Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Breast Cancer Tumor Tissue
2.2. Isolation of Human CD34+ Stem Cells from Umbilical Cord Blood
2.3. Generation of Humanized Patient-Derived Tumor Mice (hPDX)
2.4. Ethic Statements
2.5. Flow Cytometry
2.6. Statistical Analyses
3. Results
3.1. CD34+ Isolation from Umbilical Cord Blood and Cell Recovery after Cryopreservation
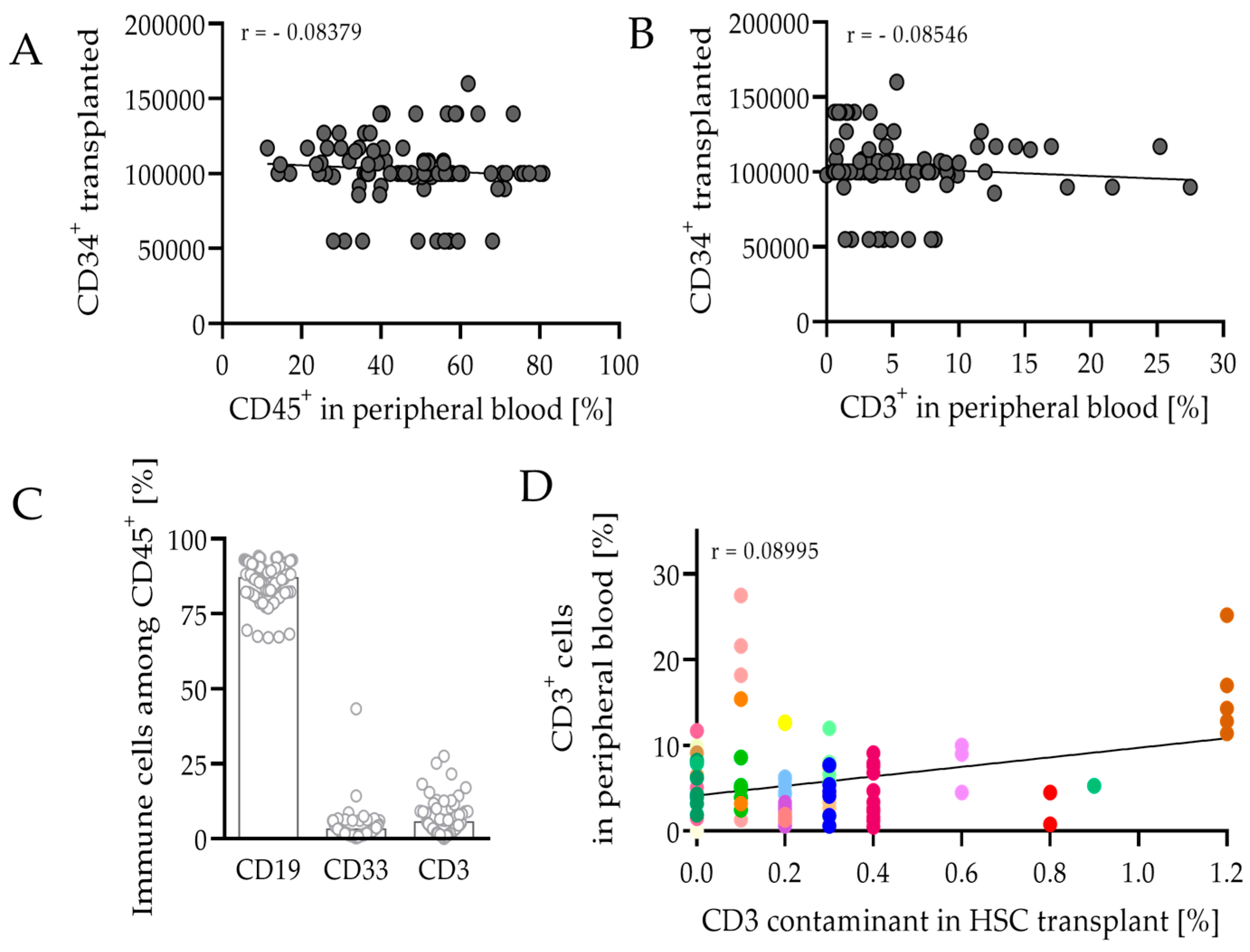
3.2. HSCT and Engraftment Success of the Human Immune System in Mice
3.3. Immunophenotyping of Splenocytes in hPDX by Multiparametric Flow Cytometry
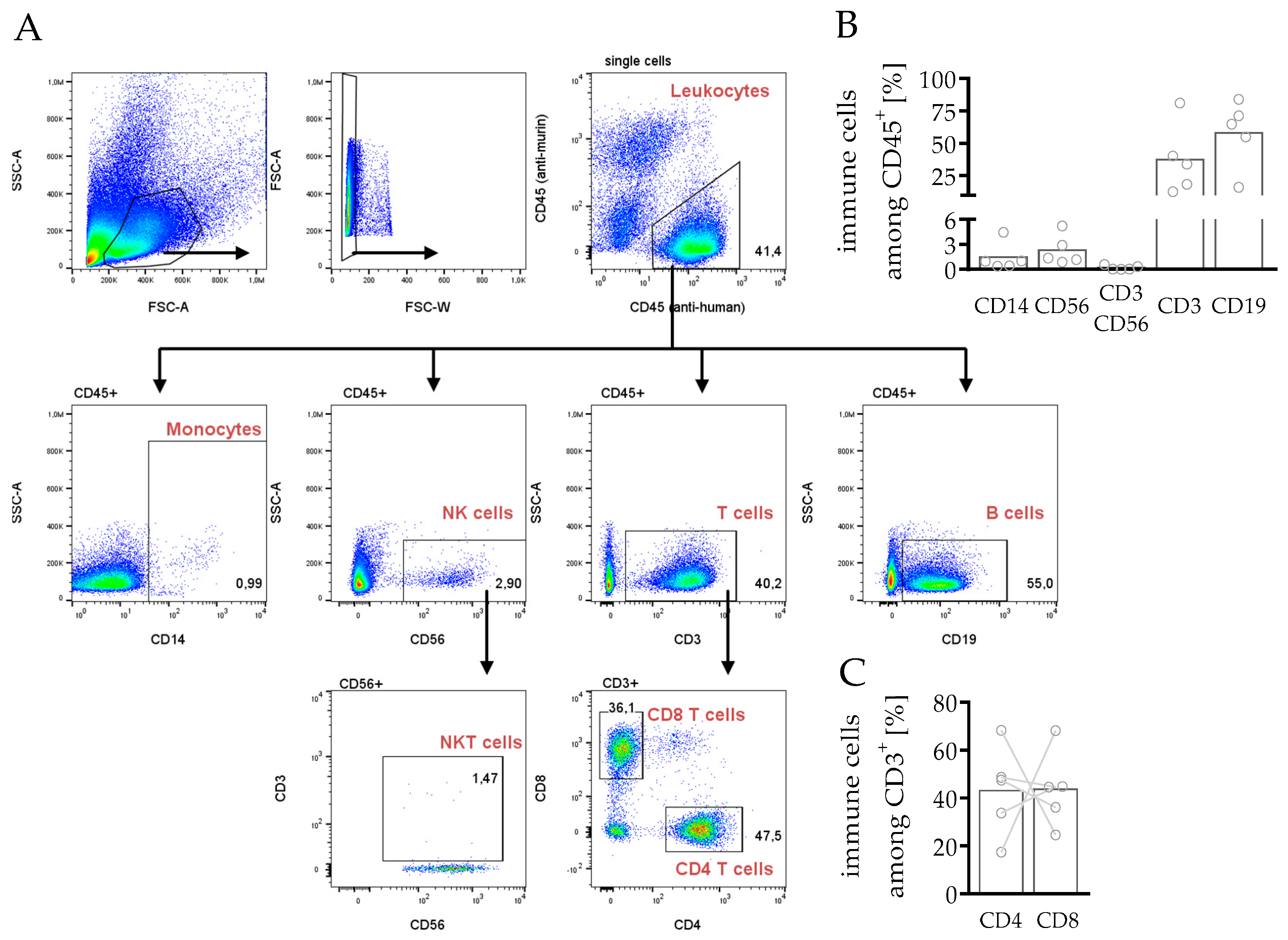
3.3.1. Composition of Leukocytes Reveals Capability to Fight Cancer in hPDX
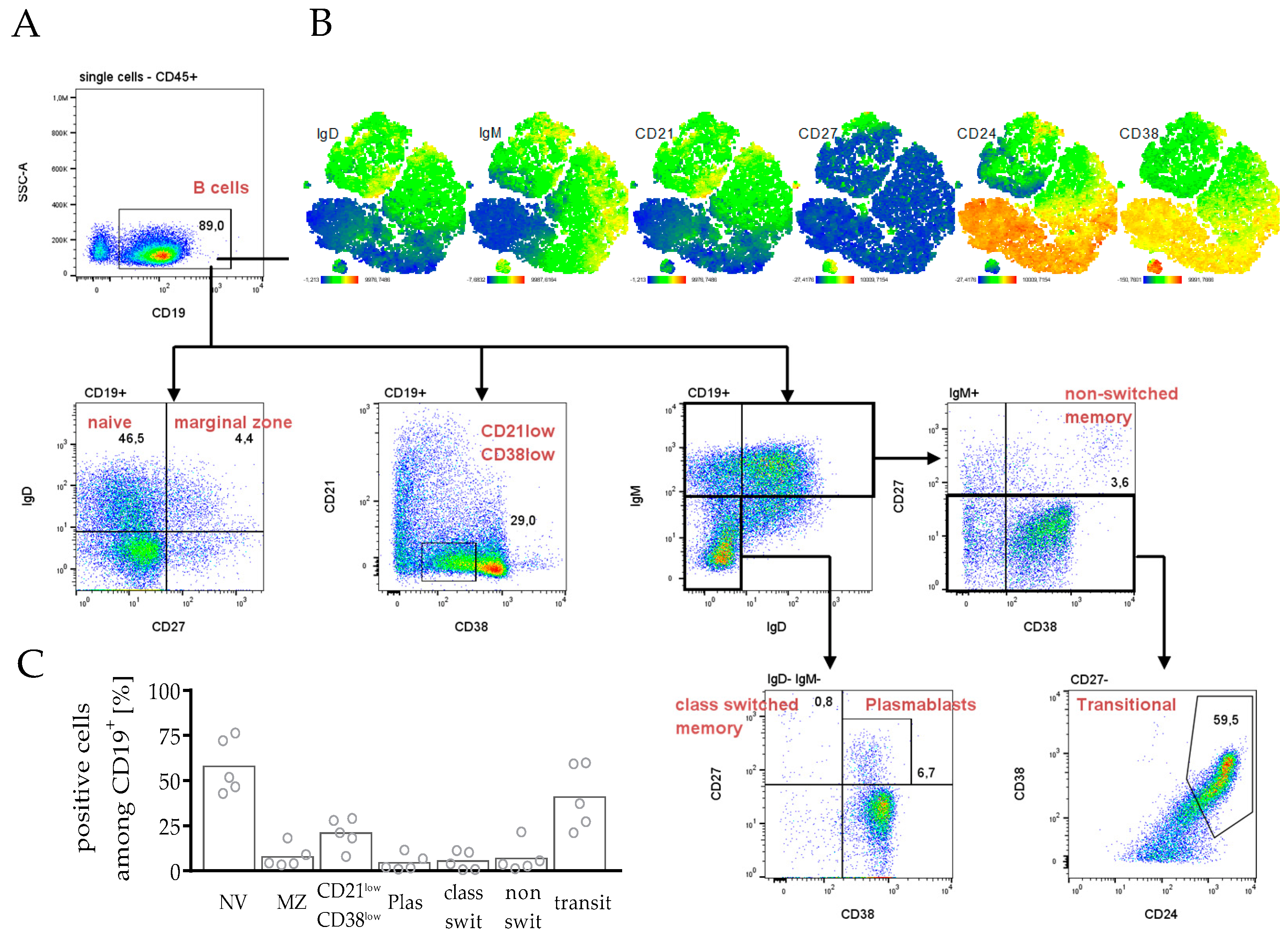
3.3.2. Differentiation and Maturation of B Cells in hPDX Are Comparable to the Known Human-like Heterogeneity
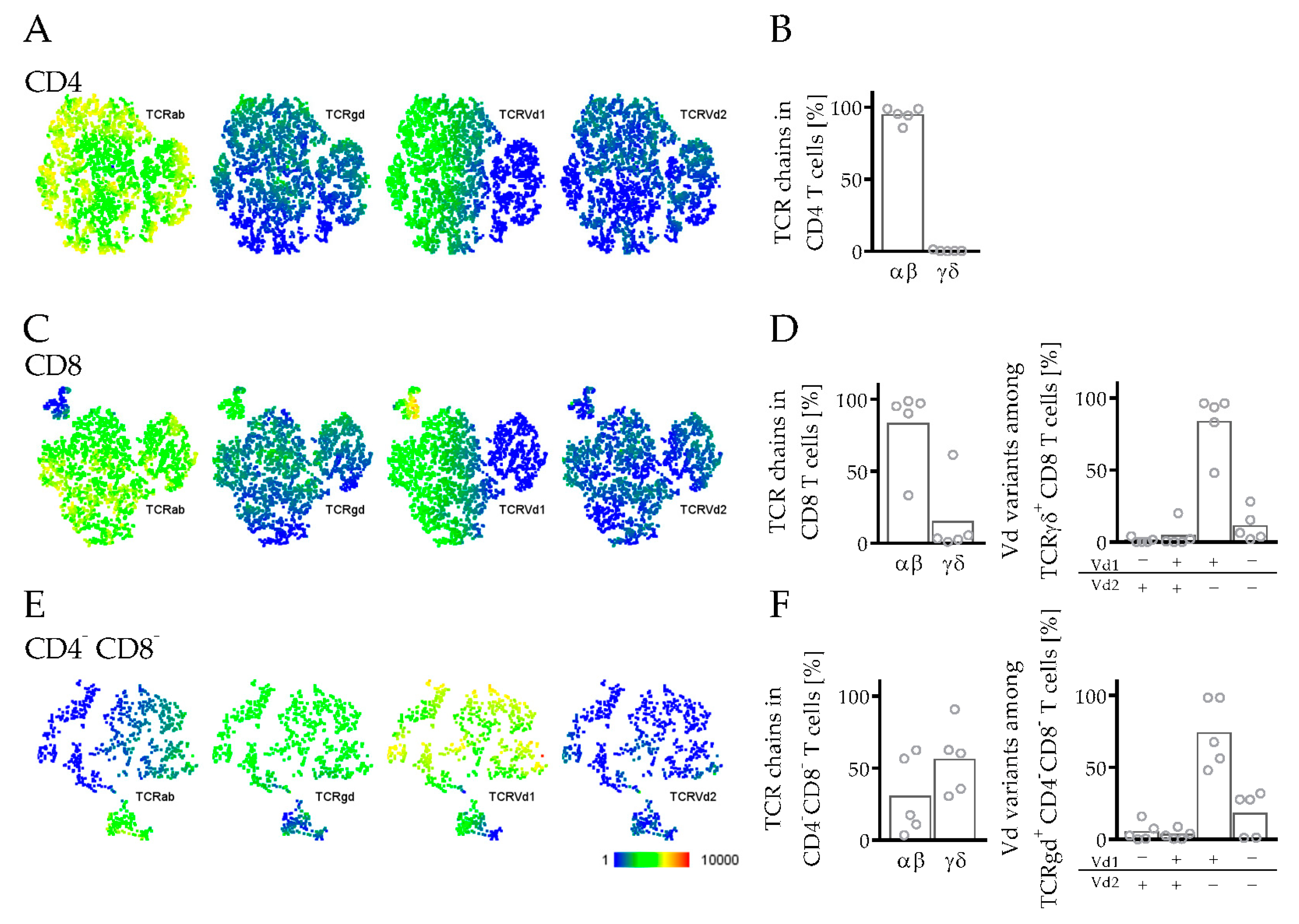
3.3.3. Phenotyping and Characterization of CD4 and CD8 T Cells in hPDX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balar, A.; Weber, J.S. PD-1 and PD-L1 antibodies in cancer: Current status and future directions. Cancer Immunol. Immunother. 2017, 66, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: Current concepts and future developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol. Rev. 2019, 290, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Subramanian, S. Intrinsic Resistance of Solid Tumors to Immune Checkpoint Blockade Therapy. Cancer Res. 2017, 77, 817–822. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.M.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients with Advanced Non-Small Cell Lung Cancer Treated with PD-1/PD-L1 Inhibitors or with Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards Daniel, R.; McDonald-Smith Grace, P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Pearson, T.; Greiner, D.L.; Shultz, L.D. Creation of “Humanized” Mice to Study Human Immunity. Curr. Protoc. Immunol. 2008, 81, 15.21.1–15.21.21. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/γcnull mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-scid IL2R gamma; null Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.G.; Cooke, A. Complement Lytic Activity Has no Role in the Pathogenesis of Autoimmune Diabetes in NOD Mice. Diabetes 1993, 42, 1574–1578. [Google Scholar] [CrossRef]
- Kataoka, S.; Satoh, J.; Fujiya, H.; Toyota, T.; Suzuki, R.; Itoh, K.; Kumagai, K. Immunologic Aspects of the Nonobese Diabetic (NOD) Mouse: Abnormalities of Cellular Immunity. Diabetes 1983, 32, 247–253. [Google Scholar] [CrossRef]
- Serreze, D.V.; Gaedeke, J.W.; Leiter, E.H. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: Defective regulation of cytokine receptors and protein kinase C. Proc. Natl. Acad. Sci. USA 1993, 90, 9625–9629. [Google Scholar] [CrossRef]
