapy?—An In Silico Study on the Role of Chronic Hypoxia in Prostate Cancer. Cancers 2021, 13, 3429. [Google Scholar] [CrossRef]
- Carles, M.; Fechter, T.; Grosu, A.; Sörensen, A.; Thomann, B.; Stoian, R.; Wiedenmann, N.; Rühle, A.; Zamboglou, C.; Ruf, J.; et al. 18F-FMISO-PET Hypoxia Monitoring for Head-and-Neck Cancer Patients: Radiomics Analyses Predict the Outcome of Chemo-Radiotherapy. Cancers 2021, 13, 3449. [Google Scholar] [CrossRef]
- Song, C.W.; Shakil, A.; Osborn, J.L.; Iwata, K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 1996, 12, 367–373. [Google Scholar] [CrossRef]
- Vaupel, P.; Horsman, M.R. Tumour perfusion and associated physiology: Characterization and significance for hyperthermia. Int. J. Hyperth. 2010, 26, 209–210. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P.; Mueller-Klieser, W.; Ott, J.; Manz, R. Impact of various thermal doses on the oxygenation and blood flow in malignant tumors upon localized hyperthermia. In Oxygen Transport to Tissue; Lubbers, D.W., Acker, H., Leniger-Follert, E., Goldstick, T.K., Eds.; Plenum Publishing Corp.: New York, NY, USA, 1984; Volume V, pp. 621–629. [Google Scholar]
- Herman, T.S.; Stickney, D.G.; Gerner, E.W. DIFFERENTIAL RATES OF HEATING INFLUENCE HYPERTHERMIA INDUCED CYTOTOXICITY IN NORMAL AND TRANSFORMED-CELLS INVITRO. Proc. Am. Assoc. Cancer Res. 1979, 20, 165. [Google Scholar]
- Dewhirst, M.; Gross, J.; Sim, D.; Arnold, P.; Boyer, D. The effect of rate of heating or cooling prior to heating on tumor and normal tissue microcirculatory blood flow. Biorheology 1984, 21, 539–558. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Gu, Y.H.; Takahashi, T.; Haswgawa, T.; Yamamoto, I.Y. Enhancement of hyperthermic effects using rapid hyperthermia. In Theoretical and Experimental Basasxcdis of Hyperthermia: Thermotherapy for Neoplasia, Inflammation and Pain; Kosaka, M., Sugahara, T., Schmidt, K.L., Eds.; Springer: Tokyo, Japan, 2003; pp. 439–444. [Google Scholar]
- Vaupel, P.; Mullerklieser, W.; Otte, J.; Manz, R.; Kallinowski, F. Blood-Flow, Tissue Oxygenation, and Ph-Distribution in Malignant-Tumors Upon Localized Hyperthermia—Basic Pathophysiological Aspects and the Role of Various Thermal Doses. Strahlentherapie 1983, 159, 73–81. [Google Scholar] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001, 61, 3027–3032. [Google Scholar] [PubMed]
- Moon, E.J.; Sonveaux, P.; Porporato, P.E.; Danhier, P.; Gallez, B.; Batinic-Haberle, I.; Nien, Y.-C.; Schroeder, T.; Dewhirst, M.W. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc. Natl. Acad. Sci. USA 2010, 107, 20477–20482. [Google Scholar] [CrossRef] [Green Version]
- Bordonaro, M.; Shirasawa, S.; Lazarova, D.L. In Hyperthermia Increased ERK and WNT Signaling Suppress Colorectal Cancer Cell Growth. Cancers 2016, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Oei, A.L.; Van Leeuwen, C.M.; Cate, R.T.; Rodermond, H.M.; Buist, M.R.; Stalpers, L.J.A.; Crezee, J.; Kok, H.; Medema, J.P.; Franken, N.A.P. Hyperthermia Selectively Targets Human Papillomavirus in Cervical Tumors via p53-Dependent Apoptosis. Cancer Res. 2015, 75, 5120–5129. [Google Scholar] [CrossRef] [Green Version]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Vujaskovic, Z.; Poulson, J.M.; Gaskin, A.A.; Thrall, D.E.; Page, R.L.; Charles, H.C.; MacFall, J.R.; Brizel, D.M.; Meyer, R.E.; Prescott, D.M.; et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 179–185. [Google Scholar] [CrossRef]
