Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement
Abstract
:Simple Summary
Abstract
1. Introduction
2. Preoperative Assessment
3. Technical Considerations
3.1. Thrombectomy
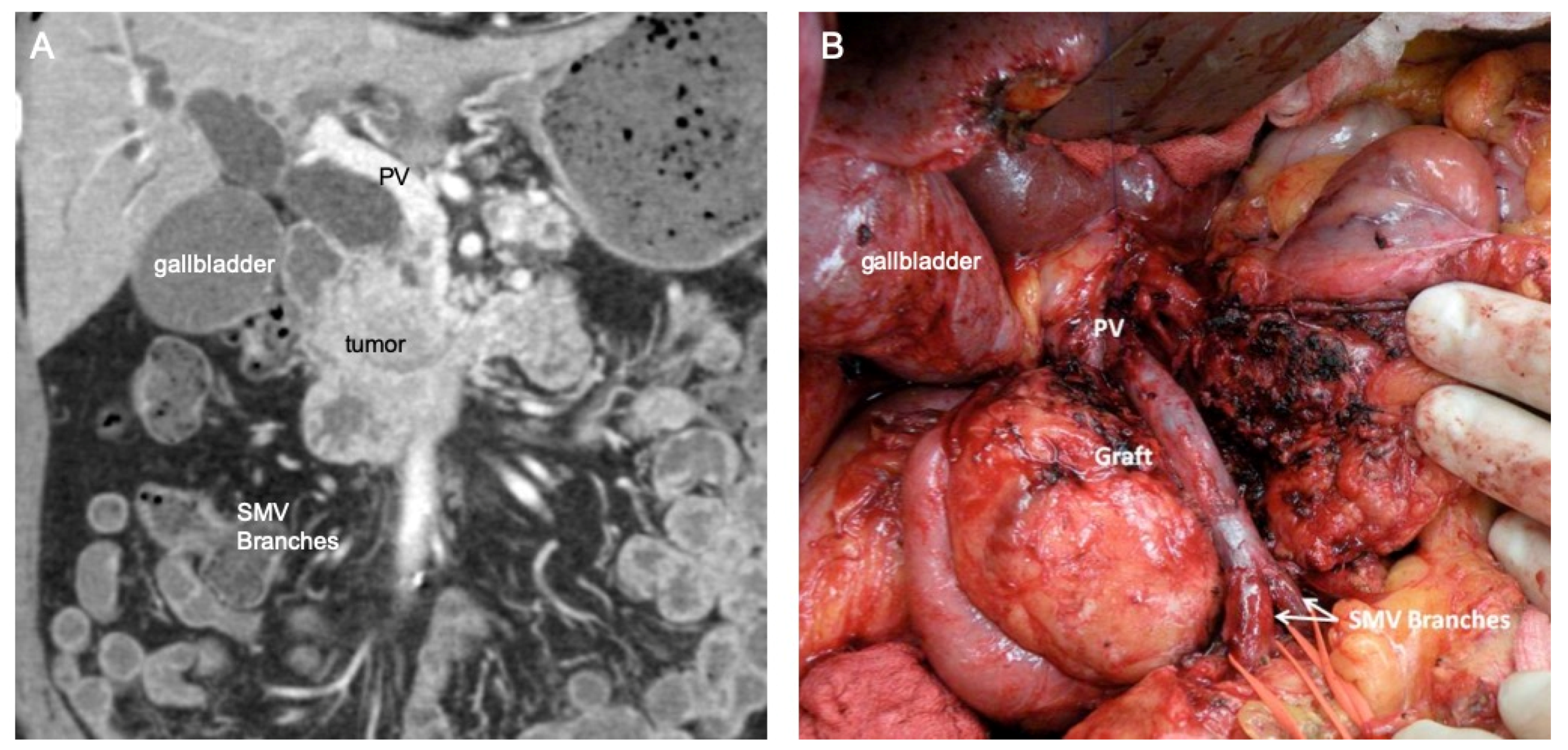
3.2. Traditional Vein Reconstruction
3.3. Complete Venous Occlusion with Collaterals
3.3.1. Early Vein Reconstruction for Left-Sided pNETs
3.3.2. Mesocaval Shunting
4. Reported Outcomes
5. Summary
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Sharma, P.; Zakalik, D. Comparison of Demographics, Tumor Characteristics, and Survival Between Pancreatic Adenocarcinomas and Pancreatic Neuroendocrine Tumors: A population-based study. Am. J. Clin. Oncol. 2018, 41, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Poultsides, G.A. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J. Gastroenterol. 2015, 21, 9512–9525. [Google Scholar] [CrossRef] [PubMed]
- Kamilaris, C.D.C.; Stratakis, C.A. Multiple Endocrine Neoplasia Type 1 (MEN1): An Update and the Significance of Early Genetic and Clinical Diagnosis. Front. Endocrinol. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, D.C.; Jensen, R.T. Gastrointestinal Neuroendocrine Tumors: Pancreatic Endocrine Tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, R.T.; Bodei, L.; Capdevila, J.; Couvelard, A.; Falconi, M.; Glasberg, S.; Kloppel, G.; Lamberts, S.; Peeters, M.; Rindi, G.; et al. Unmet Needs in Functional and Nonfunctional Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2019, 108, 26–36. [Google Scholar] [CrossRef]
- Evans, D.B.; Farnell, M.B.; Lillemoe, K.D.; Vollmer, C.; Strasberg, S.M.; Schulick, R.D. Surgical treatment of resectable and borderline resectable pancreas cancer: Expert consensus statement. Ann. Surg. Oncol. 2009, 16, 1736–1744. [Google Scholar] [CrossRef]
- Siriwardana, H.P.P.; Siriwardena, A.K. Systematic review of outcome of synchronous portal–superior mesenteric vein resection during pancreatectomy for cancer. Br. J. Surg. 2006, 93, 662–673. [Google Scholar] [CrossRef]
- Dua, M.M.; Tran, T.B.; Klausner, J.; Hwa, K.J.; Poultsides, G.A.; Norton, J.A.; Visser, B.C. Pancreatectomy with vein reconstruction: Technique matters. HPB 2015, 17, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Demir, I.E.; Schorn, S.; Jäger, C.; Scheufele, F.; Friess, H.; Ceyhan, G.O. Venous resection during pancreatectomy for pancreatic cancer: A systematic review. Transl. Gastroenterol. Hepatol. 2019, 4, 46. [Google Scholar] [CrossRef]
- Haugvik, S.P.; Labori, K.J.; Waage, A.; Line, P.D.; Mathisen, Ø.; Gladhaug, I.P. Pancreatic Surgery with Vascular Reconstruction in Patients with Locally Advanced Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2013, 17, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.C.; Chilcott, M.; Poletti, P.A.; Buhler, L.H.; Morel, P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. World J. Gastroenterol. 2010, 16, 818. [Google Scholar] [CrossRef] [PubMed]