- Pearson, T.; Markees, T.G.; Serreze, D.V.; Pierce, M.A.; Marron, M.P.; Wicker, L.S.; Peterson, L.B.; Shultz, L.D.; Mordes, J.P.; Rossini, A.A.; et al. Genetic Disassociation of Autoimmunity and Resistance to Costimulation Blockade-Induced Transplantation Tolerance in Nonobese Diabetic Mice. J. Immunol. 2003, 171, 185–195. [Google Scholar] [CrossRef]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef]
- Cao, X.; Shores, E.W.; Hu-Li, J.; Anver, M.R.; Kelsail, B.L.; Russell, S.; Drago, J.; Noguchi, M.; Grinberg, A.; Bloom, E.T.; et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995, 2, 223–238. [Google Scholar] [CrossRef]
- Augsberger, C.; Hänel, G.; Xu, W.; Pulko, V.; Hanisch, L.J.; Augustin, A.; Challier, J.; Hunt, K.; Vick, B.; Rovatti, P.E.; et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC-specific T-cell bispecific antibody. Blood 2021, 138, 2655–2669. [Google Scholar] [CrossRef]
- Bacac, M.; Colombetti, S.; Herter, S.; Sam, J.; Perro, M.; Chen, S.; Bianchi, R.; Richard, M.; Schoenle, A.; Nicolini, V.; et al. CD20-TCB with Obinutuzumab Pretreatment as Next-Generation Treatment of Hematologic Malignancies. Clin. Cancer Res. 2018, 24, 4785–4797. [Google Scholar] [CrossRef] [PubMed]
- Rios-Doria, J.; Stevens, C.; Maddage, C.; Lasky, K.; Koblish, H.K. Characterization of human cancer xenografts in humanized mice. J. Immunother. Cancer 2020, 8, e000416. [Google Scholar] [CrossRef] [PubMed]
- Wege, A.K.; Weber, F.; Kroemer, A.; Ortmann, O.; Nimmerjahn, F.; Brockhoff, G. IL-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (HTM). Oncotarget 2016, 8, 2731–2744. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.L.; Harrison, L.C.; Goodman, D.J.; Cowan, P.J.; Hawthorne, W.J.; O’Connell, P.J.; Sutherland, R.M.; Lew, A.M. Preclinical screening for acute toxicity of therapeutic monoclonal antibodies in a hu-SCID model. Clin. Transl. Immunol. 2014, 3, e29. [Google Scholar] [CrossRef]
- Cai, S.; Wang, H.; Bailey, B.; Ernstberger, A.; Juliar, B.E.; Sinn, A.L.; Chan, R.J.; Jones, D.R.; Mayo, L.D.; Baluyut, A.R.; et al. Humanized Bone Marrow Mouse Model as a Preclinical Tool to Assess Therapy-Mediated Hematotoxicity. Clin. Cancer Res. 2011, 17, 2195–2206. [Google Scholar] [CrossRef]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of immune responses to anti-PD-1 mono and combination immunotherapy in hematopoietic humanized mice implanted with tumor xenografts. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef]
- Jespersen, H.; Lindberg, M.; Donia, M.; Söderberg, E.M.V.; Andersen, R.; Keller, U.; Ny, L.; Svane, I.M.; Nilsson, L.M.; Nilsson, J.A. Clinical responses to adoptive T-cell transfer can be modeled in an autologous immune-humanized mouse model. Nat. Commun. 2017, 8, 707. [Google Scholar] [CrossRef]
