A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies?
Abstract
:Simple Summary
Abstract
1. Gastrointestinal Cancers—General Characteristics
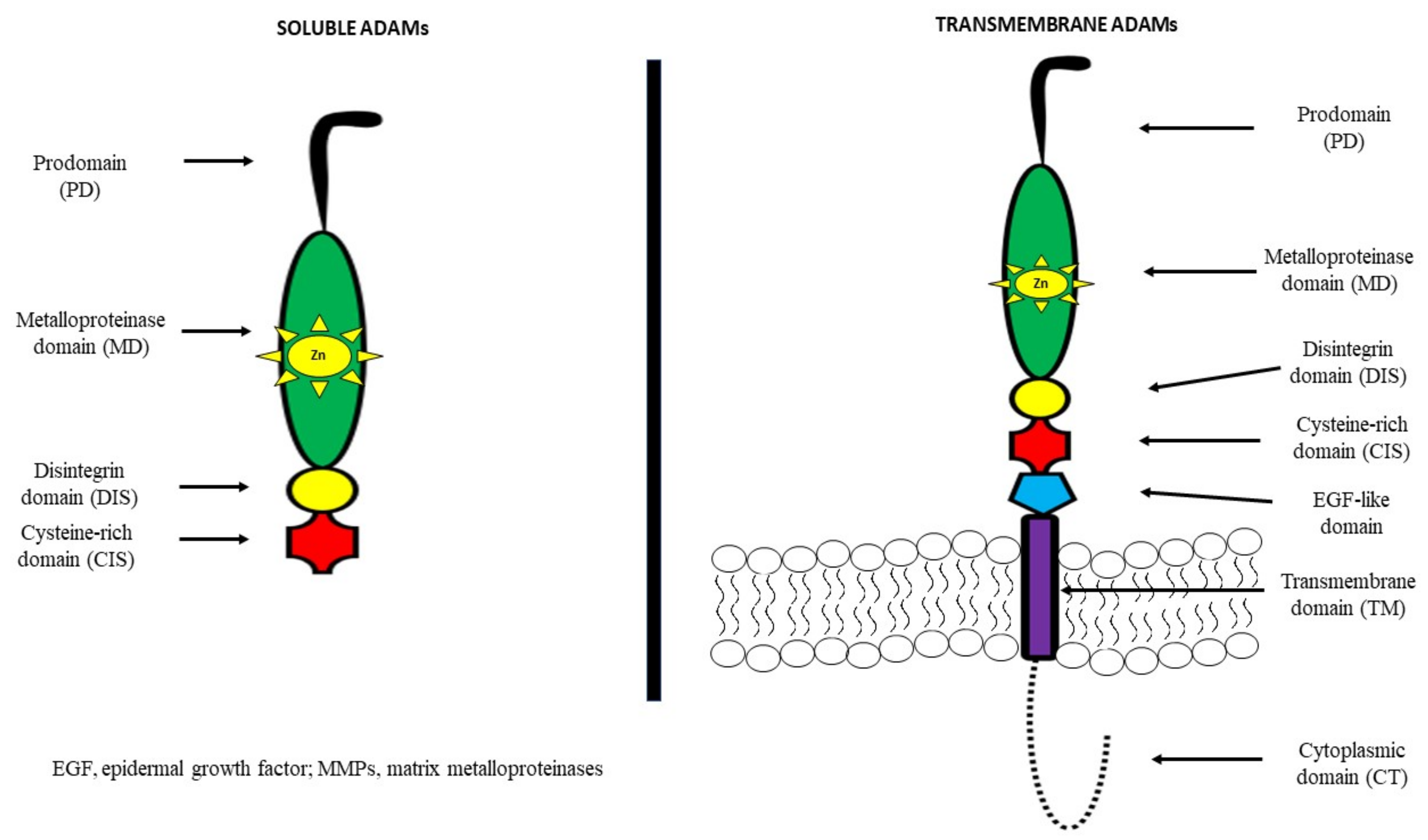
2. A Disintegrin and Metalloproteinase (ADAM)—General Information
3. A Disintegrin and Metalloproteinase (ADAM)—Their Role in Tumour Development
4. A Disintegrin and Metalloproteinase (ADAMs)—Their Role in the Development and Prognosis of Gastrointestinal Cancers (GI)
5. A Disintegrin and Metalloproteinase 8 (ADAM8)
6. A Disintegrin and Metalloproteinase 9 (ADAM9)
7. A Disintegrin and Metalloproteinase 10 (ADAM10)
8. A Disintegrin and Metalloproteinase 12 (ADAM12)
9. A Disintegrin and Metalloproteinase 15 (ADAM15)
10. A Disintegrin and Metalloproteinase 17 (ADAM17)
11. A Disintegrin and Metalloproteinase 28 (ADAM28)
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Facts & Figures 2018. Atlanta: American Cancer Society. 2018. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf (accessed on 26 April 2022).
