Regulation of Carcinogenesis by Sensory Neurons and Neuromediators
Abstract
Simple Summary
Abstract
1. Introduction
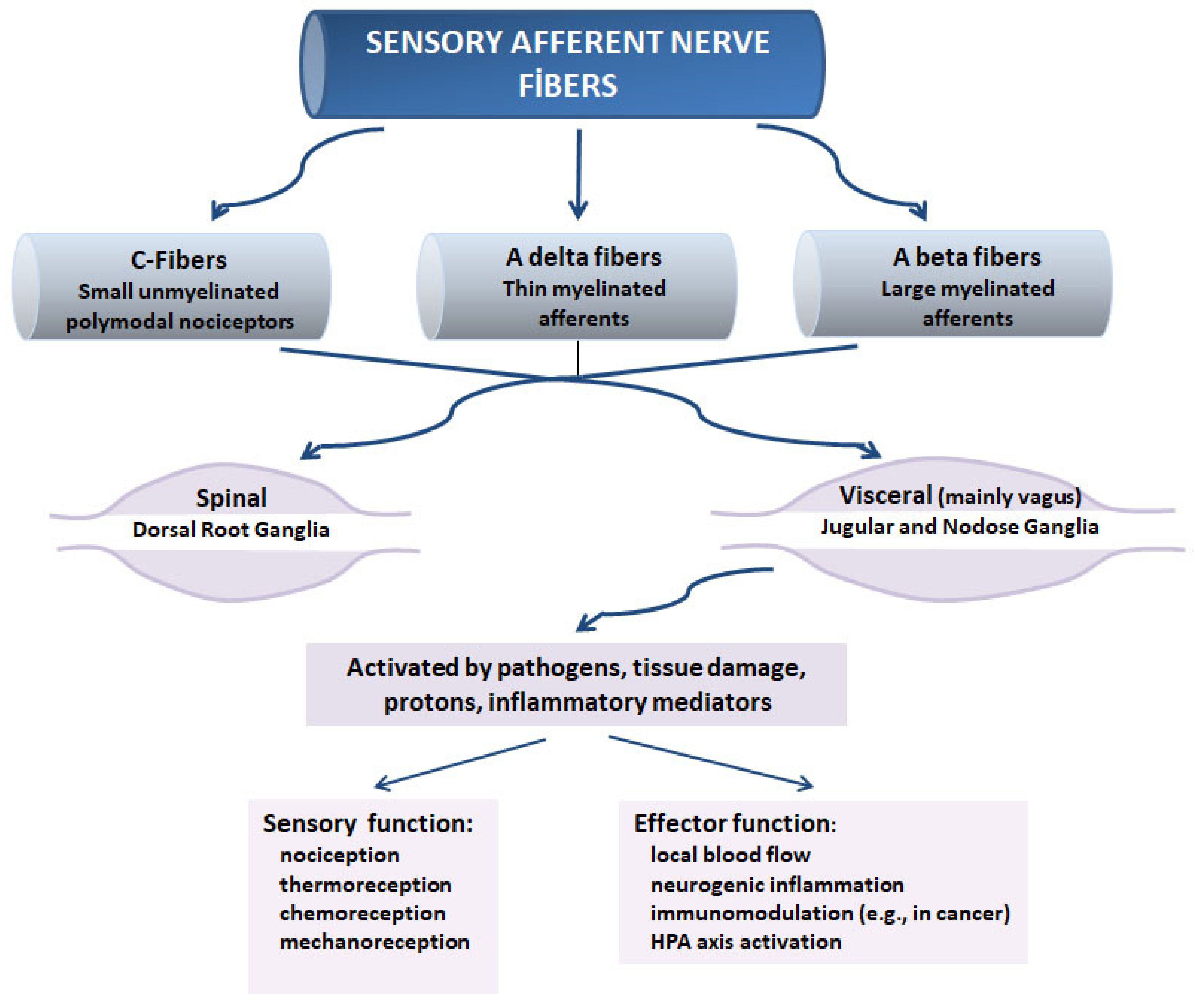
2. Sensory Neurons
3. Capsaicin-Sensitive Sensory Neurons and Their Responses to Pathogens and Cancer
4. Sensory Nerve Activity and the Antitumor Immune Response
5. Sensory Neuromediators
5.1. Substance P
5.2. CGRP in Inflammation and Immune Regulation
6. Glial Cells, Cancer, and Sensory Neuromediators
7. Sensory Nerves and Carcinogenesis: Contradictory Findings
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, C.; Clausen, J.; Sturn, D.H.; Djanani, A.; Gunsilius, E.; Wiedermann, C.J.; Kähler, C.M. Natural killer cell functions mediated by the neuropeptide substance P. Regul. Pept. 2003, 116, 119–126. [Google Scholar] [CrossRef]
- Monaco-Shawver, L.; Schwartz, L.; Tuluc, F.; Guo, C.J.; Lai, J.P.; Gunnam, S.M.; Kilpatrick, L.E.; Banerjee, P.P.; Douglas, S.D.; Orange, J.S. Substance P inhibits natural killer cell cytotoxicity through the neurokinin-1 receptor. J. Leukoc. Biol. 2010, 89, 113–125. [Google Scholar] [CrossRef]
- Johnson, M.B.; Young, A.D.; Marriott, I. The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front. Cell. Neurosci. 2017, 10, 296. [Google Scholar] [CrossRef]
- Lang, K.; Iv, T.L.D.; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S.; Entschladen, F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 2004, 112, 231–238. [Google Scholar] [CrossRef]
- Ordovas-Montanes, J.; Rakoff-Nahoum, S.; Huang, S.; Riol-Blanco, L.; Barreiro, O.; von Andrian, U.H. The Regulation of Immunological Processes by Peripheral Neurons in Homeostasis and Disease. Trends Immunol. 2015, 36, 578–604. [Google Scholar] [CrossRef]
- Mundt, S.; Greter, M.; Flügel, A.; Becher, B. The CNS Immune Landscape from the Viewpoint of a T Cell. Trends Neurosci. 2019, 42, 667–679. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Antoni, M.H.; Dhabhar, F.S. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer 2019, 125, 1417–1431. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Oh, P.J.; Shin, S.R.; Ahn, H.S.; Kim, H.J. Meta-analysis of psychosocial interventions on survival time in patients with cancer. Psychol. Health 2015, 31, 396–419. [Google Scholar] [CrossRef]
- Mueller, S.N. Neural control of immune cell trafficking. J. Exp. Med. 2022, 219, e20211604. [Google Scholar] [CrossRef] [PubMed]
- Kolter, J.; Feuerstein, R.; Zeis, P.; Hagemeyer, N.; Paterson, N.; D’Errico, P.; Baasch, S.; Amann, L.; Masuda, T.; Lösslein, A.; et al. A Subset of Skin Macrophages Contributes to the Surveillance and Regeneration of Local Nerves. Immunity 2019, 50, 1482–1497.e7. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.P.; Jabangwe, C.; Lamghari, M.; Alves, C.J. The Neuroimmune Interplay in Joint Pain: The Role of Macrophages. Front. Immunol. 2022, 13, 812962. [Google Scholar] [CrossRef]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Yun, H.; Yin, X.-T.; Stuart, P.M.; Leger, A.J.S. Sensory Nerve Retraction and Sympathetic Nerve Innervation Contribute to Immunopathology of Murine Recurrent Herpes Stromal Keratitis. Investig. Opthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef]
- Hilderman, M.; Bruchfeld, A. The cholinergic anti-inflammatory pathway in chronic kidney disease—Review and vagus nerve stimulation clinical pilot study. Nephrol. Dial. Transplant. 2020, 35, 1840–1852. [Google Scholar] [CrossRef]
- Lv, J.; Ji, X.; Li, Z.; Hao, H. The role of the cholinergic anti-inflammatory pathway in autoimmune rheumatic diseases. Scand. J. Immunol. 2021, 94, e13092. [Google Scholar] [CrossRef]
- Falvey, A.; Metz, C.N.; Tracey, K.J.; Pavlov, V.A. Peripheral nerve stimulation and immunity: The expanding opportunities for providing mechanistic insight and therapeutic intervention. Int. Immunol. 2021, 34, 107–118. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Alen, N.V. The cholinergic anti-inflammatory pathway in humans: State-of-the-art review and future directions. Neurosci. Biobehav. Rev. 2022, 136, 104622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Wang, Y. Evaluation of Platelet-Rich Plasma Therapy for Peripheral Nerve Regeneration: A Critical Review of Literature. Front. Bioeng. Biotechnol. 2022, 10, 80824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Restaino, A.C.; Vermeer, P.D. Neural regulations of the tumor microenvironment. FASEB Bioadv. 2021, 4, 29–42. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Chen, N.; Niu, C.; Li, Z.; Hu, J.; Cui, J. Nerves in the Tumor Microenvironment: Origin and Effects. Front. Cell Dev. Biol. 2020, 8, 601738. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yoon, W.; Lee, A.; Han, Y.; Byun, Y.; Kang, J.S.; Kim, H.; Kwon, W.; Suh, Y.-A.; Kim, Y.; et al. CORRIGENDUM: Addition of data source: Diagnostic model for pancreatic cancer using a multi-biomarker panel. Ann. Surg. Treat. Res. 2021, 100, 252. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Wang, X.; Ji, T. Calcitonin gene-related peptide: A promising bridge between cancer development and cancer-associated pain in oral squamous cell carcinoma (Review). Oncol. Lett. 2020, 20, 253. [Google Scholar] [CrossRef]
- Griffin, N.; Faulkner, S.; Jobling, P.; Hondermarck, H. Targeting neurotrophin signaling in cancer: The renaissance. Pharmacol. Res. 2018, 135, 12–17. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhou, J.; Yang, W.; Cui, H.; Xu, M.; Yi, L. Oncogenic role of neurotensin and neurotensin receptors in various cancers. Clin. Exp. Pharmacol. Physiol. 2017, 44, 841–846. [Google Scholar] [CrossRef]