- Thrall, D.E.; Maccarini, P.; Stauffer, P.; MacFall, J.; Hauck, M.; Snyder, S.; Case, B.; Linder, K.; Lan, L.; McCall, L.; et al. Thermal dose fractionation affects tumour physiological response. Int. J. Hyperth. 2012, 28, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Hannon, G.; Tansi, F.L.; Hilger, I.; Prina-Mello, A. The Effects of Localized Heat on the Hallmarks of Cancer. Adv. Ther. 2021, 4, 2000267. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secomb, T.; Hsu, R.; Ong, E.T.; Gross, J.F.; Dewhirst, M.W. Analysis of the Effects of Oxygen Supply and Demand on Hypoxic Fraction in Tumors. Acta Oncol. 1995, 34, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W.; Hsu, R.; Park, E.Y.H.; Dewhirst, M.W. Green’s Function Methods for Analysis of Oxygen Delivery to Tissue by Microvascular Networks. Ann. Biomed. Eng. 2004, 32, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Secomb, T.W.; Hsu, R.; Dewhirst, M.W. Synergistic effects of hyperoxic gas breathing and reduced oxygen consumption on tumor oxygenation: A theoretical model. Int. J. Radiat. Oncol. 2004, 59, 572–578. [Google Scholar] [CrossRef]
- Snyder, S.A.; Lanzen, J.L.; Braun, R.D.; Rosner, G.; Secomb, T.; Biaglow, J.; Brizel, D.; Dewhirst, M.W. Simultaneous administration of glucose and hyperoxic gas achieves greater improvement in tumor oxygenation than hyperoxic gas alone. Int. J. Radiat. Oncol. 2001, 51, 494–506. [Google Scholar] [CrossRef]
- Griffin, R.; Okajima, K.; Barrios, B.; Song, C.W. Mild temperature hyperthermia combined with carbogen breathing increases tumor partial pressure of oxygen (pO2) and radiosensitivity. Cancer Res. 1996, 56. [Google Scholar]
- Griffin, R.J.; Okajima, K.; Ogawa, A.; Song, C.W. Radiosensitization of two murine rumours with mild temperature hyperthermia and carbogen breathing. Int. J. Radiat. Oncol. Biol. Phys. 1999, 75, 1299–1306. [Google Scholar]
- Ohara, M.D.; Hetzel, F.W.; Frinak, S. Thermal Distributions in a Water Bath Heated Mouse-Tumor. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 817–822. [Google Scholar] [CrossRef]
- Oleson, J.; Dewhirst, M.; Harrelson, J.; Leopold, K.; Samulski, T.; Tso, C. Tumor temperature distributions predict hyperthermia effect. Int. J. Radiat. Oncol. 1989, 16, 559–570. [Google Scholar] [CrossRef]
- Bakker, J.F.; Paulides, M.M.; Obdeijn, I.M.; Van Rhoon, G.C.; A Van Dongen, K.W. An ultrasound cylindrical phased array for deep heating in the breast: Theoretical design using heterogeneous models. Phys. Med. Biol. 2009, 54, 3201–3215. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, G.; Paulides, M.M.; Drizdal, T.; O’Loughlin, D.; O’Halloran, M.; Glavin, M.; Van Rhoon, G.; Jones, E. Robustness of Time-Multiplexed Hyperthermia to Temperature Dependent Thermal Tissue Properties. IEEE J. Electromagn. RF Microwaves Med. Biol. 2019, 4, 126–132. [Google Scholar] [CrossRef]