- Dizdar, Ö.; Kılıçkap, S. Global Epidemiology of Gastrointestinal Cancers; Yalcin, S., Philip, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–12. [Google Scholar]
- Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf (accessed on 26 April 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.; Schmid, J.; Ebert, M.; Soucek, P.; Kunicka, T.; Liska, V.; Bruha, J.; Neary, P.; Dezeeuw, J.; Tommasino, M.; et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tomita, T.; Dixon, M.F.; Axon, A.T.R.; Robinson, P.A.; Crabtree, J.E. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in Helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J. Infect. Dis. 2002, 185, 332–340. [Google Scholar] [CrossRef] [Green Version]
- D’Elia, L.; Galletti, F.; Strazzullo, P. Dietary salt intake and risk of gastric cancer. Cancer Treat. Res. 2014, 159, 83–95. [Google Scholar]
- Yavuzsen, T.; Kazaz, N.; Tanriverdi, Ö.; Akman, T.; Davis, M.P. Symptom Management in Gastrointestinal Cancers; Yalcin, S., Philip, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 669–685. [Google Scholar]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Perex-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Yennu, S.; Kang, J.K.; Bruera, E. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015, 121, 3027–3035. [Google Scholar] [CrossRef]
- Karaosmanoglu, A.D.; Onur, M.R.; Arellano, R.S. Imaging in Gastrointestinal Cancers; Yalcin, S., Philip, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 445–464. [Google Scholar]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Gryko, M.; Pączek, S.; Szmitkowski, M.; Kędra, B.; Mroczko, B. Matrix metalloproteinase 2 (MMP-2) and its tissue inhibitor 2 (TIMP-2) in pancreatic cancer (PC). Oncotarget 2019, 10, 395–403. [Google Scholar] [CrossRef]
- Mroczko, B.; Kozłowski, M.; Groblewska, M.; Łukaszewicz, M.; Nikliński, J.; Jelski, W.; Laudański, J.; Chyczewski, L.; Szmitkowski, M. The diagnostic value of the measurement of matrix metalloproteinase 9 (MMP-9), squamous cell cancer antigen (SCC) and carcinoembryonic antigen (CEA) in the sera of esophageal cancer patients. Clin. Chim. Acta 2008, 389, 61–66. [Google Scholar] [CrossRef]
- Mroczko, B.; Łukaszewicz-Zając, M.; Wereszczyńska-Siemiątkowska, U.; Groblewska, M.; Gryko, M.; Kędra, B.; Jurkowska, G.; Szmitkowski, M. Clinical significance of the measurements of serum matrix metalloproteinase-9 and its inhibitor (tissue inhibitor of metalloproteinase-1) in patients with pancreatic cancer. Metalloproteinase-9 as an independent prognostic factor. Pancreas 2009, 38, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Łukaszewicz-Zając, M.; Guzińska-Ustymowicz, K.; Gryko, M.; Czyżewska, J.; Kemona, A.; Kędra, B.; Szmitkowski, M. Expression of matrix metalloproteinase-9 in the neoplastic and interstitial inflammatory infiltrate cells in gastric cancer. Folia Histochem. Cytobiol. 2009, 47, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczko, B.; Groblewska, M.; Łukaszewicz-Zając, M.; Bandurski, R.; Kędra, B.; Szmitkowski, M. Pre-treatment serum and plasma levels of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) in gastric cancer patients. Clin. Chem. Lab. Med. 2009, 47, 1133–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroczko, B.; Łukaszewicz-Zając, M.; Gryko, M.; Kędra, B.; Szmitkowski, M. Clinical significance of serum levels of matrix metalloproteinase 2 (MMP-2) and its tissue inhibitor (TIMP-2) in gastric cancer. Folia Histochem. Cytobiol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melton, S.D.; Genta, R.M.; Souza, R.F. Biomarkers and Molecular Diagnostic Tests in Gastrointestinal Tract and Pancreatic Neoplasms. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łukaszewicz-Zając, M.; Mroczko, B. Circulating Biomarkers of Colorectal Cancer (CRC)—Their Utility in Diagnosis and Prognosis. J. Clin. Med. 2021, 10, 2391. [Google Scholar] [CrossRef]
