A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective
Abstract
:Simple Summary
Abstract
1. Introduction
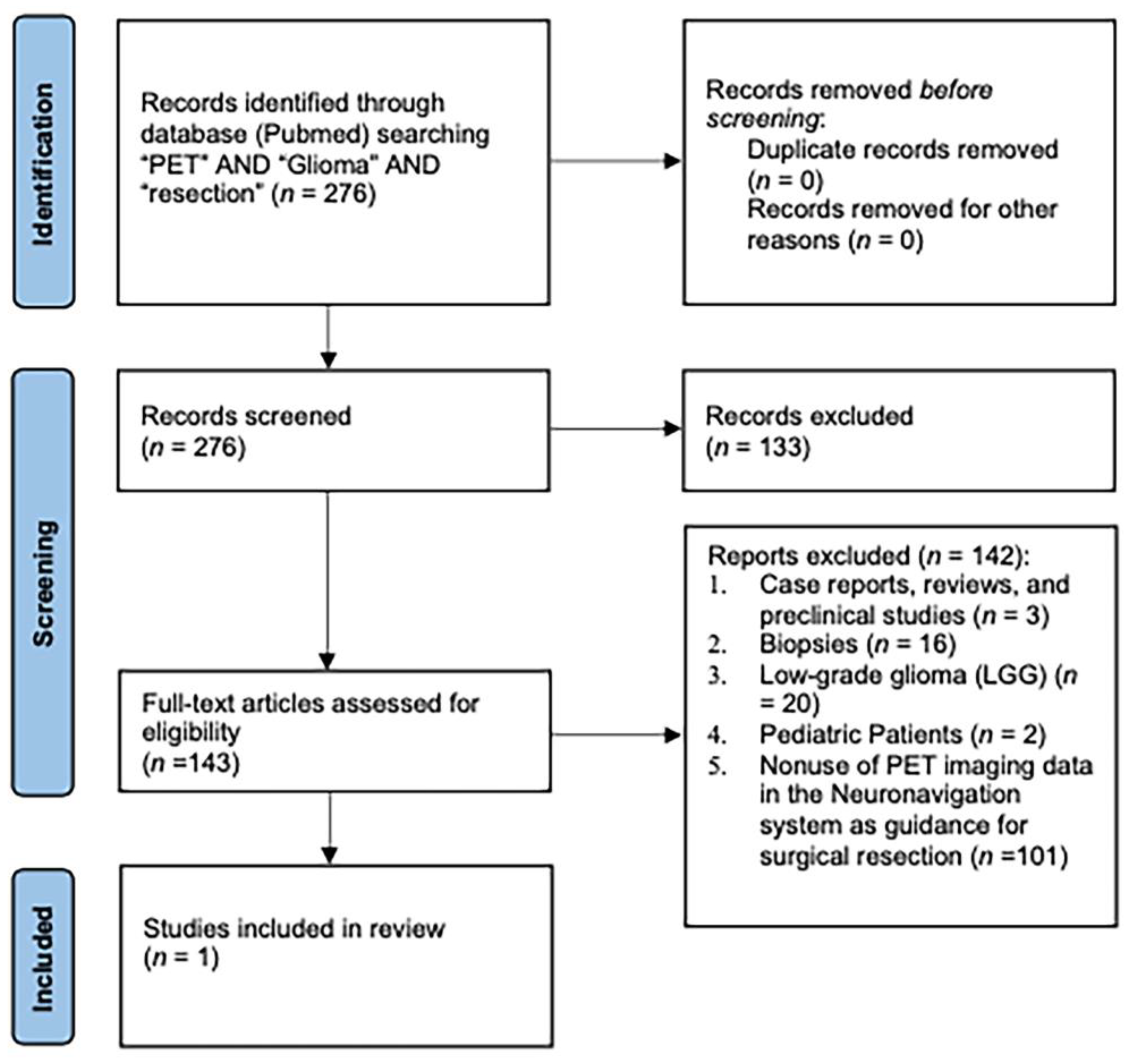
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas: Clinical article. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Karschnia, P.; Vogelbaum, M.A.; van den Bent, M.; Cahill, D.P.; Bello, L.; Narita, Y.; Berger, M.S.; Weller, M.; Tonn, J.C. Evidence-based recommendations on categories for extent of resection in diffuse glioma. Eur. J. Cancer 2021, 149, 23–33. [Google Scholar] [CrossRef]
- Gandhi, S.; Meybodi, A.T.; Belykh, E.; Cavallo, C.; Zhao, X.; Pasha Syed, M.; Borba Moreira, L.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.J.; Gohil, K.; Thompson, C.M.; Naik, A.; Hassaneen, W. Fluorescein-Guided Resection of High Grade Gliomas: A Meta-Analysis. World Neurosurg. 2021, 155, 181–188.e7. [Google Scholar] [CrossRef]
- Willems, P.W.A.; Taphoorn, M.J.B.; Burger, H.; Van Der Sprenkel, J.W.B.; Tulleken, C.A.F. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: A randomized controlled trial. J. Neurosurg. 2006, 104, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Hatiboglu, M.A.; Weinberg, J.S.; Suki, D.; Rao, G.; Prabhu, S.S.; Shah, K.; Jackson, E.; Sawaya, R. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: A prospective volumetric analysis. Neurosurgery 2009, 64, 1073–1081. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet. Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Moiyadi, A.V.; Shetty, P.M.; Mahajan, A.; Udare, A.; Sridhar, E. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir. 2013, 155, 2217–2225. [Google Scholar] [CrossRef]
- Prada, F.; Del Bene, M.; Fornaro, R.; Vetrano, I.G.; Martegani, A.; Aiani, L.; Sconfienza, L.M.; Mauri, G.; Solbiati, L.; Pollo, B.; et al. Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg. Focus 2016, 40, E7. [Google Scholar] [CrossRef] [Green Version]