- Verhaart, R.F.; Rijnen, Z.; Fortunati, V.; Verduijn, G.M.; Van Walsum, T.; Veenland, J.F.; Paulides, M.M. Temperature simulations in hyperthermia treatment planning of the head and neck region. Strahlenther. und Onkol. 2014, 190, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Rodrigues, D.B.; Bellizzi, G.G.; Sumser, K.; Curto, S.; Neufeld, E.; Montanaro, H.; Kok, H.P.; Trefna, H.D. ESHO benchmarks for computational modeling and optimization in hyperthermia therapy. Int. J. Hyperth. 2021, 38, 1425–1442. [Google Scholar] [CrossRef]
- Gavazzi, S.; van Lier, A.L.H.M.W.; Zachiu, C.; Jansen, E.; Lagendijk, J.J.W.; A Stalpers, L.J.; Crezee, H.; Kok, H.P. Advanced patient-specific hyperthermia treatment planning. Int. J. Hyperth. 2020, 37, 992–1007. [Google Scholar] [CrossRef]
- Lüdemann, L.; Wlodarczyk, W.; Nadobny, J.; Weihrauch, M.; Gellermann, J.; Wust, P. Non-invasive magnetic resonance thermography during regional hyperthermia. Int. J. Hyperth. 2010, 26, 273–282. [Google Scholar] [CrossRef]
- Craciunescu, O.I.; Das, S.K.; McCauley, R.L.; MacFall, J.R.; Samulski, T.V. 3D numerical reconstruction of the hyperthermia induced temperature distribution in human sarcomas using DE-MRI measured tissue perfusion: Validation against non-invasive MR temperature measurements. Int. J. Hyperth. 2001, 17, 221–239. [Google Scholar] [CrossRef]
- Li, Z.; Vogel, M.; Maccarini, P.F.; Stakhursky, V.; Soher, B.J.; Craciunescu, O.I.; Das, S.; Arabe, O.A.; Joines, W.T.; Stauffer, P.R. Improved hyperthermia treatment control using SAR/temperature simulation and PRFS magnetic resonance thermal imaging. Int. J. Hyperth. 2010, 27, 86–99. [Google Scholar] [CrossRef] [Green Version]
- DeWhirst, M.; Phillips, T.; Samulski, T.; Stauffer, P.; Shrivastava, P.; Paliwal, B.; Pajak, T.; Gillim, M.; Sapozink, M.; Myerson, R.; et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int. J. Radiat. Oncol. 1990, 18, 1249–1259. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J. Temperature and the catalytic activity of enzymes: A fresh understanding. FEBS Lett. 2013, 587, 2738–2743. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Engel, T.; Vaupel, P.W. Changes in microregional perfusion, oxygenation, ATP and lactate distribution in subcutaneous rat tumours upon water-filtered IR-A hyperthermia. Int. J. Hyperth. 1995, 11, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schaefer, C.; Okunieff, P. Intracellular acidosis in murine fibrosarcomas coincides with ATP depletion, hypoxia, and high levels of lactate and total Pi. NMR Biomed. 1994, 7, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sijens, P.E.; Bovee, W.M.M.J.; Koole, P.; Schipper, J. Phosphorus NMR study of the response of a murine tumour to hyperthermia as a function of treatment time and temperature. Int. J. Hyperth. 1989, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Prescott, D.M.; Charles, H.C.; Sostman, H.D.; Dodge, R.K.; Thrall, D.E.; Page, R.L.; Tucker, J.A.; Harrelson, J.M.; Leopold, K.A.; Oleson, J.R.; et al. Therapy monitoring in human and canine soft tissue sarcomas using magnetic resonance imaging and spectroscopy. Int. J. Radiat. Oncol. 1994, 28, 415–423. [Google Scholar] [CrossRef]