- Xie, L.; Moroi, Y.; Tsuji, G.; Liu, M.; Hayashida, S.; Takahara, M.; Fukagawa, S.; Takeuchi, S.; Shan, B.; Nakahara, T.; et al. CD10-bearing fibroblast inhibits matrigel invasive potency of interleukin-1α-producing squamous cell carcinoma by diminishing substance P levels in the tumor microenvironment. Cancer Sci. 2010, 101, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-Related Axonogenesis and Neurogenesis in Prostate Cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, C.; Phillips, J.A.; Djamgoz, M.B.A. Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188411. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, L.; Mravec, B. Effect of the autonomic nervous system on cancer progression depends on the type of tumor: Solid are more affected then ascitic tumors. Endocr. Regul. 2016, 50, 215–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Sternini, C. Organization of the peripheral nervous system: Autonomic and sensory ganglia. J. Investig. Dermatol. Symp. Proc. 1997, 2, 1–7. [Google Scholar] [CrossRef]
- Dalsgaard, C. The sensory system. In Hatldbook of Chemical Neuroatlatomy; Björklund, A.H.T., Owman, C., Eds.; The Peripheral Nervous System; Elsevier: Amsterdam, The Netherlands, 1988; Volume 6, pp. 599–636. [Google Scholar]
- Kruger, L. Morphological features of thin sensory afferent fibers: A new interpretation of ‘nociceptor’ function. Prog. Brain Res. 1988, 74, 253–257. [Google Scholar]
- Mazzone, S.B.; Undem, B.J. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Gottschaldt, K.M. Structure and function of avian somatosensory receptors. In Form and Function in Birds; King, J.M.A.S., Ed.; Academic Press: London, UK, 1985; Volume 3, pp. 375–461. [Google Scholar]
- De Groat, W.C. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog. Brain Res. 1986, 67, 165–187. [Google Scholar] [CrossRef]
- Dockray, G.; Sharkey, K. Neurochemistry of visceral afferent neurones. Prog. Brain Res. 1986, 67, 133–148. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Woolf, C.J.; Fitzgerald, M.; Lindsay, R.M.; Molander, C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 1989, 32, 493–502. [Google Scholar] [CrossRef]
- Stemini, C. Neurochemistry of spinal afferents supplying the gastrointestinal tract and pancreas. In Brain-Gut Interactions; Tache, D.L.W.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 45–55. [Google Scholar]
- Horváth, A.; Borbély, E.; Bölcskei, K.; Szentes, N.; Kiss, T.; Belák, M.; Rauch, T.; Glant, T.; Zákány, R.; Juhász, T.; et al. Regulatory role of capsaicin-sensitive peptidergic sensory nerves in the proteoglycan-induced autoimmune arthritis model of the mouse. J. Neuroinflam. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Zhao, W.; Bylander, J.; Chase, G.; Clawson, G. Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res. Treat. 2006, 99, 351–364. [Google Scholar] [CrossRef]
- Holzer, P. Local effector functions of capsaicin-sensitive sensory nerve endings: Involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 1988, 24, 739–768. [Google Scholar] [CrossRef]
- Holzer, P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr. Opin. Pharmacol. 2007, 7, 563–569. [Google Scholar] [CrossRef]
- Aberdeen, J.; Corr, L.; Milner, P.; Lincoln, J.; Burnstock, G. Marked increases in calcitonin gene-related peptide-containing nerves in the developing rat following long-term sympathectomy with guanethidine. Neuroscience 1990, 35, 175–184. [Google Scholar] [CrossRef]
- Kruger, L. Somatic and visceral afferent innervation: Neuroanatomical and neurochemical considerations. In Basic and Clinical Aspects of Chronic Abdominal Pain; Mayer, H.E.R., Ed.; Elsevier: New York, NY, USA, 1993; pp. 29–34. [Google Scholar]
- Jancso, G.; Kiraly, E.; Jancsó-Gábor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef]
- Szallasi, A.; Fowler, C.J. After a decade of intravesical vanilloid therapy: Still more questions than answers. Lancet Neurol. 2002, 1, 167–172. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Erin, N. Role of sensory neurons, neuroimmune pathways, and transient receptor potential vanilloid 1 (TRPV1) channels in a murine model of breast cancer metastasis. Cancer Immunol. Immunother. 2020, 69, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Boyer, P.J.; Bonneau, R.H.; Clawson, G.A.; Welch, D.R. Capsaicin-mediated denervation of sensory neurons pro-motes mammary tumor metastasis to lung and heart. Anticancer Res. 2004, 24, 1003–1009. [Google Scholar] [PubMed]
- Erin, N.; Clawson, G.A. Parameters affecting substance P measurement in heart, lung, and skin. BioTechniques 2004, 37, 232–239. [Google Scholar] [CrossRef]
- Erin, N.; Ulusoy, O. Differentiation of neuronal from non-neuronal Substance P. Regul. Pept. 2009, 152, 108–113. [Google Scholar] [CrossRef]
- Erin, N.; Zık, B.; Sarıgül, M.; Tütüncü, S. The effects of chronic low-dose capsaicin treatment on substance P levels. Regul. Pept. 2009, 153, 83–87. [Google Scholar] [CrossRef]
- Helliwell, R.J.; McLatchie, L.M.; Clarke, M.; Winter, J.; Bevan, S.; McIntyre, P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998, 250, 177–180. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Buck, S.H.; Burks, T.F. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar]
- Cervero, F.; McRitchie, H.A. Neonatal capsaicin does not affect unmyelinated efferent fibers of the autonomic nervous system: Functional evidence. Brain Res. 1982, 239, 283–288. [Google Scholar] [CrossRef]
- Nagy, J.I.; Iversen, L.L.; Goedert, M.; Chapman, D.; Hunt, S.P. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J. Neurosci. 1983, 3, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Helke, C.J.; DiMicco, J.A.; Jacobowitz, D.M.; Kopin, I.J. Effect of capsaicin administration to neonatal rats on the substance P content of discrete CNS regions. Brain Res. 1981, 222, 428–431. [Google Scholar] [CrossRef]
- Gamse, R.; Leeman, S.E.; Holzer, P.; Lembeck, F. Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn-Schmiedeberg Arch. Pharmacol. 1981, 317, 140–148. [Google Scholar] [CrossRef]
- Hammond, D.L.; Ruda, M.A. Developmental alterations in nociceptive threshold, immunoreactive calcitonin gene-related peptide and substance P, and fluoride-resistant acid phosphatase in neonatally capsaicin-treated rats. J. Comp. Neurol. 1991, 312, 436–450. [Google Scholar] [CrossRef]