- Łukaszewicz-Zając, M.; Mroczko, B.; Gryko, M.; Kędra, B.; Szmitkowski, M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin. Exp. Med. 2011, 11, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Pawluczuk, E.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Mroczko, B. Serum CXCL8 and Its Specific Receptor (CXCR2) in Gastric Cancer. Cancers 2021, 13, 5186. [Google Scholar] [CrossRef]
- Valacca, C.; Tassone, E.; Mignatti, P. TIMP-2 Interaction with MT1-MMP Activates the AKT Pathway and Protects Tumor Cells from Apoptosis. PLoS ONE 2015, 10, e0136797. [Google Scholar] [CrossRef]
- Schumacher, N.; Rose-John, S.; Schmidt-Arras, D. ADAM-Mediated Signalling Pathways in Gastrointestinal Cancer Formation. Int. J. Mol. Sci. 2020, 21, 5133. [Google Scholar] [CrossRef]
- Edwards, D.R.; Handsley, M.M.; Pennington, C.J. The ADAM metalloproteinases. Mol. Asp. Med. 2008, 29, 258–289. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Hattermann, K.; Held-Feindt, J. Lost in disruption: Role of proteases in glioma invasion and progression. Biochim. Biophys. Acta 2012, 1825, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Englund, A.T.; Geffner, M.E.; Nagel, R.A.; Lippe, B.M.; Braunstein, G.D. Pediatric germ cell and human chorionic gonadotropin producing tumors. Clinical and laboratory features. Am. J. Dis. Child. 1991, 145, 1294–1297. [Google Scholar] [CrossRef]
- Uhm, J.H.; Dooley, N.P.; Villemure, J.G.; Yong, V.W. Glioma invasion in vitro: Regulation by matrix metalloprotease-2 and protein kinase C. Clin. Exp. Metastasis 1996, 14, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Haoyuan, M.A.; Yanshu, L.I. Structure, regulatory factors and cancer-related physiological effects of ADAM9. Cell Adhes. Migr. 2020, 14, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Giebeler, N.; Zigrino, P.A. Disintegrin and Metalloprotease (ADAM): Historical Overview of Their Functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef]
- Mochizuki, S.; Okada, Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007, 98, 621–628. [Google Scholar] [CrossRef]
- Lorenzen, I.; Lokau, J.; Düsterhöft, S.; Trad, A.; Garbers, C.; Scheller, J.; Rose-John, S.; Grötzinger, J. The membrane-proximal domain of A Disintegrin and Metalloprotease 17 (ADAM17) is responsible for recognition of the interleukin-6 receptor and interleukin-1 receptor II. FEBS Lett. 2012, 586, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Düsterhöft, S.; Michalek, M.; Kordowski, F.; Oldefest, M.; Sommer, A.; Röseler, J.; Reiss, K.; Grötzinger, J.; Lorenzen, I. Extracellular Juxtamembrane Segment of ADAM17 Interacts with Membranes and Is Essential for Its Shedding Activity. Biochemistry (Moscow) 2015, 54, 5791–5800. [Google Scholar] [CrossRef]
- Schlondorff, J.; Blobel, C.P. Metalloprotease-disintegrins: Modular proteins capable of promoting cell–cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 1999, 112, 3603–3617. [Google Scholar] [CrossRef]