- Maguire, P.D.; Samulski, T.V.; Prosnitz, L.R.; Jones, E.L.; Rosner, G.L.; Powers, B.; Layfield, L.W.; Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; et al. A phase II trial testing the thermal dose parameter CEM43° T90 as a predictor of response in soft tissue sarcomas treated with pre-operative thermoradiotherapy. Int. J. Hyperth. 2001, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Poulson, J.M.; Yu, D.; Sanders, L.; Lora-Michiels, M.; Vujaskovic, Z.; Jones, E.L.; Samulski, T.V.; Powers, B.E.; Brizel, D.M.; et al. Relation between pO2, 31P magnetic resonance spectroscopy parameters and treatment outcome in patients with high-grade soft tissue sarcomas treated with thermoradiotherapy. Int. J. Radiat. Oncol. 2005, 61, 480–491. [Google Scholar] [CrossRef]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Sonveaux, P.; Rabbani, Z.N.; Liu, S.; Yan, B.; Huang, Q.; Vujaskovic, Z.; Dewhirst, M.W.; Li, C.-Y. Regulation of HIF-1α Stability through S-Nitrosylation. Mol. Cell 2007, 26, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Kim, M.-S.; Kim, H.-J.; Lee, E.; Jeong, J.-H.; Park, I.; Jeong, Y.K.; Jang, W.I. Role of HIF-1α in response of tumors to a combination of hyperthermia and radiation in vivo. Int. J. Hyperth. 2017, 34, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, M.; Omloo, J.M.T.; Sloof, G.W.; Hulshof, M.C.C.M.; Hoekstra, O.S.; Crezee, H.; Boellaard, R.; Vervenne, W.L.; Kate, F.J.W.T.; Van Lanschot, J.J.B. Monitoring of response to pre-operative chemoradiation in combination with hyperthermia in oesophageal cancer by FDG-PET. Int. J. Hyperth. 2006, 22, 149–160. [Google Scholar] [CrossRef]
- Murata, H.; Okamoto, M.; Takahashi, T.; Motegi, M.; Ogoshi, K.; Shoji, H.; Onishi, M.; Takakusagi, Y.; Okonogi, N.; Kawamura, H.; et al. SUVmax-based Parameters of FDG-PET/CT Reliably Predict Pathologic Complete Response After Preoperative Hyperthermo-chemoradiotherapy in Rectal Cancer. Anticancer Res. 2018, 38, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Lehmann, M.; Todica, A.; Herrmann, K.; Knösel, T.; Angele, M.K.; Dürr, H.R.; Rauch, J.; Bartenstein, P.; Cyran, C.C.; et al. PET Response Criteria in Solid Tumors Predicts Progression-Free Survival and Time to Local or Distant Progression After Chemotherapy with Regional Hyperthermia for Soft-Tissue Sarcoma. J. Nucl. Med. 2015, 56, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Rosner, G.L.; Clegg, S.T.; Prescott, D.M.; Dewhirst, M.W. Estimation of cell survival in tumours heated to nonuniform temperature distributions. Int. J. Hyperth. 1996, 12, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Shakil, A.; Osborn, J.L.; Song, C.W. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int. J. Radiat. Oncol. 1999, 43, 859–865. [Google Scholar] [CrossRef]
- Iwata, K.; Shakil, A.; Hur, W.J.; Makepeace, C.M.; Griffin, R.J.; Song, C.W. Tumour pO(2) can be increased markedly by mild hyperthermia. Br. J. Cancer 1996, 74, S217–S221. [Google Scholar]
- Song, C.W.; Park, H.; Griffin, R.J. Improvement of Tumor Oxygenation by Mild Hyperthermia. Radiat. Res. 2001, 155, 515–528. [Google Scholar] [CrossRef]
- Oleson, J.R. Eugene Robertson Special Lecture Hyperthermia from the clinic to the laboratory: A hypothesis. Int. J. Hyperth. 1995, 11, 315–322. [Google Scholar] [CrossRef]
- Okajima, K.; Griffin, R.J.; Iwata, K.; Shakil, A.; Song, C.W. Tumor oxygenation after mild-temperature hyperthermia in combination with carbogen breathing: Dependence on heat dose and tumor type. Radiat. Res. 1998, 149, 294. [Google Scholar] [CrossRef]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Dodge, R.K.; Charles, H.C.; Samulski, T.V.; Prosnitz, L.R.; Dewhirst, M.W. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996, 56, 5347–5350. [Google Scholar]