- Pintér, E.; Pozsgai, G.; Hajna, Z.; Helyes, Z.; Szolcsányi, J. Neuropeptide receptors as potential drug targets in the treatment of inflammatory conditions. Br. J. Clin. Pharmacol. 2013, 77, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Crosson, T.; Roversi, K.; Balood, M.; Othman, R.; Ahmadi, M.; Wang, J.C.; Pereira, P.J.S.; Tabatabaei, M.; Couture, R.; Eichwald, T.; et al. Profiling of how nociceptor neurons detect danger—New and old foes. J. Intern. Med. 2019, 286, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, M. The axon reflex. Neuroanatomy 2008, 7, 17–19. [Google Scholar]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Lai, N.Y.; Mills, K.; Chiu, I.M. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J. Intern. Med. 2017, 282, 5–23. [Google Scholar] [CrossRef]
- Holzer, P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; La Russa, F.; Bennett, D.L. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci. 2015, 16, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef]
- Reinshagen, M.; Patel, A.; Sottili, M.; Nast, C.; Davis, W.; Mueller, K.; Eysselein, V.E. Protective function of extrinsic sensory neurons in acute rabbit experimental colitis. Gastroenterology 1994, 106, 1208–1214. [Google Scholar] [CrossRef]
- O’Mahony, C.; van der Kleij, H.; Bienenstock, J.; Shanahan, F.; O’Mahony, L. Loss of vagal anti-inflammatory effect: In vivo visualization and adoptive transfer. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R1118–R1126. [Google Scholar] [CrossRef]
- Prechtl, J.C.; Powley, T.L. The fiber composition of the abdominal vagus of the rat. Anat. Embryol. 1990, 181, 101–115. [Google Scholar] [CrossRef]
- Hanes, W.M.; Olofsson, P.S.; Talbot, S.; Tsaava, T.; Ochani, M.; Imperato, G.H.; Levine, Y.A.; Roth, J.; Pascal, M.A.; Foster, S.L.; et al. Neuronal Circuits Modulate Antigen Flow Through Lymph Nodes. Bioelectron. Med. 2016, 3, 18–28. [Google Scholar] [CrossRef]
- Qu, L. Neuronal Fc gamma receptor I as a novel mediator for IgG immune complex-induced peripheral sensitization. Neural Regen. Res. 2012, 7, 2075–2079. [Google Scholar] [CrossRef]
- Naafs, B. Current views on reactions in leprosy. Indian J. Lepr. 2000, 72, 97–122. [Google Scholar]
- Karanth, S.S.; Springall, D.R.; Lucas, S.; Levy, D.; Ashby, P.; Levene, M.M.; Polak, J.M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 1989, 157, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.; Lightman, S.L.; Heijnen, C.J. Can nerve damage disrupt neuroendocrine immune homeostasis? Leprosy as a case in point. Trends Immunol. 2002, 23, 18–22. [Google Scholar] [CrossRef]
- Karanth, S.S.; Springall, D.R.; Kar, S.; Gibson, S.J.; Royston, J.P.; Banerjee, D.K.; Polak, J.M. Time-related decrease of substance P and CGRP in central and peripheral projections of sensory neurones inMycobacterium leprae infected nude mice: A model for lepromatous leprosy in man. J. Pathol. 1990, 160, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Ansel, J.C.; Armstrong, C.A.; Song, I.; Quinlan, K.L.; Olerud, J.E.; Caughman, S.W.; Bunnett, N.W. Interactions of the skin and nervous system. J. Investig. Dermatol. Symp. Proc. 1997, 2, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Susaki, Y.; Shimizu, S.; Katakura, K.; Watanabe, N.; Kawamoto, K.; Matsumoto, M.; Tsudzuki, M.; Furusaka, T.; Kitamura, Y.; Matsuda, H. Functional properties of murine macrophages promoted by nerve growth factor. Blood 1996, 88, 4630–4637. [Google Scholar] [CrossRef]
- Facer, P.; Mann, D.; Mathur, R.; Pandya, S.; Ladiwala, U.; Singhal, B.; Hongo, J.-A.; Sinicropi, D.V.; Terenghi, G.; Anand, P. Do nerve growth factor-related mechanisms contribute to loss of cutaneous nociception in leprosy? Pain 2000, 85, 231–238. [Google Scholar] [CrossRef]
- Bockman, D.E.; Büchler, M.; Beger, H.G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994, 107, 219–230. [Google Scholar] [CrossRef]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D., Jr.; et al. Melanoma-induced reprogramming of Schwann cell signaling aids tumor growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef]
- Petrou, A.; Soonawalla, Z.; Silva, M.-A.; Manzelli, A.; Moris, D.; Tabet, P.P.; Friend, P. Prognostic indicators following curative pancreatoduodenectomy for pancreatic carcinoma: A retrospective multivariate analysis of a single centre experience. J. Balk. Union Oncol. 2016, 21, 874–882. [Google Scholar]
- Mitsunaga, S.; Hasebe, T.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Nakagohri, T.; Ochiai, A. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am. J. Surg. Pathol. 2007, 31, 1636–1644. [Google Scholar] [CrossRef]
- Lee, T.L.; Chiu, P.H.; Li, W.Y.; Yang, M.H.; Wei, P.Y.; Chu, P.Y.; Wang, Y.F.; Tai, S.K. Nerve-tumour interaction enhances the aggressiveness of oral squamous cell carcinoma. Clin. Otolaryngol. 2019, 44, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Lailler, C.; Clatot, F.; Galmiche, A. Perineural invasion in head and neck squamous cell carcinoma: Background, mechanisms, and prognostic implications. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Bapat, A.A.; Hostetter, G.; Von Hoff, D.D.; Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Cancer 2011, 11, 695–707. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhang, B.-Y.; Zhou, B.; Zhu, C.-Z.; Sun, L.-Q.; Feng, Y.-J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar] [PubMed]
- Nakao, A.; Harada, A.; Nonami, T.; Kaneko, T.; Takagi, H. clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas 1996, 12, 357–361. [Google Scholar] [CrossRef]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Neural plasticity in pancreatitis and pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 649–659. [Google Scholar] [CrossRef]
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural invasion in pancreatic cancer: The past, present and future. Cancers 2010, 2, 1513–1527. [Google Scholar] [CrossRef]