- Hellman, P.; Andersson, M.; Rastad, J.; Juhlin, C.; Karacagil, S.; Eriksson, B.; Skogseid, B.; Åkerström, G. Surgical Strategy for Large or Malignant Endocrine Pancreatic Tumors. World J. Surg. 2000, 24, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Titan, A.L.; Norton, J.A.; Fisher, A.T.; Foster, D.S.; Harris, E.J.; Worhunsky, D.J.; Worth, P.J.; Dua, M.M.; Visser, B.C.; Poultsides, G.A.; et al. Evaluation of Outcomes Following Surgery for Locally Advanced Pancreatic Neuroendocrine Tumors. JAMA Netw. Open 2020, 3, e2024318. [Google Scholar] [CrossRef]
- Engelbrecht, M.; Akin, O.; Dixit, D.; Schwartz, L. Bland and tumor thrombi in abdominal malignancies: Magnetic resonance imaging assessment in a large oncologic patient population. Abdom. Imaging 2010, 36, 62–68. [Google Scholar] [CrossRef]
- Naswa, N.; Kumar, R.; Bal, C.; Malhotra, A. Vascular thrombosis as a cause of abdominal pain in a patient with neuroendocrine carcinoma of pancreas: Findings on 68Ga-DOTANOC PET/CT. Indian J. Nucl. Med. 2012, 27, 35. [Google Scholar] [CrossRef] [Green Version]
- De Robertis, R.; Paiella, S.; Cardobi, N.; Landoni, L.; Tinazzi Martini, P.; Ortolani, S.; De Marchi, G.; Gobbo, S.; Giardino, A.; Butturini, G.; et al. Tumor thrombosis: A peculiar finding associated with pancreatic neuroendocrine neoplasms. A pictorial essay. Abdom. Radiol. 2018, 43, 613–619. [Google Scholar] [CrossRef]
- Younan, G.; Tsai, S.; Evans, D.B.; Christians, K.K. Techniques of Vascular Resection and Reconstruction in Pancreatic Cancer. Surg. Clin. N. Am. 2016, 96, 1351–1370. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Dua, M.M.; Visser, B.C. Early Vein Reconstruction and Right-to-Left Dissection for Left-Sided Pancreatic Tumors with Portal Vein Occlusion. J. Gastrointest. Surg. 2014, 18, 2034–2037. [Google Scholar] [CrossRef]
- Bedirli, A.; Patiroglu, T.E.; Sakrak, O.; Aritas, Y. Portal vein resection for a portal vein thrombus caused by nonfunctioning islet cell carcinoma: Report of a case. Surg. Today 2004, 34, 802–804. [Google Scholar] [CrossRef]
- Sakamoto, E.; Hasegawa, H.; Ogiso, S.; Igami, T.; Mori, T.; Mizuno, T.; Hattori, K.; Sugimoto, M.; Fukami, Y. Curative resection for a pancreatic endocrine carcinoma involving the portal vein. Hepatogastroenterology 2004, 51, 1849–1851. [Google Scholar] [PubMed]
- Kawakami, H.; Kuwatani, M.; Hirano, S.; Kondo, S.; Nakanishi, Y.; Itoh, T.; Asaka, M. Pancreatic Endocrine Tumors with Intraductal Growth into the Main Pancreatic Duct and Tumor Thrombus within the Portal Vein: A Case Report and Review of the Literature. Intern. Med. 2007, 46, 273–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamato, H.; Kawakami, H.; Kuwatani, M.; Shinada, K.; Kondo, S.; Kubota, K.; Asaka, M. Pancreatic Carcinoma Associated with Portal Vein Tumor Thrombus: Three Case Reports. Intern. Med. 2009, 48, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, T.; Masuda, T.; Nishizawa, M.; Ito, H.; Igari, K.; Aihara, A.; Kumagai, Y.; Iida, M.; Odajima, H.; Arii, S.; et al. Curative resection of a huge malignant pancreatic endocrine tumor by pancreatoduodenectomy with portal and superior mesenteric vein resection and reconstruction using the right ovarian vein: Report of a case. Surg. Today 2011, 41, 1260–1265. [Google Scholar] [CrossRef]