- Vujaskovic, Z.; Rosen, E.L.; Blackwell, K.L.; Jones, E.L.; Brizel, D.M.; Prosnitz, L.R.; Samulski, T.V.; Dewhirst, M.W. Ultrasound guided pO 2 measurement of breast cancer reoxygenation after neoadjuvant chemotherapy and hyperthermia treatment. Int. J. Hyperth. 2003, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Matteucci, M.L.; Anyarambhatla, G.; Rosner, G.; Azuma, C.; E Fisher, P.; Dewhirst, M.W.; Needham, D.; E Thrall, D. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin. Cancer Res. 2000, 6, 3748–3755. [Google Scholar] [PubMed]
- Jones, E.L.; Prosnitz, L.R.; Dewhirst, M.W.; Marcom, P.K.; Hardenbergh, P.H.; Marks, L.B.; Brizel, D.M.; Vujaskovic, Z. Thermochemoradiotherapy Improves Oxygenation in Locally Advanced Breast Cancer. Clin. Cancer Res. 2004, 10, 4287–4293. [Google Scholar] [CrossRef] [Green Version]
- Thrall, D.E.; LaRue, S.M.; Yu, D.; Samulski, T.; Sanders, L.; Case, B.; Rosner, G.; Azuma, C.; Poulson, J.; Pruitt, A.F.; et al. Thermal Dose Is Related to Duration of Local Control in Canine Sarcomas Treated with Thermoradiotherapy. Clin. Cancer Res. 2005, 11, 5206–5214. [Google Scholar] [CrossRef] [Green Version]
- Lora-Michiels, M.; Yu, D.; Sanders, L.; Poulson, J.M.; Azuma, C.; Case, B.; Vujaskovic, Z.; Thrall, D.E.; Charles, H.C.; Dewhirst, M.W. Extracellular pH and P-31 Magnetic Resonance Spectroscopic Variables are Related to Outcome in Canine Soft Tissue Sarcomas Treated with Thermoradiotherapy. Clin. Cancer Res. 2006, 12, 5733–5740. [Google Scholar] [CrossRef] [Green Version]
- Cline, J.; Rosner, G.L.; Raleigh, J.A.; Thrall, D.E. Quantification of CCI-103F labeling heterogeneity in canine solid tumors. Int. J. Radiat. Oncol. 1997, 37, 655–662. [Google Scholar] [CrossRef]
- Cline, J.M.; Thrall, D.E.; Rosner, G.L.; Raleigh, J.A. DISTRIBUTION OF THE HYPOXIA MARKER CCI-103F IN CANINE TUMORS. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 921–933. [Google Scholar] [CrossRef]
- Chi, J.-T.; Thrall, D.E.; Jiang, C.; Snyder, S.; Fels, D.; Landon, C.; McCall, L.; Lan, L.; Hauck, M.; MacFall, J.R.; et al. Comparison of Genomics and Functional Imaging from Canine Sarcomas Treated with Thermoradiotherapy Predicts Therapeutic Response and Identifies Combination Therapeutics. Clin. Cancer Res. 2011, 17, 2549–2560. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, D.; Weng, Y.; Weng, S.; Yan, C.; Xu, X.; Chen, J.; Ye, R.; Hong, J. Early Diffusion-Weighted Imaging and Proton Magnetic Resonance Spectroscopy Features of Liver Transplanted Tumors Treated with Radiation in Rabbits: Correlation with Histopathology. Radiat. Res. 2018, 191, 52–59. [Google Scholar] [CrossRef]