- Ghigna, C.; Giordano, S.; Shen, H.; Benvenuto, F.; Castiglioni, F.; Comoglio, P.M.; Green, M.R.; Riva, G.; Biamonti, S. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol. Cell 2005, 20, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Takehara, T.; Liu, X.; Fujimoto, J.; Friedman, S.L.; Takahashi, H. Expression and role of Bcl-xL in human hepatocellular carcinomas. Hepatology 2001, 34, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Line, A.; Slucka, Z.; Stengrevics, A.; Li, G.; Rees, R.C. Altered splicing pattern of TACC1 mRNA in gastric cancer. Cancer Genet. Cytogenet. 2002, 139, 78–83. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-S. Alternative Splicing and Its Impact as a Cancer Diagnostic Marker. Genom. Inform. 2012, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chute, M.; Jana, S.; Kassiri, Z. Disintegrin and metalloproteinases (ADAMs and ADAM-TSs), the emerging family of proteases in heart physiology and pathology. Curr. Opin. Physiol. 2018, 1, 34–45. [Google Scholar] [CrossRef]
- Duffy, M.J.; Mullooly, M.; O’Donovan, N.; Sukor, S.; Crown, J.; Pierce, A.; McGowan, P.M. The ADAMs family of proteases: New biomarkers and therapeutic targets for cancer? Clin. Proteom. 2011, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, M.J.; McKiernan, E.; O’Donovan, N.; McGowan, P.M. Role of ADAMs in cancer formation and progression. Clin. Cancer Res. 2009, 15, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Walkiewicz, K.; Kozieł, P.; Bednarczyk, M.; Błażelonis, A.; Mazurek, U.; Muc-Wierzgoń, M. Expression of Migration-Related Genes in Human Colorectal Cancer and Activity of a Disintegrin and Metalloproteinase 17. Biomed. Res. Int. 2016, 2016, 8208904. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, S.; Shimoda, M.; Shiomi, T.; Fujii, Y.; Okada, Y. ADAM28 is activated by MMP-7 (matrilysin-1) and cleaves insulin-like growth factor binding protein-3. Biochem. Biophys. Res. Commun. 2004, 315, 79–84. [Google Scholar] [CrossRef]
- Gao, M.Q.; Kim, B.G.; Kang, S.; Choi, Y.P.; Yoon, J.H.; Cho, N.H. Human breast cancer-associated fibroblasts enhance cancer cell proliferation through increased TGF-α cleavage by ADAM17. Cancer Lett. 2013, 336, 240–246. [Google Scholar] [CrossRef]
- Carl-McGrath, S.; Lendeckel, U.; Ebert, M.; Roessner, A.; Röcken, C. The disintegrin-metalloproteinases ADAM9, ADAM12, and ADAM15 are upregulated in gastric cancer. Int. J. Oncol. 2005, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, L.; Kelwick, R.; Decock, J.; Edwards, D.R. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front. Biosci. 2011, 16, 1861–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herat, L.; Rudnicka, C.; Okada, Y.; Mochizuki, S.; Schlaich, M.; Matthews, V. The Metalloproteinase ADAM28 Promotes Metabolic Dysfunction in Mice. Int. J. Mol. Sci. 2017, 18, 884. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Moses, M.A. ADAM12 induces estrogen-independence in breast cancer cells. Breast Cancer Res. Treat. 2012, 131, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Rodig, S.; Bielenberg, D.; Zurakowski, D.; Moses, M.A. ADAM12 transmembrane and secreted isoforms promote breast tumor growth: A distinct role for ADAM12-S protein in tumor metastasis. J. Biol. Chem. 2011, 286, 20758–20768. [Google Scholar] [CrossRef] [Green Version]