- Wang, M.; Yao, L.C.; Cheng, M.; Cai, D.; Martinek, J.; Pan, C.X.; Shi, W.; Ma, A.H.; De Vere White, R.W.; Airhart, S.; et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018, 32, 1537–1549. [Google Scholar] [CrossRef]
- Park, N.; Pandey, K.; Chang, S.K.; Kwon, A.Y.; Bin Cho, Y.; Hur, J.; Katwal, N.B.; Kim, S.K.; Lee, S.A.; Son, G.W.; et al. Preclinical platform for long-term evaluation of immuno-oncology drugs using hCD34+ humanized mouse model. J. Immunother. Cancer 2020, 8, e001513. [Google Scholar] [CrossRef]
- Horowitz, N.B.; Mohammad, I.; Moreno-Nieves, U.Y.; Koliesnik, I.; Tran, Q.; Sunwoo, J.B. Humanized Mouse Models for the Advancement of Innate Lymphoid Cell-Based Cancer Immunotherapies. Front. Immunol. 2021, 12, 648580. [Google Scholar] [CrossRef]
- Meehan, T.F.; Conte, N.; Goldstein, T.; Inghirami, G.; Murakami, M.A.; Brabetz, S.; Gu, Z.; Wiser, J.A.; Dunn, P.; Begley, D.A.; et al. PDX-MI: Minimal Information for Patient-Derived Tumor Xenograft Models. Cancer Res. 2017, 77, e62–e66. [Google Scholar] [CrossRef] [PubMed]
- Stripecke, R.; Münz, C.; Schuringa, J.J.; Bissig, K.; Soper, B.; Meeham, T.; Yao, L.; Di Santo, J.P.; Brehm, M.; Rodriguez, E.; et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol. Med. 2020, 12, e8662. [Google Scholar] [CrossRef] [PubMed]
- Streitz, M.; Miloud, T.; Kapinsky, M.; Reed, M.R.; Magari, R.; Geissler, E.K.; Hutchinson, J.A.; Vogt, K.; Schlickeiser, S.; Kverneland, A.H.; et al. Standardization of whole blood immune phenotype monitoring for clinical trials: Panels and methods from the ONE study. Transpl. Res. 2013, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.A.; Kronenberg, K.; Riquelme, P.; Wenzel, J.J.; Glehr, G.; Schilling, H.L.; Zeman, F.; Evert, K.; Schmiedel, M.; Mickler, M.; et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 2021, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Wege, A.K.; Ernst, W.; Eckl, J.; Frankenberger, B.; Vollmann-Zwerenz, A.; Männel, D.N.; Ortmann, O.; Kroemer, A.; Brockhoff, G. Humanized tumor mice-A new model to study and manipulate the immune response in advanced cancer therapy. Int. J. Cancer 2011, 129, 2194–2206. [Google Scholar] [CrossRef]
- Wege, A.K.; Rom-Jurek, E.; Jank, P.; Denkert, C.; Ugocsai, P.; Solbach, C.; Blohmer, J.; Sinn, B.; van Mackelenbergh, M.; Möbus, V.; et al. mdm2 gene amplification is associated with luminal breast cancer progression in humanized PDX mice and a worse outcome of estrogen receptor positive disease. Int. J. Cancer 2021, 150, 1357–1372. [Google Scholar] [CrossRef]
- Kronenberg, K.; Riquelme, P.; Hutchinson, J.A. Standard protocols for immune profiling of peripheral blood leucocyte subsets by flow cytometry using DuraClone IM reagents. Protoc. Exch. 2021. [Google Scholar] [CrossRef]
- Lang, J.; Weiss, N.; Freed, B.M.; Torres, R.M.; Pelanda, R. Generation of hematopoietic humanized mice in the newborn BALB/c-Rag2null Il2rγnull mouse model: A multivariable optimization approach. Clin. Immunol. 2011, 140, 102–116. [Google Scholar] [CrossRef]