- Morse, D.L.; Galons, J.-P.; Payne, C.M.; Jennings, D.L.; Day, S.; Xia, G.; Gillies, R.J. MRI-measured water mobility increases in response to chemotherapy via multiple cell-death mechanisms. NMR Biomed. 2007, 20, 602–614. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.S.; Fan, S.J.; Gao, D.S.; Chow, A.M.; Man, K.; Wu, E.X. Diffusion tensor imaging of liver fibrosis in an experimental model. J. Magn. Reson. Imaging 2010, 32, 1141–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Geng, D.; Zhan, S.; Li, H.; Xu, X.; Yi, C. Magnetic resonance imaging characteristics of hepatocyte apoptosis (induced by right portal vein ligation) and necrosis (induced by combined right portal vein and right hepatic artery ligation) in rats. J. Int. Med. Res. 2014, 43, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Thrall, D. Data obtained during conduct of Thermal Dose Equivalence Trial, studying companion canine patients with soft tissue sarcomas. 2010. Unpublished work. [Google Scholar]
- Moeller, B.J.; Dreher, M.R.; Rabbani, Z.; Schroeder, T.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell 2005, 8, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrall, D.E.; LaRue, S.M.; Pruitt, A.F.; Case, B.; Dewhirst, M.W. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int. J. Hyperth. 2006, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Viglianti, B.L.; Lora-Michiels, M.; Poulson, J.M.; Plantenga, J.P.; Yu, D.; Sanders, L.L.; I Craciunescu, O.; Vujaskovic, Z.; Thrall, D.E.; MacFall, J.R.; et al. Dynamic Contrast-enhanced Magnetic Resonance Imaging as a Predictor of Clinical Outcome in Canine Spontaneous Soft Tissue Sarcomas Treated with Thermoradiotherapy. Clin. Cancer Res. 2009, 15, 4993–5001. [Google Scholar] [CrossRef] [Green Version]
- Leopold, K.A.; Dewhirst, M.; Samulski, T.; Harrelson, J.; Tucker, J.A.; George, S.L.; Dodge, R.K.; Grant, W.; Clegg, S.; Prosnitz, L.R.; et al. Relationships among Tumor Temperature, Treatment Time, and Histopathological Outcome Using Preoperative Hyperthermia with Radiation in Soft-Tissue Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 989–998. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; A Sim, D.; Sapareto, S.; Connor, W.G. Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res. 1984, 44. [Google Scholar]
- Thomsen, A.R.; Saalmann, M.A.; Nicolay, N.H.; Grosu, A.-L.; Vaupel, P. Improved oxygenation of human skin, subcutis and superficial cancers upon mild hyperthermia delivered by wIRA-irradiation. Adv. Exp. Med. Biol. 2021, in press. [Google Scholar]
- Waterman, F.M.; Nerlinger, R.E.; Moylan, D.J., 3rd; Leeper, D.B. Response of human tumor blood flow to local hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 75–82. [Google Scholar] [CrossRef]
- Yuan, H.; Schroeder, T.; E Bowsher, J.; Hedlund, L.W.; Wong, T.; Dewhirst, M.W. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2006, 47, 989–998. [Google Scholar] [PubMed]
- Hoogsteen, I.J.; Lok, J.; Marres, H.A.; Takes, R.P.; Rijken, P.F.; van der Kogel, A.J.; Kaanders, J.H. Hypoxia in larynx carcinomas assessed by pimonidazole binding and the value of CA-IX and vascularity as surrogate markers of hypoxia. Eur. J. Cancer 2009, 45, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- E Hansen, A.; Kristensen, A.T.; Jørgensen, J.T.; McEvoy, F.J.; Busk, M.; Van Der Kogel, A.J.; Bussink, J.; A Engelholm, S.; Kjær, A. 64Cu-ATSM and 18FDG PET uptake and 64Cu-ATSM autoradiography in spontaneous canine tumors: Comparison with pimonidazole hypoxia immunohistochemistry. Radiat. Oncol. 2012, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- Vaishnavi, S.N.; Vlassenko, A.G.; Rundle, M.M.; Snyder, A.Z.; Mintun, M.A.; Raichle, M.E. Regional aerobic glycolysis in the human brain. Proc. Natl. Acad. Sci. 2010, 107, 17757–17762. [Google Scholar] [CrossRef] [Green Version]