- Tsuchikawa, T.; Kondo, S.; Hirano, S.; Tanaka, E.; Kawasaki, R.; Kato, K.; Matsumoto, J.; Shichinohe, T. Distal pancreatectomy and portal vein resection without vascular reconstruction for endocrine tumors with massive intraportal growth: Report of a case. Hepatogastroenterology 2011, 58, 1029–1031. [Google Scholar]
- Akatsu, T.; Aiura, K.; Shimazu, M.; Ueda, M.; Wakabayashi, G.; Tanabe, M.; Kawachi, S.; Shinoda, M.; Kameyama, K.; Kitajima, M.; et al. Successful pancreatectomy with en-bloc resection of the celiac artery and portal vein for pancreatic endocrine carcinoma. Hepatogastroenterology 2007, 54, 1269–1271. [Google Scholar]
- Gundara, J.S.; Alvarado-Bachmann, R.; Williams, N.; Gananadha, S.; Gill, A.; Hugh, T.J.; Samra, J.S. Multivisceral resection of pancreatic neuroendocrine tumours: A report of two cases. World J. Surg.Oncol. 2011, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.B.; Skibber, J.M.; Lee, J.E.; Cleary, K.R.; Ajani, J.A.; Gagel, R.F.; Sellin, R.V.; Fenoglio, C.J.; Merrell, R.C.; Hickey, R.C. Nonfunctioning islet cell carcinoma of the pancreas. Surgery 1993, 114, 1175–1182. [Google Scholar] [CrossRef]
- Norton, J.A.; Kivlen, M.; Li, M.; Schneider, D.; Chuter, T.; Jensen, R.T.; Mulvihill, S.J.; O’Connell, T.X.; Clark, O.H.; Reber, H.A.; et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch. Surg. 2003, 138, 859–866. [Google Scholar] [CrossRef] [Green Version]
- Norton, J.A.; Harris, E.J.; Chen, Y.; Visser, B.C.; Poultsides, G.A.; Kunz, P.C.; Fisher, G.A.; Jensen, R.T. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch. Surg. 2011, 146, 724–732. [Google Scholar] [CrossRef] [Green Version]
- Kleine, M.; Schrem, H.; Vondran, F.W.R.; Krech, T.; Klempnauer, J.; Bektas, H. Extended surgery for advanced pancreatic endocrine tumours. Br. J. Surg. 2012, 99, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Fratini, G.; Giudici, F.; Bellucci, F.; Batignani, G.; Tonelli, F. Feasibility of mesenteric vein reconstruction with PTFE prosthesis for non functioning endocrine pancreatic tumor surgery. Pancreat. Dis. Ther. 2012, 2, 1. [Google Scholar]
- ElLakis, M.; Gianakou, A.; Nockel, P.; Wiseman, D.; Tirosh, A.; Quezado, M.A.; Patel, D.; Nilubol, N.; Pacak, K.; Sadowski, S.M.; et al. Radioguided Surgery with Gallium 68 Dotatate for Patients with Neuroendocrine Tumors. JAMA Surg. 2019, 154, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloyd, J.M.; Wiseman, J.T.; Pawlik, T.M. Surgical management of pancreatic neuroendocrine liver metastases. J. Gastrointest. Oncol. 2020, 11, 590–600. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.Y.; Visser, B.C.; Dua, M.M. Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement. Cancers 2022, 14, 2312. https://doi.org/10.3390/cancers14092312
Li AY, Visser BC, Dua MM. Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement. Cancers. 2022; 14(9):2312. https://doi.org/10.3390/cancers14092312
Chicago/Turabian StyleLi, Amy Y., Brendan C. Visser, and Monica M. Dua. 2022. "Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement" Cancers 14, no. 9: 2312. https://doi.org/10.3390/cancers14092312
APA StyleLi, A. Y., Visser, B. C., & Dua, M. M. (2022). Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement. Cancers, 14(9), 2312. https://doi.org/10.3390/cancers14092312