- Salvo, E.; Campana, W.M.; Scheff, N.N.; Nguyen, T.H.; Jeong, S.-H.; Wall, I.; Wu, A.K.; Zhang, S.; Kim, H.; Bhattacharya, A.; et al. Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 2020, 161, 2592–2602. [Google Scholar] [CrossRef]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Okui, T. Crosstalk between Sensory Nerves and Cancer in Bone. Curr. Osteoporos. Rep. 2018, 16, 648–656. [Google Scholar] [CrossRef]
- Sternini, C. Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann. N. Y. Acad. Sci. 1992, 657, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Brodin, E.; Hua, X.; Saria, A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol. Scand. 1984, 120, 217–227. [Google Scholar] [CrossRef]
- Okajima, K.; Harada, N. Regulation of inflammatory responses by sensory neurons: Molecular mechanism(s) and possible therapeutic applications. Curr. Med. Chem. 2006, 13, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Byun, J.S.; Gardner, K. Wounds that will not heal: Pervasive cellular reprogramming in cancer. Am. J. Pathol. 2013, 182, 1055–1064. [Google Scholar] [CrossRef]
- Goertzen, C.; Mahdi, H.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047–29063. [Google Scholar] [CrossRef]
- Lasfar, A.; Zloza, A.; Silk, A.W.; Lee, L.Y.; Cohen-Solal, K.A. Interferon Lambda: Toward a Dual Role in Cancer. J. Interf. Cytokine Res. 2019, 39, 22–29. [Google Scholar] [CrossRef]
- Berger, E.; Markers, E.G.; Delpierre, C.; Hosnijeh, F.S.; Kelly-Irving, M.; Portengen, L.; Bergdahl, I.A.; Johansson, A.-S.; Krogh, V.; Palli, D.; et al. Association between low-grade inflammation and Breast cancer and B-cell Myeloma and Non-Hodgkin Lymphoma: Findings from two prospective cohorts. Sci. Rep. 2018, 8, 10805. [Google Scholar] [CrossRef]
- Praveen, T.K.; Gangadharappa, H.V.; Abu Lila, A.S.; Moin, A.; Mehmood, K.; Krishna, K.L.; Hussain, T.; Alafanan, A.; Shakil, S.; Rizvi, S.M.D. Inflammation targeted nanomedicines: Patents and applications in cancer therapy. Semin. Cancer Biol. 2022; in press. [Google Scholar] [CrossRef]
- Beck, S.; Hochreiter, B.; Schmid, J.A. Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks. Front. Cell Dev. Biol. 2022, 10, 859863. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Péoc’H, M.; Sundaralingam, A.; Rahman, N.; Froudarakis, M.E. Inflammation of the Pleural Cavity: A Review on Pathogenesis, Diagnosis and Implications in Tumor Pathophysiology. Cancers 2022, 14, 1415. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.H.; Schnitzlein, H.N. The numbers of nerve fibers in the vagus nerve of man. Anat. Rec. 1961, 139, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R. Anatomy and function of sensory hepatic nerves. Anat. Rec. 2004, 280, 827–835. [Google Scholar] [CrossRef]
- Erin, N.; Barkan, G.A.; Harms, J.F.; Clawson, G.A. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters Substance P level. Regul. Pept. 2008, 151, 35–42. [Google Scholar] [CrossRef]
- Erin, N.; Barkan, G.A.; Clawson, G.A. Vagus nerve regulates breast cancer metastasis to the adrenal gland. Anticancer Res. 2013, 33, 3675–3682. [Google Scholar]
- Partecke, L.I.; Käding, A.; Trung, D.N.; Diedrich, S.; Sendler, M.; Weiss, F.; Kühn, J.-P.; Mayerle, J.; Beyer, K.; Von Bernstorff, W.; et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget 2017, 8, 22501–22512. [Google Scholar] [CrossRef]
- Ogawa, T.; Makino, T.; Mizumoto, K.; Nakayama, F. Promoting effect of truncal vagotomy on pancreatic carcinogenesis initiated with N-nitrosobis(2-oxopropyl)amine in Syrian golden hamsters. Carcinogenesis 1991, 12, 1227–1230. [Google Scholar] [CrossRef]
- Tatsuta, M.; Yamamura, H.; Iishi, H.; Ichii, M.; Noguchi, S.; Baba, M.; Taniguchi, H. Promotion by vagotomy of gastric car-cinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1985, 45, 194–197. [Google Scholar]
- Tatsuta, M.; Iishi, H.; Yamamura, H.; Baba, M.; Taniguchi, H. Effects of bilateral and unilateral vagotomy on gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in wistar rats. Int. J. Cancer 1988, 42, 414–418. [Google Scholar] [CrossRef]
- Ekbom, A.; Lundegårdh, G.; McLaughlin, J.K.; Nyrén, O. Relation of vagotomy to subsequent risk of lung cancer: Population based cohort study. BMJ 1998, 316, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Caygill, C.P.J.; Knowles, R.L.; Hall, R. Increased risk of cancer mortality after vagotomy for peptic ulcer. Eur. J. Cancer Prev. 1991, 1, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, T.; Yoshihara, K.; Asano, Y.; Sudo, N. Protective Role of the Hepatic Vagus Nerve against Liver Metastasis in Mice. Neuroimmunomodulation 2017, 24, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chapleau, M.W.; Sabharwal, R. Methods of assessing vagus nerve activity and reflexes. Heart Fail. Rev. 2011, 16, 109–127. [Google Scholar] [CrossRef]
- De Couck, M.; Van Brummelen, D.; Schallier, D.; De Greve, J.; Gidron, Y. The relationship between vagal nerve activity and clinical outcomes in prostate and non-small cell lung cancer patients. Oncol. Rep. 2013, 30, 2435–2441. [Google Scholar] [CrossRef]
- Mouton, C.; Ronson, A.; Razavi, D.; Delhaye, F.; Kupper, N.; Paesmans, M.; Moreau, M.; Nogaret, J.-M.; Hendlisz, A.; Gidron, Y. The relationship between heart rate variability and time-course of carcinoembryonic antigen in colorectal cancer. Auton. Neurosci. 2012, 166, 96–99. [Google Scholar] [CrossRef]
- De Couck, M.; Maréchal, R.; Moorthamers, S.; Van Laethem, J.-L.; Gidron, Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2016, 40, 47–51. [Google Scholar] [CrossRef]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Cailotto, C.; Costes, L.M.M.; van der Vliet, J.; VAN Bree, S.H.W.; VAN Heerikhuize, J.J.; Buijs, R.M.; Boeckxstaens, G.E. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol. Motil. 2011, 24, 191-e93. [Google Scholar] [CrossRef]