- Abramovitch, R.; Dafni, H.; Smouha, E.; E Benjamin, L.; Neeman, M. In vivo prediction of vascular susceptibility to vascular susceptibility endothelial growth factor withdrawal: Magnetic resonance imaging of C6 rat glioma in nude mice. Cancer Res. 1999, 59, 5012–5016. [Google Scholar]
- Huhnt, W. Growth, microvessel density and tumor cell invasion of human colon adenocarcinoma under repeated treatment with hyperthermia and serotonin. J. Cancer Res. Clin. Oncol. 1995, 121, 423–428. [Google Scholar] [CrossRef]
- Li, K.; Shen, S.-Q.; Xiong, C.-L. Microvessel Damage May Play an Important Role in Tumoricidal Effect for Murine H22 Hepatoma Cells with Hyperthermia In Vivo. J. Surg. Res. 2008, 145, 97–104. [Google Scholar] [CrossRef]
- Ting, Z.; Dan, Z.; Luo, Q.M.; Yang, W. Dynamics of blood flow in normal tissue and tumor during local hyperthermia. In Proceedings of the 3rd International Conference on Photonics and Imaging in Biology and Medicine, Wuhan, China, 08–11 June 2003; pp. 484–491. [Google Scholar]
- Shan, S.; Rosner, G.; Braun, R.; Hahn, J.; Pearce, C.; Dewhirst, M. Effects of diethylamine/nitric oxide on blood perfusion and oxygenation in the R3230Ac mammary carcinoma. Br. J. Cancer 1997, 76, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Zlotecki, R.A.; Baxter, L.T.; Boucher, Y.; Jain, R.K. Pharmacologic Modification of Tumor Blood Flow and Interstitial Fluid Pressure in a Human Tumor Xenograft: Network Analysis and Mechanistic Interpretation. Microvasc. Res. 1995, 50, 429–443. [Google Scholar] [CrossRef]
- Lüdemann, L.; Sreenivasa, G.; Amthauer, H.; Michel, R.; Gellermann, J.; Wust, P. Use of H215O-PET for investigating perfusion changes in pelvic tumors due to regional hyperthermia. Int. J. Hyperth. 2009, 25, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.G.; Palmer, G.M.; Dewhirst, M.W. Clinical and Pre-clinical Methods for Quantifying Tumor Hypoxia. In Hypoxia and Cancer Metastasis; Springer: Cham, Switzerland, 2019; Volume 1136, pp. 19–41. [Google Scholar] [CrossRef]
- Loshek, D.D.; Orr, J.S.; Solomonidis, E. Interaction of hyperthermia and radiation: The survival surface. Br. J. Radiol. 1977, 50, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.N.; Tran, E.; Berthelet, E.; Wu, J.; Olson, R. Early (90-day) mortality after radical radiotherapy for head and neck squamous cell carcinoma: A population-based analysis. Head Neck 2018, 40, 2432–2440. [Google Scholar] [CrossRef]
- Munira, A.; Fang, C.Z. MANAGEMENT AND PROGNOSTIC FACTORS OF SQUAMOUS CELL CARCINOMA AND ADENOCARCINOMA OF THE UTERINE CERVIX. Int. J. Pharm. Sci. Rev. Res. 2019, 6, 10281–10287. [Google Scholar] [CrossRef]
- Lassen, P.; Huang, S.H.; Su, J.; O’Sullivan, B.; Waldron, J.; Andersen, M.; Primdahl, H.; Johansen, J.; Kristensen, C.; Andersen, E.; et al. Impact of tobacco smoking on radiotherapy outcomes in 1875 HPV-positive oropharynx cancer patients. J. Clin. Oncol. 2019, 37, 6047. [Google Scholar] [CrossRef]