- Lund, F.E. Cytokine-producing B lymphocytes—Key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef]
- Shen, P.; Fillatreau, S. Antibody-independent functions of B cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Lino, A.C.; Dorner, T.; Bar-Or, A.; Fillatreau, S. Cytokine-producing B cells: A translational view on their roles in human and mouse autoimmune diseases. Immunol. Rev. 2015, 269, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Du, W.; Lan, H.; Liu, Y.; Mao, C.; Du, J.; Mou, X. Development of humanized mouse with patient-derived xenografts for cancer immunotherapy studies: A comprehensive review. Cancer Sci. 2021, 112, 2592–2606. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Takahashi, T.; Okajima, A.; Shiokawa, M.; Ishii, N.; Katano, I.; Ito, R.; Ito, M.; Minegishi, M.; Minegishi, N.; et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γcnull (NOG) mice (hu-HSC NOG mice). Int. Immunol. 2009, 21, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.G.; Troyer, R.M.; Devi, S.; Akkina, R. Dengue virus infection and immune response in humanized RAG2(−/−)gamma(c)(−/−) (RAG-hu) mice. Virology 2007, 369, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Biswas, S.; Tallarico, A.S.C.; Sarkis, P.T.N.; Geng, S.; Panditrao, M.M.; Zhu, Q.; Marasco, W.A. Human B-cell ontogeny in humanized NOD/SCID γcnull mice generates a diverse yet auto/poly- and HIV-1-reactive antibody repertoire. Genes Immun. 2012, 13, 399–410. [Google Scholar] [CrossRef]
- Gorantla, S.; Sneller, H.; Walters, L.; Sharp, J.G.; Pirruccello, S.J.; West, J.T.; Wood, C.; Dewhurst, S.; Gendelman, H.E.; Poluektova, L. Human Immunodeficiency Virus Type 1 Pathobiology Studied in Humanized BALB/c-Rag2−/−gammac−/−mice. J. Virol. 2007, 81, 2700–2712. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.D.; Legrand, N.; Van Geelen, C.M.M.; Noerder, M.; Huntington, N.; Lim, A.; Yasuda, E.; Diehl, S.A.; Scheeren, F.A.; Ott, M.; et al. Generation of Human Antigen-Specific Monoclonal IgM Antibodies Using Vaccinated “Human Immune System” Mice. PLoS ONE 2010, 5, e13137. [Google Scholar] [CrossRef]
- Wege, A.K.; Schmidt, M.; Ueberham, E.; Ponnath, M.; Ortmann, O.; Brockhoff, G.; Lehmann, J. Co-transplantation of human hematopoietic stem cells and human breast cancer cells in NSG mice: A novel approach to generate tumor cell specific human antibodies. MAbs 2014, 6, 968–977. [Google Scholar] [CrossRef]
- Tonomura, N.; Habiro, K.; Shimizu, A.; Sykes, M.; Yang, Y.G. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood 2008, 111, 4293–4296. [Google Scholar] [CrossRef]