- Matsunaga, K.; Klein, T.W.; Friedman, H.; Yamamoto, Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages tolegionella pneumophilainfection by nicotine. J. Immunol. 2001, 167, 6518–6524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, W.J.; Van Der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; Van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.-R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Yoshikawa, K.; Fujii, Y.X.; Moriwaki, Y.; Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007, 80, 2314–2319. [Google Scholar] [CrossRef]
- Nouri-Shirazi, M.; Tinajero, R.; Guinet, E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs). Immunol. Lett. 2007, 109, 155–164. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef]
- Das, R.; Langou, S.; Le, T.T.; Prasad, P.; Lin, F.; Nguyen, T.D. Electrical Stimulation for Immune Modulation in Cancer Treatments. Front. Bioeng. Biotechnol. 2022, 9, 795300. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Watanabe, Y.; Shinagawa, T.; Kai, M.; Miyazawa, M.; Okuda, T.; Kawashima, K.; Yabashi, A.; Waguri, S.; Misawa, H. Primary sensory neuronal expression of SLURP-1, an endogenous nicotinic acetylcholine receptor ligand. Neurosci. Res. 2009, 64, 403–412. [Google Scholar] [CrossRef]
- Gallowitsch-Puerta, M.; Pavlov, V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007, 80, 2325–2329. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, G.; Shrivastava, B.; Dahiya, R.; Tiwari, J.; Ashwathanarayana, M.; Sharma, R.K.; Agrawal, M.; Mishra, A.; Dua, K. Calcitonin gene-related peptide (CGRP): A novel target for Alzheimer’s disease. CNS Neurosci. Ther. 2017, 23, 457–461. [Google Scholar] [CrossRef]
- Liou, J.-C.; Fu, W.-M. Additive effect of ADP and CGRP in modulation of the acetylcholine receptor channel in Xenopus embryonic myocytes. Br. J. Pharmacol. 1995, 115, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hintze, T.H. cAMP signal transduction cascade, a novel pathway for the regulation of endothelial nitric oxide production in coronary blood vessels. Arter. Thromb. Vasc. Biol. 2001, 21, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Koth, C.M.; Abdul-Manan, N.; Lepre, C.A.; Connolly, P.J.; Yoo, S.; Mohanty, A.K.; Lippke, J.A.; Zwahlen, J.; Coll, J.T.; Doran, J.D.; et al. Refolding and characterization of a soluble ectodomain complex of the calcitonin gene-related peptide receptor. Biochemistry 2010, 49, 1862–1872. [Google Scholar] [CrossRef]
- Moss, S.J.; Harkness, P.C.; Mason, I.J.; Barnard, E.A.; Mudge, A.W. Evidence that CGRP and cAMP increase transcription of AChR α-subunit gene, but not of other subunit genes. J. Mol. Neurosci. 1991, 3, 101–108. [Google Scholar] [CrossRef]
- Prato, V.; Taberner, F.J.; Hockley, J.R.F.; Callejo, G.; Arcourt, A.; Tazir, B.; Hammer, L.; Schad, P.; Heppenstall, P.A.; Smith, E.S.; et al. Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep. 2017, 21, 3102–3115. [Google Scholar] [CrossRef]
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin. Immunopathol. 2018, 40, 237–247. [Google Scholar] [CrossRef]
- Mantyh, P.W. Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry 2002, 63, 6–10. [Google Scholar]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the Immune response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- De Camilli, P.; Jahn, R. Pathways to regulated exocytosis in neurons. Annu. Rev. Physiol. 1990, 52, 625–645. [Google Scholar] [CrossRef]
- Donkin, J.J.; Turner, R.J.; Hassan, I.; Vink, R. Substance P in traumatic brain injury. Prog. Brain Res. 2007, 161, 97–109. [Google Scholar] [CrossRef]
- Ho, W.-Z.; Douglas, S.D. Substance P and neurokinin-1 receptor modulation of HIV. J. Neuroimmunol. 2004, 157, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Lescoulié, P.L.; Yassine-Diab, B.; Enault, G.; Mazières, B.; De Préval, C.; Cantagrel, A. Substance P enhances cytokine-induced vascular cell adhesion molecule-1 (VCAM-1) expression on cultured rheumatoid fibroblast-like synoviocytes. Clin. Exp. Immunol. 1998, 113, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Azzolina, A.; Bongiovanni, A.; Lampiasi, N. Substance P induces TNF-α and IL-6 production through NFκB in peritoneal mast cells. Biochim. Biophys. Acta 2003, 1643, 75–83. [Google Scholar] [CrossRef]
- Lorton, D.; Bellinger, D.L.; Felten, S.Y.; Felten, D.L. Substance P innervation of the rat thymus. Peptides 1990, 11, 1269–1275. [Google Scholar] [CrossRef]
- Hukkanen, M.; Konttinen, Y.T.; Rees, R.G.; Gibson, S.J.; Santavirta, S.; Polak, J.M. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J. Rheumatol. 1992, 19, 1252–1259. [Google Scholar] [PubMed]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Payan, D.G.; Brewster, D.R.; Goetzl, E.J. Specific stimulation of human T lymphocytes by substance P. J. Immunol. 1983, 131, 1613–1615. [Google Scholar]
- Stanisz, A.M.; Scicchitano, R.; Dazin, P.; Bienenstock, J.; Payan, D.G. Distribution of substance P receptors on murine spleen and Peyer’s patch T and B cells. J. Immunol. 1987, 139, 749–754. [Google Scholar]
- Ho, W.Z.; Lai, J.P.; Zhu, X.H.; Uvaydova, M.; Douglas, S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997, 159, 5654–5660. [Google Scholar]
- Marriott, I.; Bost, K.L. Substance P receptor mediated macrophage responses. Adv. Exp. Med. Biol. 2001, 493, 247–254. [Google Scholar] [CrossRef]
- Wozniak, A.; McLennan, G.; Betts, W.H.; Murphy, G.A.; Scicchitano, R. Activation of human neutrophils by substance P: Effect on FMLP-stimulated oxidative and arachidonic acid metabolism and on antibody-dependent cell-mediated cytotoxicity. Immunology 1989, 68, 359–364. [Google Scholar] [PubMed]
- Shanahan, F.; Denburg, J.A.; Fox, J.; Bienenstock, J.; Befus, D. Mast cell heterogeneity: Effects of neuroenteric peptides on histamine release. J. Immunol. 1985, 135, 1331–1337. [Google Scholar] [PubMed]
- Bar-Shavit, Z.; Goldman, R.; Stabinsky, Y.; Gottlieb, P.; Fridkin, M.; Teichberg, V.I.; Blumberg, S. Enhancement of phagocytosis—A newly found activity of Substance P residing in its N-terminal tetrapeptide sequence. Biochem. Biophys. Res. Commun. 1980, 94, 1445–1451. [Google Scholar] [CrossRef]