- Fritzsche, F.R.; Wassermann, K.; Jung, M.; Tölle, A.; Kristiansen, I.; Lein, M.; Johannsen, M.; Dietel, M.; Jung, K.; Kristiansen, G. ADAM9 is highly expressed in renal cell cancer and is associated with tumour progression. BMC Cancer 2008, 26, 179. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Li, Z.; Gao, W.; Yu, G.; Liu, D.; Pan, F. ADAM-12 as a diagnostic marker for the proliferation, migration and invasion in patients with small cell lung cancer. PLoS ONE 2014, 9, e85936. [Google Scholar] [CrossRef]
- Ni, P.; Yu, M.; Zhang, R.; He, M.; Wang, H.; Chen, S.; Duan, G. Prognostic Significance of ADAM17 for Gastric Cancer Survival: A Meta-Analysis. Medicina 2020, 56, 322. [Google Scholar] [CrossRef]
- Huang, J.; Bai, Y.; Huo, L.; Xiao, J.; Fan, X.; Yang, Z.; Chen, H.; Yang, Z. Upregulation of a disintegrin and metalloprotease 8 is associated with progression and prognosis of patients with gastric cancer. Transl. Res. 2015, 166, 602–613. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeung, H.C.; Rha, S.Y.; Yu, E.J.; Kim, T.S.; Shin, Y.K.; Zhang, X.; Park, K.H.; Park, S.W.; Chung, H.C.; et al. The effect of disintegrin-metalloproteinase ADAM9 in gastric cancer progression. Mol. Cancer Ther. 2014, 13, 3074–3085. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Ye, Z.Y.; Li, L.; Zhao, Z.S.; Shao, Q.S.; Tao, H.Q. ADAM 10 is associated with gastric cancer progression and prognosis of patients. J. Surg. Oncol. 2011, 103, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, H.; Zhang, C.; He, J.; Wei, H.; Zhou, M.; Lu, Y.; Sun, Y.; Ding, J.W.; Zeng, J.; et al. ADAM17 promotes epithelial-mesenchymal transition via TGF-β/Smad pathway in gastric carcinoma cells. Int. J. Oncol. 2016, 49, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Gu, J.; Qi, Y.; Lu, Y.; Yang, L.; Liu, J.; Liang, X. ADAM28 from both endothelium and gastric cancer cleaves von Willebrand Factor to eliminate von Willebrand Factor-induced apoptosis of gastric cancer cells. Eur. J. Pharmacol. 2021, 898, 173994. [Google Scholar] [CrossRef] [PubMed]
- Dosch, J.; Ziemke, E.; Wan, S.; Luker, K.; Welling, T.; Hardiman, K.; Fearon, E.; Thomas, S.; Flynn, M.; Rios-Doria, J.; et al. Targeting ADAM17 inhibits human colorectal adenocarcinoma progression and tumor-initiating cell frequency. Oncotarget 2017, 8, 65090–65099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Bai, Y.; Huo, L.; Chen, H.; Huang, J.; Li, J.; Fan, X.; Yang, Z.; Wang, L.; Wang, J. Expression of A disintegrin and metalloprotease 8 is associated with cell growth and poor survival in colorectal cancer. BMC Cancer 2014, 14, 568. [Google Scholar] [CrossRef] [Green Version]
- Walkiewicz, K.; Strzelczyk, J.; Waniczek, D.; Biernacki, K.; Muc-Wierzgoń, M.; Copija, A.; Nowakowska-Zajdel, E. Adamalysines as Biomarkers and a Potential Target of Therapy in Colorectal Cancer Patients: Preliminary Results. Dis. Markers 2019, 2019, 5035234. [Google Scholar] [CrossRef]
- Toquet, C.; Colson, A.; Jarry, A.; Bezieau, S.; Volteau, C.; Boisseau, P.; Merlin, D.; Laboisse, C.L.; Mosnier, J.F. ADAM15 to α5β1 integrin switch in colon carcinoma cells: A late event in cancer progression associated with tumor dedifferentiation and poor prognosis. Int. J. Cancer 2012, 130, 278–287. [Google Scholar] [CrossRef]
- Yamada, D.; Ohuchida, K.; Mizumoto, K.; Ohhashi, S.; Yu, J.; Egami, T.; Fujita, H.; Nagai, E.; Tanaka, M. Increased expression of ADAM 9 and ADAM 15 mRNA in pancreatic cancer. Anticancer Res. 2007, 27, 793–799. [Google Scholar]
- Valkovskaya, N.; Kayed, H.; Felix, K.; Hartmann, D.; Giese, N.A.; Osinsky, S.P.; Friess, H.; Kleeff, J. ADAM8 expression is associated with increased invasiveness and reduced patient survival in pancreatic cancer. J. Cell Mol. Med. 2007, 11, 1162–1174. [Google Scholar] [CrossRef]