- Lang, J.; Kelly, M.; Freed, B.M.; McCarter, M.D.; Kedl, R.M.; Torres, R.M.; Pelanda, R. Studies of Lymphocyte Reconstitution in a Humanized Mouse Model Reveal a Requirement of T Cells for Human B Cell Maturation. J. Immunol. 2013, 190, 2090–2101. [Google Scholar] [CrossRef]
- Seung, E.; Tager, A.M. Humoral Immunity in Humanized Mice: A Work in Progress. J. Infect. Dis. 2013, 208 (Suppl. S2), S155–S159. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, Z.; Li, G.; Li, F.; Wang, L.; Zhang, L.; Zurawski, S.M.; Zurawski, G.; Levy, Y.; Su, L. Human innate responses and adjuvant activity of TLR ligands in vivo in mice reconstituted with a human immune system. Vaccine 2017, 35, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Borsotti, C.; Schickel, J.N.; Zhu, S.; Strowig, T.; Eynon, E.E.; Frleta, D.; Gurer, C.; Murphy, A.J.; Yancopoulos, G.D.; et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood 2017, 129, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Billerbeck, E.; Barry, W.T.; Mu, K.; Dorner, M.; Rice, C.M.; Ploss, A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor–, granulocyte-macrophage colony-stimulating factor–, and interleukin-3–expressing NOD-SCID IL2Rγnull humanized mice. Blood 2011, 117, 3076–3086. [Google Scholar] [CrossRef] [PubMed]
- Jangalwe, S.; Shultz, L.D.; Mathew, A.; Brehm, M.A. Improved B cell development in humanized NOD-scid IL2Rγ null mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 2016, 4, 427–440. [Google Scholar] [CrossRef]
- Song, Y.; Rongvaux, A.; Taylor, A.; Jiang, T.; Tebaldi, T.; Balasubramanian, K.; Bagale, A.; Terzi, Y.K.; Gbyli, R.; Wang, X.; et al. A highly efficient and faithful MDS patient-derived xenotransplantation model for pre-clinical studies. Nat. Commun. 2019, 10, 366. [Google Scholar] [CrossRef]
- Kumar, S.; Koenig, J.; Schneider, A.; Wermeling, F.; Boddul, S.; Theobald, S.; Vollmer, M.; Kloos, D.; Lachmann, N.; Klawonn, F.; et al. In Vivo Lentiviral Gene Delivery of HLA-DR and Vaccination of Humanized Mice for Improving the Human T and B Cell Immune Reconstitution. Biomedicines 2021, 9, 961. [Google Scholar] [CrossRef]
- Coronella-Wood, J.A.; Hersh, E.M. Naturally occurring B-cell responses to breast cancer. Cancer Immunol. Immunother. 2003, 52, 715–738. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-Evoked Regulatory B Cells Promote Breast Cancer Metastasis by Converting Resting CD4+ T Cells to T-Regulatory Cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef]
- Akkina, R. Humanized Mice for Studying Human Immune Responses and Generating Human Monoclonal Antibodies. Microbiol. Spectr. 2014, 2, 157–171. [Google Scholar] [CrossRef]
- Halkias, J.; Yen, B.; Taylor, K.T.; Reinhartz, O.; Winoto, A.; Robey, E.A.; Melichar, H.J. Conserved and divergent aspects of human T-cell development and migration in humanized mice. Immunol. Cell Biol. 2015, 93, 716–726. [Google Scholar] [CrossRef]
