Novel Intraoperative Navigation Using Ultra-High-Resolution CT in Robot-Assisted Partial Nephrectomy
Abstract
:Simple Summary
Abstract
1. Introduction
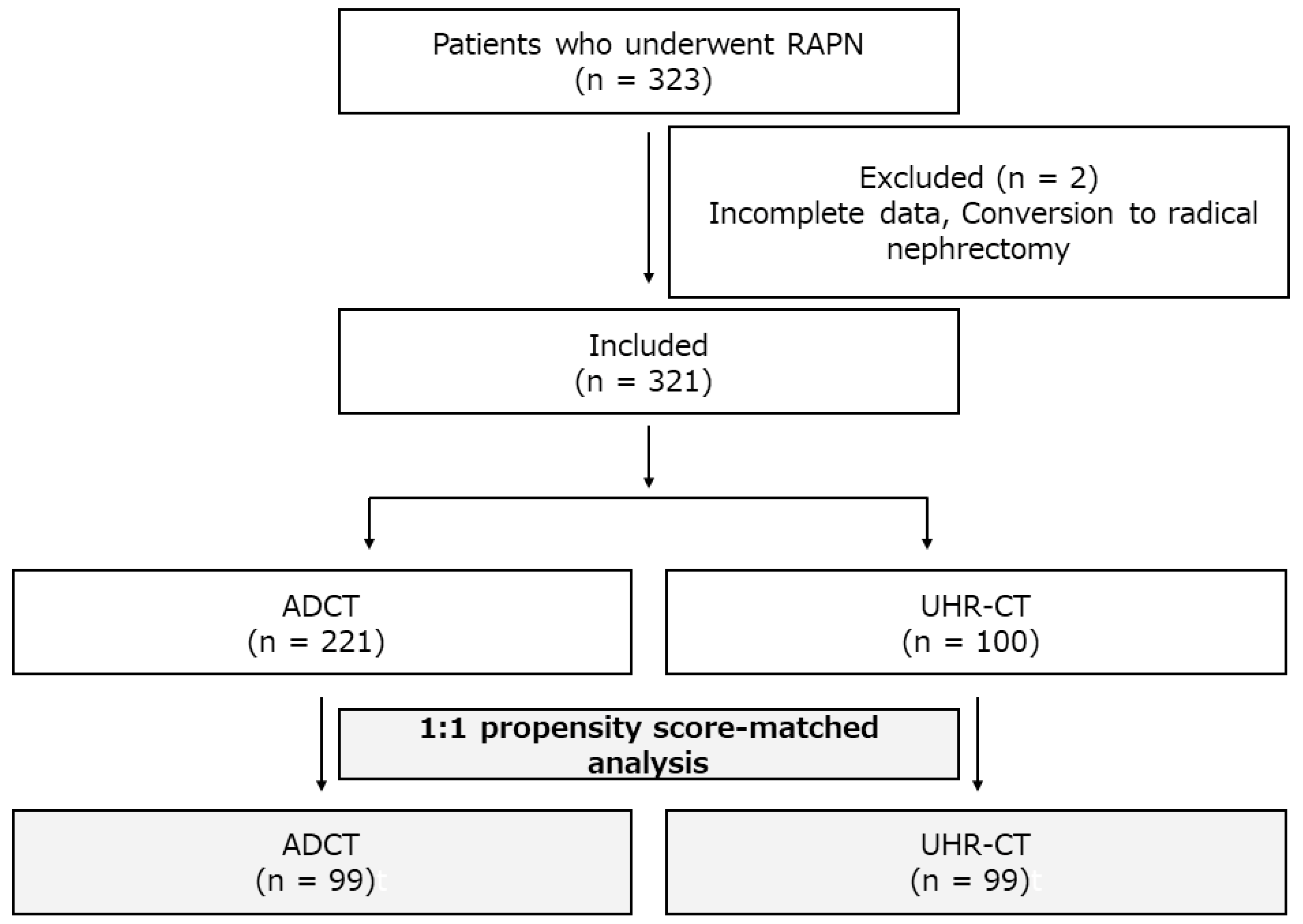
2. Materials and Methods
2.1. Patient Population
2.2. CT Examination
2.3. Data Collection
2.4. Surgery
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for management of the clinical T1 renal mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: A systematic review and meta-analysis. Eur. Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Herrmann, T.R.; Shariat, S.F.; Kyriazis, I.; Nagele, U.; Traxer, O.; Liatsikos, E.N.; European Association of Urology. EAU guidelines on robotic and single-site surgery in urology. Eur. Urol. 2013, 64, 277–291. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Song, S.; Ye, H.; Yang, Q.; Liu, B.; Cai, C.; Yang, B.; Xiao, L.; Chen, Q.; et al. Propensity-score matched analysis comparing robot-assisted with laparoscopic partial nephrectomy. BJU Int. 2015, 115, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel Raheem, A.; Alatawi, A.; Kim, D.K.; Sheikh, A.; Alabdulaali, I.; Han, W.K.; Choi, Y.D.; Rha, K.H. Outcomes of high-complexity renal tumours with a Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score of >/=10 after robot-assisted partial nephrectomy with a median 46.5-month follow-up: A tertiary centre experience. BJU Int. 2016, 118, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Yanagawa, M.; Honda, O.; Kikuchi, N.; Miyata, T.; Tsukagoshi, S.; Uranishi, A.; Tomiyama, N. Effect of Matrix Size on the Image Quality of Ultra-high-resolution CT of the Lung: Comparison of 512 × 512, 1024 × 1024, and 2048 ×. Acad. Radiol. 2018, 25, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Hino, T.; Kamitani, T.; Sagiyama, K.; Yamasaki, Y.; Matsuura, Y.; Tsutsui, S.; Sakai, Y.; Furuyama, T.; Yabuuchi, H. Detectability of the artery of Adamkiewicz on computed tomography angiography of the aorta by using ultra-high-resolution computed tomography. Jpn. J. Radiol. 2020, 38, 658–665. [Google Scholar] [CrossRef]
- Kakinuma, R.; Moriyama, N.; Muramatsu, Y.; Gomi, S.; Suzuki, M.; Nagasawa, H.; Kusumoto, M.; Aso, T.; Muramatsu, Y.; Tsuchida, T.; et al. Ultra-High-Resolution Computed Tomography of the Lung: Image Quality of a Prototype Scanner. PLoS ONE 2015, 10, e0137165. [Google Scholar] [CrossRef]