- Alldinger, I.; Dittert, D.; Peiper, M.; Fusco, A.; Chiappetta, G.; Staub, E.; Lohr, M.; Jesnowski, R.; Baretton, G.; Ockert, D.; et al. Gene expression analysis of pancreatic cell lines reveals genes overexpressed in pancreatic cancer. Pancreatology 2005, 5, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Lei, S.; Wu, S. ADAM10 is overexpressed in human hepatocellular carcinoma and contributes to the proliferation, invasion and migration of HepG2 cells. Oncol. Rep. 2013, 30, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiu, J.S.; Hsieh, M.J.; Chiou, H.L.; Wang, H.L.; Yeh, C.B.; Yang, S.F.; Chou, Y.E. Impact of ADAM10 gene polymorphisms on hepatocellular carcinoma development and clinical characteristics. Int. J. Med. Sci. 2018, 15, 1334–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tan, Y.F.; Jiang, C.; Zhang, K.; Zha, T.Z.; Zhang, M. High ADAM8 expression is associated with poor prognosis in patients with hepatocellular carcinoma. Pathol. Oncol. Res. 2013, 19, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, S.; Liu, K.; Wang, Y.; Ji, B.; Zhang, X.; Liu, Y. A disintegrin and metalloprotease (ADAM)10 is highly expressed in hepatocellular carcinoma and is associated with tumor progression. J. Int. Med. Res. 2014, 42, 611–618. [Google Scholar] [CrossRef]
- Grutzmann, R.; Luttges, J.; Sipos, B.; Ammerpohl, O.; Dobrowolski, F.; Alldinger, I.; Kersting, S.; Ockert, D.; Koch, R.; Kalthoff, H.; et al. ADAM9 expression in pancreatic cancer is associated with tumour type and is a prognostic factor in ductal adenocarcinoma. Br. J. Cancer 2004, 90, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Qian, J.; Wu, Q.; Chen, Y.; Yu, G. ADAM-17 expression is enhanced by FoxM1 and is a poor prognostic sign in gastric carcinoma. J. Surg. Res. 2017, 220, 223–233. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, J.; Lu, K.; Chen, Q.; Tao, D.; Chen, Z. Therapeutic potential of ADAM17 modulation in gastric cancer through regulation of the EGFR and TNF-alpha signalling pathways. Mol. Cell. Biochem. 2017, 426, 17–26. [Google Scholar] [CrossRef]
- Romagnoli, M.; Mineva, N.D.; Polmear, M.; Conrad, C.; Srinivasan, S.; Loussouarn, D.; Barillé-Nion, S.; Georgakoudi, I.; Dagg, A.; McDermott, E.W.; et al. ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis. EMBO Mol. Med. 2014, 6, 278–294. [Google Scholar] [CrossRef]
- Guaiquil, V.H.; Swendeman, S.; Zhou, W.; Guaiquil, P.; Weskamp, G.; Bartsch, J.W.; Blobel, C.P. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J. Mol. Med. 2010, 88, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wan, M.; Ma, L.; Liu, X.; He, J. Protective effects of ADAM8 against cisplatin-mediated apoptosis in non-small-cell lung cancer. Cell Biol. Int. 2013, 37, 47–53. [Google Scholar] [CrossRef]
- Fröhlich, C.; Nehammer, C.; Albrechtsen, R.; Kronqvist, P.; Kveiborg, M.; Sehara-Fujisawa, A.; Mercurio, A.M.; Wewer, U.M. ADAM12 produced by tumor cells rather than stromal cells accelerates breast tumor progression. Mol. Cancer Res. 2011, 9, 1449–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossello, A.; Nuti, E.; Ferrini, S.; Fabbi, M. Targeting ADAM17 Sheddase Activity in Cancer. Curr. Drug Targets 2016, 17, 1908–1927. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; D’Amore, M.; Sisto, M. ADAM17 at the interface between inflammation and autoimmunity. Immunol. Lett. 2014, 162, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.L.; Minond, D. Recent Advances in ADAM17 Research: A Promising Target for Cancer and Inflammation. Mediat. Inflamm. 2017, 2017, 9673537. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, D.; Sun, X.; Zhang, Y.; Wang, L.; Suo, J. ADAM17 promotes lymph node metastasis in gastric cancer via activation of the Notch and Wnt signaling pathways. Int. J. Mol. Med. 2019, 43, 914–926. [Google Scholar] [CrossRef]