- Majji, S.; Wijayalath, W.; Shashikumar, S.; Pow-Sang, L.; Villasante, E.; Brumeanu, T.D.; Casares, S. Differential effect of HLA class-I versus class-II transgenes on human T and B cell reconstitution and function in NRG mice. Sci. Rep. 2016, 6, 28093. [Google Scholar] [CrossRef] [PubMed]
- Masse-Ranson, G.; Dusséaux, M.; Fiquet, O.; Darche, S.; Boussand, M.; Li, Y.; Lopez-Lastra, S.; Legrand, N.; Corcuff, E.; Toubert, A.; et al. Accelerated thymopoiesis and improved T-cell responses in HLA-A2/-DR2 transgenic BRGS-based human immune system mice. Eur. J. Immunol. 2019, 49, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Saito, Y.; Najima, Y.; Tanaka, S.; Ochi, T.; Tomizawa, M.; Doi, T.; Sone, A.; Suzuki, N.; Fujiwara, H.; et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc. Natl. Acad. Sci. USA 2010, 107, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Covassin, L.; Laning, J.; Abdi, R.; Langevin, D.L.; Phillips, N.E.; Shultz, L.D.; Brehm, M.A. Human peripheral blood CD4 T cell-engrafted non-obese diabetic-scid IL2rγnull H2-Ab1 tm1Gru Tg (human leucocyte antigen D-related 4) mice: A mouse model of human allogeneic graft-versus-host disease. Clin. Exp. Immunol. 2011, 166, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer immunotherapy with γδ T cells: Many paths ahead of us. Cell. Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- André, M.C.; Erbacher, A.; Gille, C.; Schmauke, V.; Goecke, B.; Hohberger, A.; Mang, P.; Wilhelm, A.; Mueller, I.; Herr, W.; et al. Long-Term Human CD34+ Stem Cell-Engrafted Nonobese Diabetic/SCID/IL-2R gamma(null) mice Show Impaired CD8+ T Cell Maintenance and a Functional Arrest of Immature NK Cells. J. Immunol. 2010, 185, 2710–2720. [Google Scholar] [CrossRef]
- Sato, Y.; Takata, H.; Kobayashi, N.; Nagata, S.; Nakagata, N.; Ueno, T.; Takiguchi, M. Failure of Effector Function of Human CD8+ T Cells in NOD/SCID/JAK3−/−Immunodeficient Mice Transplanted with Human CD34+ Hematopoietic Stem Cells. PLoS ONE 2010, 5, e13109. [Google Scholar] [CrossRef][Green Version]
- Strowig, T.; Gurer, C.; Ploss, A.; Liu, Y.F.; Arrey, F.; Sashihara, J.; Koo, G.; Rice, C.M.; Young, J.; Chadburn, A.; et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J. Exp. Med. 2009, 206, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, N.G.; de Almeida, P.E.; Stack, J.P.; Nelakanti, R.V.; Diecke, S.; Shao, N.Y.; Swijnenburg, R.J.; Sanchez-Freire, V.; Matsa, E.; Liu, C.; et al. Alloimmune Responses of Humanized Mice to Human Pluripotent Stem Cell Therapeutics. Cell Rep. 2017, 20, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Wege, A.K.; Dreyer, T.F.; Teoman, A.; Ortmann, O.; Brockhoff, G.; Bronger, H. CX3CL1 Overexpression Prevents the For-mation of Lung Metastases in Trastuzumab-Treated MDA-MB-453-Based Humanized Tumor Mice (HTM). Cancers 2021, 13, 2459, ISBN 2072-6694. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Lin, S.; Huang, G.; Cheng, L.; Li, Z.; Xiao, Y.; Deng, Q.; Jiang, Y.; Li, B.; Lin, S.; Wang, S.; et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs 2018, 10, 1301–1311. [Google Scholar] [CrossRef]