- Mathers, A.R.; Tckacheva, O.A.; Janelsins, B.M.; Shufesky, W.J.; Morelli, A.E.; Larregina, A.T. In vivo signaling through the neurokinin 1 receptor favors transgene expression by langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses. J. Immunol. 2007, 178, 7006–7017. [Google Scholar] [CrossRef]
- Janelsins, B.M.; Sumpter, T.L.; Tkacheva, O.A.; Rojas-Canales, D.M.; Erdos, G.; Mathers, A.R.; Shufesky, W.J.; Storkus, W.J.; Falo, L.D., Jr.; Morelli, A.E.; et al. Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12. Blood 2013, 121, 2923–2933. [Google Scholar] [CrossRef]
- Janelsins, B.M.; Mathers, A.R.; Tkacheva, O.A.; Erdos, G.; Shufesky, W.J.; Morelli, A.E.; Larregina, A.T. Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity. Blood 2009, 113, 3017–3026. [Google Scholar] [CrossRef]
- Lighvani, S.; Huang, X.; Trivedi, P.P.; Swanborg, R.H.; Hazlett, L.D. Substance P regulates natural killer cell interferon-? production and resistance toPseudomonas aeruginosa infection. Eur. J. Immunol. 2005, 35, 1567–1575. [Google Scholar] [CrossRef]
- Kincy-Cain, T.; Bost, K.L. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 1997, 158, 2334–2339. [Google Scholar]
- Croitoru, K.; Ernst, P.B.; Bienenstock, J.; Padol, I.; Stanisz, A.M. Selective modulation of the natural killer activity of murine intestinal intraepithelial leucocytes by the neuropeptide substance P. Immunology 1990, 71, 196–201. [Google Scholar]
- Arsenescu, R.; Blum, A.M.; Metwali, A.; Elliott, D.E.; Weinstock, J.V. IL-12 Induction of mRNA encoding substance P in murine macrophages from the spleen and sites of inflammation. J. Immunol. 2005, 174, 3906–3911. [Google Scholar] [CrossRef]
- Blum, A.; Setiawan, T.; Hang, L.; Stoyanoff, K.; Weinstock, J.V. Interleukin-12 (IL-12) and IL-23 Induction of substance P synthesis in Murine T cells and macrophages is subject to IL-10 and transforming growth factor β regulation. Infect. Immun. 2008, 76, 3651–3656. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I.; Bost, K.L. IL-4 and IFN-γ Up-Regulate Substance P Receptor expression in murine peritoneal macrophages. J. Immunol. 2000, 165, 182–191. [Google Scholar] [CrossRef]
- Simeonidis, S.; Castagliuolo, I.; Pan, A.; Liu, J.; Wang, C.-C.; Mykoniatis, A.; Pasha, A.; Valenick, L.; Sougioultzis, S.; Zhao, D.; et al. Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-κB site on its promoter. Proc. Natl. Acad. Sci. USA 2003, 100, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Hartung, H.P.; Toyka, K.V. Activation of macrophages by substance P: Induction of oxidative burst and thromboxane release. Eur. J. Pharmacol. 1983, 89, 301–305. [Google Scholar] [CrossRef]
- Murris-Espin, M.; Pinelli, E.; Pipy, B.; Leophonte, P.; Didier, A. Substance P and alveolar macrophages: Effects on oxidative metabolism and eicosanoid production. Allergy 1995, 50, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.F.; Chavanel, G.; Senik, A. Substance P enhances IL-2 expression in activated human T cells. J. Immunol. 1992, 148, 3498–3504. [Google Scholar]
- Nio, D.A.; Moylan, R.N.; Roche, J.K. Modulation of T lymphocyte function by neuropeptides. Evidence for their role as local immunoregulatory elements. J. Immunol. 1993, 150, 5281–5288. [Google Scholar]
- Rameshwar, P.; Gascon, P.; Ganea, D. Stimulation of IL-2 production in murine lymphocytes by substance P and related tachykinins. J. Immunol. 1993, 151, 2484–2496. [Google Scholar]
- Scicchitano, R.; Biennenstock, J.; Stanisz, A.M. In vivo immunomodulation by the neuropeptide substance P. Immunology 1988, 63, 733–735. [Google Scholar]
- Santoni, G.; Perfumi, M.C.; Spreghini, E.; Romagnoli, S.; Piccoli, M. Neurokinin type-1 receptor antagonist inhibits enhancement of T cell functions by substance P in normal and neuromanipulated capsaicin-treated rats. J. Neuroimmunol. 1999, 93, 15–25. [Google Scholar] [CrossRef]
- Ikeda, Y.; Takei, H.; Matsumoto, C.; Mase, A.; Yamamoto, M.; Takeda, S.; Ishige, A.; Watanabe, K. Administration of substance P during a primary immune response amplifies the secondary immune response via a long-lasting effect on CD8+ T lymphocytes. Arch. Dermatol. Res. 2007, 299, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.M.; Metwali, A.; Crawford, C.; Li, J.; Qadir, K.; Elliott, D.E.; Weinstock, J.V. Interleukin 12 and antigen independently induce substance P receptor expression in T cells in murine schistosomiasis mansoni. FASEB J. 2001, 15, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.V.; Blum, A.; Metwali, A.; Elliott, D.; Arsenescu, R. IL-18 and IL-12 Signal through the NF-κB pathway to induce NK-1R Expression on T cells. J. Immunol. 2003, 170, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- Beinborn, M.; Blum, A.; Hang, L.; Setiawan, T.; Schroeder, J.C.; Stoyanoff, K.; Leung, J.; Weinstock, J.V. TGF-β regulates T-cell neurokinin-1 receptor internalization and function. Proc. Natl. Acad. Sci. USA 2010, 107, 4293–4298. [Google Scholar] [CrossRef] [PubMed]
- Eglezos, A.; Andrews, P.V.; Boyd, R.L.; Helme, R.D. Effects of capsaicin treatment on immunoglobulin secretion in the rat: Further evidence for involvement of tachykinin-containing afferent nerves. J. Neuroimmunol. 1990, 26, 131–138. [Google Scholar] [CrossRef]
- Paunicka, K.J.; Mellon, J.; Robertson, D.; Petroll, M.; Brown, J.R.; Niederkorn, J.Y. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am. J. Transplant. 2015, 15, 1490–1501. [Google Scholar] [CrossRef]