- Matsukiyo, R.; Ohno, Y.; Matsuyama, T.; Nagata, H.; Kimata, H.; Ito, Y.; Ogawa, Y.; Murayama, K.; Kato, R.; Toyama, H. Deep learning-based and hybrid-type iterative reconstructions for CT: Comparison of capability for quantitative and qualitative image quality improvements and small vessel evaluation at dynamic CE-abdominal CT with ultra-high and standard resolutions. Jpn. J. Radiol. 2020, 39, 186–197. [Google Scholar] [CrossRef]
- Morisaka, H.; Shimizu, Y.; Adachi, T.; Fukushima, K.; Arai, T.; Yamamura, W.; Koyanagi, M.; Kariyasu, T.; Machida, H.; Sano, K.; et al. Effect of Ultra High-Resolution Computed Tomography and Model-Based Iterative Reconstruction on Detectability of Simulated Submillimeter Artery. J. Comput. Assist Tomogr. 2020, 44, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Ito, H.; Sarai, M.; Nagahara, Y.; Miyajima, K.; Matsumoto, R.; Doi, Y.; Kataoka, Y.; Takahashi, H.; Ozaki, Y.; et al. Ultra-High-Resolution Computed Tomography Angiography for Assessment of Coronary Artery Stenosis. Circ. J. 2018, 82, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Murayama, K.; Suzuki, S.; Nagata, H.; Oda, J.; Nakahara, I.; Katada, K.; Fujii, K.; Toyama, H. Visualization of Lenticulostriate Arteries on CT Angiography Using Ultra-High-Resolution CT Compared with Conventional-Detector CT. Am. J. Neuroradiol. 2020, 41, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Murayama, K.; Suzuki, S.; Watanabe, A.; Hayakawa, M.; Saito, Y.; Katada, K.; Toyama, H. Initial clinical experience of a prototype ultra-high-resolution CT for assessment of small intracranial arteries. Jpn J. Radiol. 2019, 37, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Tsubamoto, M.; Hata, A.; Yanagawa, M.; Honda, O.; Miyata, T.; Yoshida, Y.; Nakayama, A.; Kikuchi, N.; Uranishi, A.; Tsukagoshi, S.; et al. Ultra high-resolution computed tomography with 1024-matrix: Comparison with 512-matrix for the evaluation of pulmonary nodules. Eur. J. Radiol. 2020, 128, 109033. [Google Scholar] [CrossRef]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Matsumoto, N.; Momosaka, D.; Nakatake, H.; Sakai, Y.; Honda, H. Ultrahigh-resolution CT scan of the temporal bone. Eur. Arch. Otorhinolaryngol. 2018, 275, 2797–2803. [Google Scholar] [CrossRef]