- Choi, B.; Lee, J.S.; Kim, S.J.; Hong, D.; Park, J.B.; Lee, K.Y. Anti-tumor effects of anti-PD-1 antibody, pembrolizumab, in humanized NSG PDX mice xenografted with dedifferentiated liposarcoma. Cancer Lett. 2020, 478, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.N.; Fong, S.Y.; Tan, W.W.S.; Tan, S.Y.; Liu, M.; Cheng, J.Y.; Lim, S.; Suteja, L.; Huang, E.K.; Chan, J.K.Y.; et al. Establishment and Characterization of Humanized Mouse NPC-PDX Model for Testing Immunotherapy. Cancers 2020, 12, 1025. [Google Scholar] [CrossRef]
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.J. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front. Immunol. 2019, 10, 1719. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2021, 11, 3739. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; Vignali, D.A.A.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008, 10, 29–37. [Google Scholar] [CrossRef]
- Sasada, T.; Kimura, M.; Yoshida, Y.; Kanai, M.; Takabayashi, A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: Possible involvement of regulatory T cells in disease progression. Cancer 2003, 98, 1089–1099. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those at Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Glotzbecker, B.; Mills, H.; Vasir, B.; Tzachanis, D.; Levine, J.D.; Joyce, R.M.; Wellenstein, K.; Keefe, W.; Schickler, M.; et al. PD-1 Blockade by CT-011, Anti-PD-1 Antibody, Enhances Ex Vivo T-cell Responses to Autologous Dendritic Cell/Myeloma Fusion Vaccine. J. Immunother. 2011, 34, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.A.; Bellinghausen, I.; Trinschek, B.; Becker, C. Translating Treg Therapy in Humanized Mice. Front. Immunol. 2015, 6, 623. [Google Scholar] [CrossRef] [PubMed]
- Onoe, T.; Kalscheuer, H.; Danzl, N.; Chittenden, M.; Zhao, G.; Yang, Y.G.; Sykes, M. Human Natural Regulatory T Cell Development, Suppressive Function, and Postthymic Maturation in a Humanized Mouse Model. J. Immunol. 2011, 187, 3895–3903. [Google Scholar] [CrossRef]
- Serr, I.; Fürst, R.W.; Achenbach, P.; Scherm, M.G.; Gökmen, F.; Haupt, F.; Sedlmeier, E.M.; Knopff, A.; Shultz, L.; Willis, R.A.; et al. Type 1 diabetes vaccine candidates promote human Foxp3+Treg induction in humanized mice. Nat. Commun. 2016, 7, 10991. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Jacobse, J.; Li, J.; Allaman, M.M.; Otipoby, K.L.; Sampson, E.R.; Wilson, K.T.; Goettel, J.A. HLA-Restriction of Human Treg Cells Is Not Required for Therapeutic Efficacy of Low-Dose IL-2 in Humanized Mice. Front. Immunol. 2021, 12, 630204. [Google Scholar] [CrossRef]