- Zhang, T.C.; Zhu, W.G.; Huang, M.D.; Fan, R.H.; Chen, X.F. Prognostic value of ADAM17 in human gastric cancer. Med. Oncol. 2012, 29, 2684–2690. [Google Scholar] [CrossRef]
- Shou, Z.X.; Jin, X.; Zhao, Z.S. Upregulated expression of ADAM17 is a prognostic marker for patients with gastric cancer. Ann. Surg. 2012, 256, 1014–1022. [Google Scholar] [CrossRef]
- Hubeau, C.; Rocks, N.; Cataldo, D. ADAM28: Another ambivalent protease in cancer. Cancer Lett. 2020, 494, 18–26. [Google Scholar] [CrossRef]
| ADAMs | Other Name | Involvement in Cancer Biology | Inhibitors |
|---|---|---|---|
| ADAM8 | MS2 (CD156) | Promotion of migration | - |
| ADAM9 | MDC9, MCMP, Meltrin-γ | Promotion of cell adhesion and invasion, binding to integrins (α6β4 and α2β1) | - |
| ADAM10 | MDAM, Kuzbanian | Type I membrane glycoprotein L1 shedding, promotion of cell growth and migration | TIMP1 TIMP3 |
| ADAM12 | Meltrin-α, MCMP, MLTN, MLTNA | HB-EGF (heparin-binding epidermal growth factor) shedding, promotion of cell growth | TIMP3 |
| ADAM15 | Metargidin, MDC15, AD56, CR II-7 | Promotion of cell growth | No data |
| ADAM17 | TACE, cSVP | TGF-β (transforming growth factor) shedding, promotion of cell growth | TIMP2 TIMP3 |
| ADAM19 | Meltrin-β, FKSG34 | No data | - |
| ADAM28 | e-MDC II, MDC-Lm, MDC-Ls | IGFBP-3 (insulin-like growth factor binding protein-3) cleavage, promotion of cell growth | TIMP3 TIMP4 |
| ADAMTS1 | C3-C5, METH1, KIAA1346 | HB-EGF (heparin-binding epidermal growth factor) and AR shedding, promotion of cell growth, survival and invasion | No data |
| ADAMTS4 | KIAA0688, aggrecanase-1, ADMP-1 | No data | TIMP3 |
| ADAMTS5 | ADAMTS11, aggrecanase-2, ADMP-2 | Brevican cleavage, promotion of invasion | TIMP3 |
| ADAMs | GI Cancers | Results | References |
| ADAM8 | GC |
| [54] |
| CRC |
| [60] | |
| PC |
| [64] | |
| LC |
| [68] | |
| ADAM9 | GC |
| [46] |
| PC |
| [63,65,70] | |
| Adam10 | GC |
| [56] |
| CRC |
| [61] | |
| HCC |
| [66,67,69] | |
| ADAM12 | GC |
| [46] |
| CRC |
| [43,61] | |
| ADAM15 | GC |
| [46] |
| CRC |
| [62] | |
| PC |
| [63] | |
| ADAM17 | GC |
| [71,72] |
| CRC |
| [43,59,61] | |
| ADAM28 | GC |
| [58] |
| CRC |
| [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukaszewicz-Zając, M.; Pączek, S.; Mroczko, B. A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies? Cancers 2022, 14, 2307. https://doi.org/10.3390/cancers14092307
Łukaszewicz-Zając M, Pączek S, Mroczko B. A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies? Cancers. 2022; 14(9):2307. https://doi.org/10.3390/cancers14092307
Chicago/Turabian StyleŁukaszewicz-Zając, Marta, Sara Pączek, and Barbara Mroczko. 2022. "A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies?" Cancers 14, no. 9: 2307. https://doi.org/10.3390/cancers14092307
APA StyleŁukaszewicz-Zając, M., Pączek, S., & Mroczko, B. (2022). A Disintegrin and Metalloproteinase (ADAM) Family—Novel Biomarkers of Selected Gastrointestinal (GI) Malignancies? Cancers, 14(9), 2307. https://doi.org/10.3390/cancers14092307