- Lapuz, C.; Kondalsamy-Chennakesavan, S.; Bernshaw, D.; Khaw, P.; Narayan, K. Stage IB cervix cancer with nodal involvement treated with primary surgery or primary radiotherapy: Patterns of failure and outcomes in a contemporary population. J. Med. Imaging Radiat. Oncol. 2015, 60, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Dressman, H.K.; Hans, C.; Bild, A.; Olson, J.A.; Rosen, E.; Marcom, P.K.; Liotcheva, V.B.; Jones, E.L.; Vujaskovic, Z.; Marks, J.; et al. Gene Expression Profiles of Multiple Breast Cancer Phenotypes and Response to Neoadjuvant Chemotherapy. Clin. Cancer Res. 2006, 12, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Jentsch, M.; Snyder, P.; Sheng, C.; Cristiano, E.; Loewer, A. p53 dynamics in single cells are temperature-sensitive. Sci. Rep. 2020, 10, 1481. [Google Scholar] [CrossRef]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 2015, 20, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, C.; Li, S.; Wang, X.; Sun, X.; Wang, P.; Zhang, B.; Ren, H. Hyperthermia induces apoptosis by targeting Survivin in esophageal cancer. Oncol. Rep. 2015, 34, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.M.; Boruta, R.J.; Viglianti, B.L.; Lan, L.; Spasojevic, I.; Dewhirst, M.W. Non-invasive monitoring of intra-tumor drug concentration and therapeutic response using optical spectroscopy. J. Control. Release 2010, 142, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-T.; Boss, M.-K.; Dewhirst, M.W. Imaging Tumor Hypoxia to Advance Radiation Oncology. Antioxidants Redox Signal. 2014, 21, 313–337. [Google Scholar] [CrossRef] [Green Version]

| Variable | Parameter Estimate | Hazard Ratio | Score p-Value | Wald p-Value |
|---|---|---|---|---|
| HF Post-Pre | −0.0643 | 0.94 | 0.0070 | 0.0340 |
| Median pO2 Post-Pre | 0.0896 | 1.09 | 0.0230 | 0.0710 |
| Pimo % area | −6.549 | 0.00 | 0.038 | 0.0510 |
| PDE/ATP | 0.2246 | 1.25 | 0.0490 | 0.0640 |
| Variable | N | CEM43T10 | CEM43T50 | CEM43T90 | |||
|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | ||
| Change ADC Pre-post * | 29 | −0.53 | 0.0030 | −0.56 | 0.0015 | 0.11 | 0.5665 |
| iAUC change 24 h ^ | 17 | 0.07 | 0.7798 | 0.23 | 0.3599 | 0.5109 | 0.0311 |
| Median pO2 change at 24 h ^ | 38 | 0.38 | 0.0214 | 0.27 | 0.1087 | −0.07 | 0.9829 |
| Change HF 24 h ^ | 38 | −0.34 | 0.0451 | −0.27 | 0.1074 | −0.07 | 0.674 |
| Volume change Pre-Post * | 38 | −0.42 | 0.0084 | −0.36 | 0.0258 | 0.2983 | 0.17 |
| Variable | N | Coefficient | p-Value |
|---|---|---|---|
| iAUC median change 24 h | 17 | −0.47 | 0.0472 |
| Median pO2 change 24 h | 38 | −0.040 | 0.0146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewhirst, M.W.; Oleson, J.R.; Kirkpatrick, J.; Secomb, T.W. Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy. Cancers 2022, 14, 1701. https://doi.org/10.3390/cancers14071701
Dewhirst MW, Oleson JR, Kirkpatrick J, Secomb TW. Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy. Cancers. 2022; 14(7):1701. https://doi.org/10.3390/cancers14071701
Chicago/Turabian StyleDewhirst, Mark W., James R. Oleson, John Kirkpatrick, and Timothy W. Secomb. 2022. "Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy" Cancers 14, no. 7: 1701. https://doi.org/10.3390/cancers14071701
APA StyleDewhirst, M. W., Oleson, J. R., Kirkpatrick, J., & Secomb, T. W. (2022). Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy. Cancers, 14(7), 1701. https://doi.org/10.3390/cancers14071701