- Erin, N.; Korcum, A.F.; Tanrıöver, G.; Kale, S.; Demir, N.; Köksoy, S. Activation of neuroimmune pathways increases therapeutic effects of radiotherapy on poorly differentiated breast carcinoma. Brain Behav. Immun. 2015, 48, 174–185. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Morteau, O.; Keates, A.C.; Valenick, L.; Wang, C.C.; Zacks, J.; Lu, B.; Gerard, N.P.; Pothoulakis, C. Protective effects of neurokinin-1 receptor during colitis in mice: Role of the epidermal growth factor receptor. Br. J. Pharmacol. 2002, 136, 271–279. [Google Scholar] [CrossRef]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Kim, S.; Piao, J.; Hwang, D.Y.; Park, J.S.; Son, Y.; Hong, H.S. Substance P accelerates wound repair by promoting neovascularization and preventing inflammation in an ischemia mouse model. Life Sci. 2019, 225, 98–106. [Google Scholar] [CrossRef]
- Hong, H.S.; Lee, J.; Lee, E.A.; Kwon, Y.S.; Lee, E.; Ahn, W.; Jiang, M.H.; Kim, J.C.; Son, Y. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat. Med. 2009, 15, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-H.; Kim, D.-Y.; Yi, J.Y.; Son, Y. Substance P accelerates intestinal tissue regeneration after γ-irradiation-induced damage. Wound Repair Regen. 2009, 17, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Exogenous administration of substance p enhances wound healing in a novel skin-injury model. Exp. Biol. Med. 2005, 230, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.; Hong, H.S.; Son, Y. Substance P promotes diabetic wound healing by modulating inflammation and restoring cellular activity of mesenchymal stem cells. Wound Repair Regen. 2016, 24, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Chung, E.; Chi, G.F.; Ahn, W.; Lim, J.E.; Hong, H.S.; Kim, D.W.; Choi, H.; Kim, J.; Son, Y. Substance P induces M2-type macrophages after spinal cord injury. NeuroReport 2012, 23, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C.; Kuchibhotla, S.; Auster, M.E.; Kokkotou, E.; Mooney, D.J.; et al. Substance P promotes Wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015, 185, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.S.; Son, Y. Substance P ameliorates collagen II-induced arthritis in mice via suppression of the inflammatory response. Biochem. Biophys. Res. Commun. 2014, 453, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Chung, E.; Son, Y. A neuropeptide, Substance-P, directly induces tissue-repairing M2 like macrophages by activating the PI3K/Akt/mTOR pathway even in the presence of IFNγ. Sci. Rep. 2017, 7, 9417. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Pioszak, A.A.; Hay, D.L. RAMPs as allosteric modulators of the calcitonin and calcitonin-like class B G protein-coupled receptors. Adv. Pharmacol. 2020, 88, 115–141. [Google Scholar] [CrossRef]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.N.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Soares, M.B.; Shoemaker, C.B.; David, J.R.; Bozza, M.T. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock 2005, 24, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef]
- Jörres, A.; Dinter, H.; Topley, N.; Gahl, G.M.; Frei, U.; Scholz, P. Inhibition of tumour necrosis factor production in endotoxin-stimulated human mononuclear leukocytes by the prostacyclin analogue iloprost: Cellular mechanisms. Cytokine 1997, 9, 119–125. [Google Scholar] [CrossRef]
- Huang, J.; Stohl, L.L.; Zhou, X.; Ding, W.; Granstein, R.D. Calcitonin gene-related peptide inhibits chemokine production by human dermal microvascular endothelial cells. Brain Behav. Immun. 2011, 25, 787–799. [Google Scholar] [CrossRef]
- Millet, I.; Phillips, R.J.; Sherwin, R.S.; Ghosh, S.; Voll, R.E.; Flavell, R.A.; Vignery, A.; Rincón, M. Inhibition of NF-κB activity and Enhancement of apoptosis by the neuropeptide calcitonin gene-related peptide. J. Biol. Chem. 2000, 275, 15114–15121. [Google Scholar] [CrossRef]
- Ding, W.; Wagner, J.A.; Granstein, R.D. CGRP, PACAP, and VIP modulate langerhans cell function by inhibiting NF-κB activation. J. Investig. Dermatol. 2007, 127, 2357–2367. [Google Scholar] [CrossRef]
- Bell, D.; McDermott, B.J. Calcitonin gene-related peptide in the cardiovascular system: Characterization of receptor popula-tions and their (patho)physiological significance. Pharmacol. Rev. 1996, 48, 253–288. [Google Scholar]
- Klebanoff, S.J.; Vadas, M.A.; Harlan, J.M.; Sparks, L.H.; Gamble, J.R.; Agosti, J.M.; Waltersdorph, A.M. Stimulation of neu-trophils by tumor necrosis factor. J. Immunol. 1986, 136, 4220–4225. [Google Scholar]
- Schneider, L.; Hartwig, W.; Flemming, T.; Hackert, T.; Fortunato, F.; Heck, M.; Gebhard, M.-M.; Nawroth, P.P.; Bierhaus, A.; Büchler, M.W.; et al. Protective effects and anti-inflammatory pathways of exogenous calcitonin gene-related peptide in severe necrotizing pancreatitis. Pancreatology 2009, 9, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Uchiba, M.; Kurihara, H.; Nakagata, N.; Okajima, K. Antithrombin reduces reperfusion-induced liver injury in mice by enhancing sensory neuron activation. Thromb. Haemost. 2006, 95, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, N.; Okajima, K.; Harada, N.; Arai, M.; Ishida, Y.; Shimada, S.; Kurihara, H.; Nakagata, N. Contribution of sensory neurons to sex difference in the development of stress-induced gastric mucosal injury in mice. Gastroenterology 2006, 131, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, N.; Okajima, K.; Harada, N. Estrogen and isoflavone attenuate stress-induced gastric mucosal injury by inhibiting decreases in gastric tissue levels of CGRP in ovariectomized rats. Am. J. Physiol. Liver Physiol. 2007, 292, G615–G619. [Google Scholar] [CrossRef]
- Grobman, M.; Graham, A.; Outi, H.; Dodam, J.R.; Reinero, C.R. Chronic neurokinin-1 receptor antagonism fails to ameliorate clinical signs, airway hyper-responsiveness or airway eosinophilia in an experimental model of feline asthma. J. Feline Med. Surg. 2015, 18, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.A.; Basbaum, A.I.; Kwiat, G.C.; Goetzl, E.J.; Levine, J.D. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience 1987, 22, 651–659. [Google Scholar] [CrossRef]
- Vasko, M.R.; Campbell, W.B.; Waite, K.J. Prostaglandin E2 enhances bradykinin-stimulated release of neuropeptides from rat sensory neurons in culture. J. Neurosci. 1994, 14, 4987–4997. [Google Scholar] [CrossRef]