- Schilling, H.L.; Glehr, G.; Kapinsky, M.; Ahrens, N.; Riquelme, P.; Cordero, L.; Bitterer, F.; Schlitt, H.J.; Geissler, E.K.; Haferkamp, S.; et al. Development of a Flow Cytometry Assay to Predict Immune Checkpoint Blockade-Related Complications. Front. Immunol. 2021, 12, 765644. [Google Scholar] [CrossRef]
- Holl, E.K.; Frazier, V.N.; Landa, K.; Beasley, G.M.; Hwang, E.S.; Nair, S.K. Examining Peripheral and Tumor Cellular Immunome in Patients with Cancer. Front. Immunol. 2019, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.R.; Dávila-González, D.; Choi, D.S.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; Dave, B.; Chang, J.C. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018, 20, 108. [Google Scholar] [CrossRef] [PubMed]


| 488 nm (Blue Laser) | 638 nm (Red Laser) | 405 nm (Violet Laser) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FITC | PE | ECD 1 | PerCP-Vio770 PerCPCy5.5 PECy5.5 | PeCy7 | APC AF647 | A700/ APC-A700 | APC-A750 | PB | KrOrange | |
| basic phenotyping | CD16 | CD56 | CD19 | CD45 2 (mouse) | CD14 | CD4 5 | CD8 7 | CD3 | --- | CD45 (human) |
| T cell subsets | CD45RA | --- | CD27 | CD45 2 (mouse) | CCR7 | CD4 5 | CD8 7 | CD3 | CD57 | CD45 (human) |
| TCR | TCRγδ | TCRαβ | --- | CD45 2 (mouse) | TCR Vδ1 | CD4 5 | CD8 7 | CD3 | TCR Vδ2 | CD45 (human) |
| B cells | IgD | CD21 | CD19 | CD45 2 (mouse) | CD27 | CD24 5 | --- | CD38 | IgM | CD45 (human) |
| exhausted CD4 T cells | CD49b | TIGIT | CD27 | KLRG1 3 | CD279 | TIM3 5 | CD8 7 + CD45 8 (mouse) | CD3 | CD4 | CD45 (human) |
| exhausted CD8 T cells | CD49b | CD160 | CD27 | CD244 4 | CD279 | CD127 5 | CD8 7 | CD3 | CD4 + CD45 (mouse) | CD45 (human) |
| TREGs | CD45RA | CD45 (mouse) | CD8 | CD25 3 | TIGIT | FoxP3 6 | CD4 7 | CD3 | Helios | CD45 (human) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruss, C.; Kellner, K.; Ortmann, O.; Seitz, S.; Brockhoff, G.; Hutchinson, J.A.; Wege, A.K. Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice. Cancers 2022, 14, 2214. https://doi.org/10.3390/cancers14092214
Bruss C, Kellner K, Ortmann O, Seitz S, Brockhoff G, Hutchinson JA, Wege AK. Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice. Cancers. 2022; 14(9):2214. https://doi.org/10.3390/cancers14092214
Chicago/Turabian StyleBruss, Christina, Kerstin Kellner, Olaf Ortmann, Stephan Seitz, Gero Brockhoff, James A. Hutchinson, and Anja Kathrin Wege. 2022. "Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice" Cancers 14, no. 9: 2214. https://doi.org/10.3390/cancers14092214
APA StyleBruss, C., Kellner, K., Ortmann, O., Seitz, S., Brockhoff, G., Hutchinson, J. A., & Wege, A. K. (2022). Advanced Immune Cell Profiling by Multiparameter Flow Cytometry in Humanized Patient-Derived Tumor Mice. Cancers, 14(9), 2214. https://doi.org/10.3390/cancers14092214