- Yanagawa, M.; Hata, A.; Honda, O.; Kikuchi, N.; Miyata, T.; Uranishi, A.; Tsukagoshi, S.; Tomiyama, N. Subjective and objective comparisons of image quality between ultra-high-resolution CT and conventional area detector CT in phantoms and cadaveric human lungs. Eur. Radiol. 2018, 28, 5060–5068. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, K.; Tanaka, R.; Takagi, H.; Ueyama, Y.; Kikuchi, K.; Chiba, T.; Arakita, K.; Schuijf, J.D.; Saito, Y. Ultra-high-resolution CT angiography of the artery of Adamkiewicz: A feasibility study. Neuroradiology 2018, 60, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Nakamura, Y.; Tatsugami, F.; Nakaura, T.; Awai, K. Improvement of image quality at CT and MRI using deep learning. Jpn. J. Radiol. 2019, 37, 73–80. [Google Scholar] [CrossRef]
- Ohno, Y.; Fujisawa, Y.; Fujii, K.; Sugihara, N.; Kishida, Y.; Seki, S.; Yoshikawa, T. Effects of acquisition method and reconstruction algorithm for CT number measurement on standard-dose CT and reduced-dose CT: A QIBA phantom study. Jpn. J. Radiol. 2019, 37, 399–411. [Google Scholar] [CrossRef]
- Ohno, Y.; Takenaka, D.; Kanda, T.; Yoshikawa, T.; Matsumoto, S.; Sugihara, N.; Sugimura, K. Adaptive iterative dose reduction using 3D processing for reduced- and low-dose pulmonary CT: Comparison with standard-dose CT for image noise reduction and radiological findings. Am. J. Roentgenol. 2012, 199, W477–W485. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Yaguchi, A.; Okazaki, T.; Aoyagi, K.; Yamagata, H.; Sugihara, N.; Koyama, H.; Yoshikawa, T.; Sugimura, K. Comparative evaluation of newly developed model-based and commercially available hybrid-type iterative reconstruction methods and filter back projection method in terms of accuracy of computer-aided volumetry (CADv) for low-dose CT protocols in phantom study. Eur. J. Radiol. 2016, 85, 1375–1382. [Google Scholar]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Dulabon, L.M.; Kaouk, J.H.; Haber, G.P.; Berkman, D.S.; Rogers, C.G.; Petros, F.; Bhayani, S.B.; Stifelman, M.D. Multi-institutional analysis of robotic partial nephrectomy for hilar versus nonhilar lesions in 446 consecutive cases. Eur. Urol. 2011, 59, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.G.; Metwalli, A.; Blatt, A.M.; Bratslavsky, G.; Menon, M.; Linehan, W.M.; Pinto, P.A. Robotic partial nephrectomy for renal hilar tumors: A multi-institutional analysis. J. Urol. 2008, 180, 2353–2356. [Google Scholar] [CrossRef] [Green Version]