- Hingtgen, C.M.; Waite, K.J.; Vasko, M.R. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3′,5′-cyclic monophosphate transduction cascade. J. Neurosci. 1995, 15, 5411–5419. [Google Scholar] [CrossRef]
- Lallemend, F.; Ernfors, P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci. 2012, 35, 373–381. [Google Scholar] [CrossRef]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp. Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef]
- Horvathova, L.; Padova, A.; Tillinger, A.; Osacka, J.; Bizik, J.; Mravec, B. Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress 2016, 19, 528–534. [Google Scholar] [CrossRef]
- Polli-Lopes, A.C.; Zucoloto, S.; Cunha, F.D.Q.; Figueiredo, L.A.D.S.; Garcia, S.B. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003, 190, 45–50. [Google Scholar] [CrossRef]
- Sinha, S.; Fu, Y.-Y.; Grimont, A.; Ketcham, M.; Lafaro, K.; Saglimbeni, J.A.; Askan, G.; Bailey, J.M.; Melchor, J.P.; Zhong, Y. PanIN neuroendocrine cells promote tumorigenesis via neuronal cross-talk. Cancer Res. 2017, 77, 1868–1879. [Google Scholar] [CrossRef]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Vermeer, D.W.; Madeo, M.; Reavis, H.; Vermeer, S.J.; Williamson, C.S.; Rickel, A.; Stamp, J.; Lucido, C.T.; Cain, J. Tumor-infiltrating nerves create an electro-physiologically active microenvironment and contribute to treatment resistance. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lackovicova, L.; Banovska, L.; Bundzikova, J.; Janega, P.; Bizik, J.; Kiss, A.; Mravec, B. Chemical sympathectomy suppresses fibrosarcoma development and improves survival of tumor-bearing rats. Neoplasma 2011, 58, 424–429. [Google Scholar] [CrossRef]
- Kessler, J.A. Parasympathetic, sympathetic, and sensory interactions in the iris: Nerve growth factor regulates cholinergic ciliary ganglion innervation in vivo. J. Neurosci. 1985, 5, 2719–2725. [Google Scholar] [CrossRef]
- Ahrén, B.; Taborsky, G.J.; Porte, D. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia 1986, 29, 827–836. [Google Scholar] [CrossRef]
- Wu, G.; Ringkamp, M.; Murinson, B.H.F.; Pogatzki, E.M.; Hartke, T.V.; Weerahandi, H.M.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002, 22, 7746–7753. [Google Scholar] [CrossRef]
- Wu, G.; Ringkamp, M.; Hartke, T.V.; Murinson, B.H.F.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J. Neurosci. 2001, 21, RC140. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Blennerhassett, P.; Collins, S.M. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Liver Physiol. 2007, 293, G560–G567. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Blennerhassett, P.; El-Sharkawy, R.T.; Collins, S.M. The protective effect of the vagus nerve in a murine model of chronic relapsing colitis. Am. J. Physiol. Liver Physiol. 2007, 293, G711–G718. [Google Scholar] [CrossRef]
- Oszlács, O.; Jancsó, G.; Kis, G.; Dux, M.; Sántha, P. Perineural capsaicin induces the uptake and transganglionic transport of choleratoxin b subunit by nociceptive c-fiber primary afferent neurons. Neuroscience 2015, 311, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Jancso´, G.; Lawson, S.N. Transganglionic degeneration of capsaicin-sensitive C-fiber primary afferent terminals. Neuroscience 1990, 39, 501–511. [Google Scholar] [CrossRef]
- Pini, A.; Lynn, B. C-fibre Function during the 6 Weeks Following Brief Application of Capsaicin to a Cutaneous Nerve in the Rat. Eur. J. Neurosci. 1991, 3, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Woolf, C.J. The time course and specificity of the changes in the behavioural and dorsal horn cell responses to noxious stimuli following peripheral nerve capsaicin treatment in the rat. Neuroscience 1982, 7, 2051–2056. [Google Scholar] [CrossRef]
- Jancso, G.; Király, E.; Jancsó-Gábor, A. Direct evidence for an axonal site of action of capsaicin. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1980, 313, 91–94. [Google Scholar] [CrossRef]
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggstrom, J.Z.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153. [Google Scholar] [CrossRef]
- Roberson, D.P.; Gudes, S.; Sprague, J.M.; Patoski, H.A.W.; Robson, V.K.; Blasl, F.; Duan, B.; Oh, S.B.; Bean, B.P.; Ma, Q.; et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 2013, 16, 910–918. [Google Scholar] [CrossRef]
- Hockley, J.R.F.; Taylor, T.S.; Callejo, G.; Wilbrey, A.L.; Gutteridge, A.; Bach, K.; Winchester, W.J.; Bulmer, D.C.; McMurray, G.; Smith, E.S.J. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019, 68, 633–644. [Google Scholar] [CrossRef]
- Zuo, H.-D.; Zhang, X.-M.; Li, C.-J.; Cai, C.-P.; Zhao, Q.-H.; Xie, X.-G.; Xiao, B.; Tang, W. CT and MR imaging patterns for pancreatic carcinoma invading the extrapancreatic neural plexus (Part I): Anatomy, imaging of the extrapancreatic nerve. World J. Radiol. 2012, 4, 36–43. [Google Scholar] [CrossRef]
- Schonkeren, S.L.; Thijssen, M.S.; Vaes, N.; Boesmans, W.; Melotte, V. The Emerging Role of Nerves and Glia in Colorectal Cancer. Cancers 2021, 13, 152. [Google Scholar] [CrossRef]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor Innervation: Cancer Has Some Nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erin, N.; Shurin, G.V.; Baraldi, J.H.; Shurin, M.R. Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers 2022, 14, 2333. https://doi.org/10.3390/cancers14092333
Erin N, Shurin GV, Baraldi JH, Shurin MR. Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers. 2022; 14(9):2333. https://doi.org/10.3390/cancers14092333
Chicago/Turabian StyleErin, Nuray, Galina V. Shurin, James H. Baraldi, and Michael R. Shurin. 2022. "Regulation of Carcinogenesis by Sensory Neurons and Neuromediators" Cancers 14, no. 9: 2333. https://doi.org/10.3390/cancers14092333
APA StyleErin, N., Shurin, G. V., Baraldi, J. H., & Shurin, M. R. (2022). Regulation of Carcinogenesis by Sensory Neurons and Neuromediators. Cancers, 14(9), 2333. https://doi.org/10.3390/cancers14092333