- Khalifeh, A.; Autorino, R.; Hillyer, S.P.; Laydner, H.; Eyraud, R.; Panumatrassamee, K.; Long, J.A.; Kaouk, J.H. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: A single surgeon experience. J. Urol. 2013, 189, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Takahara, K.; Sumitomo, M.; Fukaya, K.; Jyoudai, T.; Nishino, M.; Hikichi, M.; Nukaya, T.; Zennami, K.; Ichino, M.; Fukami, N.; et al. Predictors for trifecta achievement of robot-assisted partial nephrectomy in high-complexity tumors (Preoperative Aspects and Dimensions Used for an Anatomical score >/=10). Asian J. Endosc. Surg. 2020, 13, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Pietrabissa, A.; Morelli, L.; Ferrari, M.; Peri, A.; Ferrari, V.; Moglia, A.; Pugliese, L.; Guarracino, F.; Mosca, F. Mixed reality for robotic treatment of a splenic artery aneurysm. Surg. Endosc. 2010, 24, 1204. [Google Scholar] [CrossRef] [Green Version]
- Rosset, A.; Spadola, L.; Ratib, O. OsiriX: An open-source software for navigating in multidimensional DICOM images. J. Digit. Imaging 2004, 17, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.M.; Vagvolgyi, B.P.; Agarwal, R.; Reiley, C.E.; Taylor, R.H.; Hager, G.D. Augmented reality during robot-assisted laparoscopic partial nephrectomy: Toward real-time 3D-CT to stereoscopic video registration. Urology 2009, 73, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef] [PubMed]


| Pre-Matching | Post-Matching | |||||
|---|---|---|---|---|---|---|
| Median (IQR) or n (%) | ADCT (n = 221) | UHR-CT (n = 100) | p Value | ADCT (n = 99) | UHR-CT (n = 99) | p Value |
| Age | 60 (49–68) | 62 (54–70) | 0.072 | 62 (50–70) | 62 (54–70) | 0.444 |
| Sex (%): Male | 166 (75.1) | 72 (72.0) | 0.583 | 73 (73.7) | 71 (71.7) | 0.873 |
| Female | 55 (24.9) | 28 (28.0) | 26 (26.3) | 28 (28.3) | ||
| BMI, kg/m2 | 24 (22–26) | 24 (22–26) | 0.334 | 24 (21–26) | 23 (22–26) | 0.175 |
| ASA score | 2 (1–2) | 2 (1–2) | 0.274 | 2 (1–2) | 2 (1–2) | 0.365 |
| eGFR, mL/min/1.73 m2 | 70 (59–80) | 68 (56–80) | 0.333 | 71 (58–80) | 68 (56–80) | 0.421 |
| Tumor side: Right | 114 (51.6) | 53 (53.0) | 0.904 | 54 (54.5) | 53 (53.5) | 0.887 |
| Left | 107 (48.4) | 47 (47.0) | 45 (45.5) | 46 (46.5) | ||
| Approach:Transperitoneal | 118 (53.4) | 47 (47.0) | 0.335 | 57 (57.6) | 47 (47.5) | 0.203 |
| Retroperitoneal | 103 (46.6) | 53 (53.0) | 42 (42.4) | 52 (52.5) | ||
| RENAL score | 7 (5–8) | 7 (5–8) | 0.375 | 7 (6–8) | 7 (5–8) | 0.133 |
| Hilar tumor | 48 (21.7) | 14 (14.0) | 0.127 | 21 (21.2) | 14 (14.1) | 0.264 |
| Cystic tumor | 36 (16.3) | 11 (11.0) | 0.237 | 16 (16.2) | 11 (11.1) | 0.408 |
| Post-Matching | |||
|---|---|---|---|
| Median (IQR) or n (%) | ADCT (n = 99) | UHR-CT (n = 99) | p Value |
| Surgical time, min | 158 (136–190) | 163 (148–190) | 0.440 |
| Console time, min | 110 (95–144) | 112 (91–133) | 0.483 |
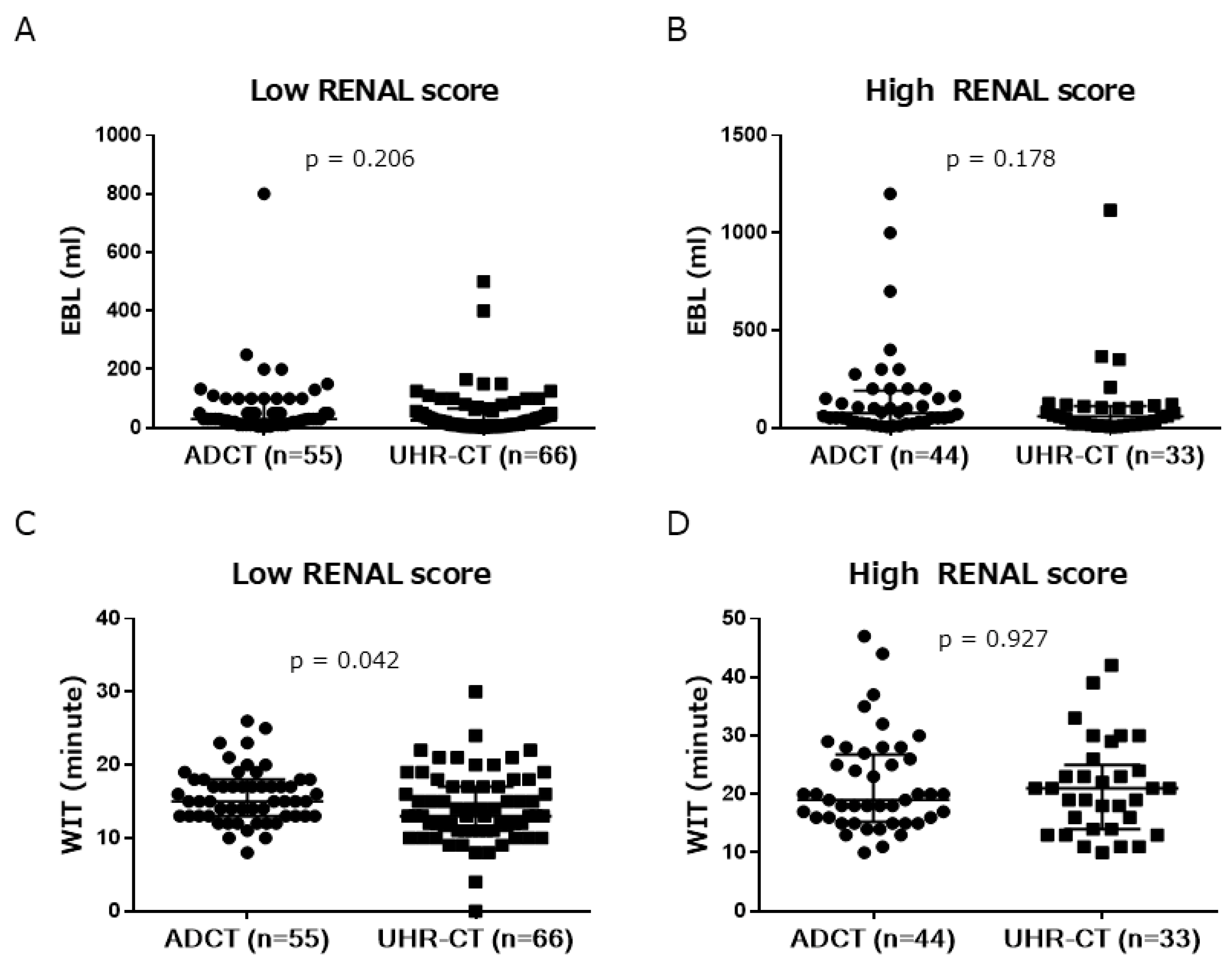
| WIT, min | 17 (14–20) | 15 (12–21) | 0.032 |
| EBL, ml | 50 (20–104) | 33 (10–85) | 0.028 |
| Transfusion | 3 (3.0) | 1 (1.0) | 0.621 |
| Negative surgical margins | 99 (100) | 98 (99.0) | 1.000 |
| Pathology, clear cell carcinoma | 80 (80.8) | 72 (72.7) | 0.246 |
| Clavien-Dindo ≥3 | 0 (0) | 2 (2.0) | 0.497 |
| Trifecta | 80 (80.8) | 81 (81.8) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahara, K.; Ohno, Y.; Fukaya, K.; Matsukiyo, R.; Nukaya, T.; Takenaka, M.; Zennami, K.; Ichino, M.; Fukami, N.; Sasaki, H.; et al. Novel Intraoperative Navigation Using Ultra-High-Resolution CT in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 2047. https://doi.org/10.3390/cancers14082047
Takahara K, Ohno Y, Fukaya K, Matsukiyo R, Nukaya T, Takenaka M, Zennami K, Ichino M, Fukami N, Sasaki H, et al. Novel Intraoperative Navigation Using Ultra-High-Resolution CT in Robot-Assisted Partial Nephrectomy. Cancers. 2022; 14(8):2047. https://doi.org/10.3390/cancers14082047
Chicago/Turabian StyleTakahara, Kiyoshi, Yoshiharu Ohno, Kosuke Fukaya, Ryo Matsukiyo, Takuhisa Nukaya, Masashi Takenaka, Kenji Zennami, Manabu Ichino, Naohiko Fukami, Hitomi Sasaki, and et al. 2022. "Novel Intraoperative Navigation Using Ultra-High-Resolution CT in Robot-Assisted Partial Nephrectomy" Cancers 14, no. 8: 2047. https://doi.org/10.3390/cancers14082047
APA StyleTakahara, K., Ohno, Y., Fukaya, K., Matsukiyo, R., Nukaya, T., Takenaka, M., Zennami, K., Ichino, M., Fukami, N., Sasaki, H., Kusaka, M., Toyama, H., Sumitomo, M., & Shiroki, R. (2022). Novel Intraoperative Navigation Using Ultra-High-Resolution CT in Robot-Assisted Partial Nephrectomy. Cancers, 14(8), 2047. https://doi.org/10.3390/cancers14082047