- Sastry, R.; Bi, W.L.; Pieper, S.; Frisken, S.; Kapur, T.; Wells, W.; Golby, A.J. Applications of Ultrasound in the Resection of Brain Tumors. J. Neuroimaging 2017, 27, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Simonelli, M.; Santoro, A.; Clerici, E.; Rossi, M.; Scorsetti, M.; Bello, L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neurooncol. 2017, 135, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, Y.; Friedman, E.; Liu, Z.; Zhu, J.J.; Hsu, S.; Tandon, N. The Survival Advantage of “supratotal” Resection of Glioblastoma Using Selective Cortical Mapping and the Subpial Technique. Neurosurgery 2017, 81, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Certo, F.; Altieri, R.; Maione, M.; Schonauer, C.; Sortino, G.; Fiumanò, G.; Tirrò, E.; Massimino, M.; Broggi, G.; Vigneri, P.; et al. FLAIRectomy in Supramarginal Resection of Glioblastoma Correlates With Clinical Outcome and Survival Analysis: A Prospective, Single Institution, Case Series. Oper. Neurosurg. 2021, 20, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Seker-Polat, F.; Degirmenci, N.P.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Bianconi, A.; Aruta, G.; Rizzo, F.; Salvati, L.F.; Zeppa, P.; Garbossa, D.; Cofano, F. Systematic Review on Tumor Microenvironment in Glial Neoplasm: From Understanding Pathogenesis to Future Therapeutic Perspectives. Int. J. Mol. Sci. 2022, 23, 4166. [Google Scholar] [CrossRef]
- Burger, P.C.; Dubois, P.J.; Schold, S.C.; Smith, K.R.; Odom, G.L.; Crafts, D.C.; Giangaspero, F. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J. Neurosurg. 1983, 58, 159–169. [Google Scholar] [CrossRef]
- De Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The influence of surgery on recurrence pattern of glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Altieri, R.; Barbagallo, D.; Certo, F.; Broggi, G.; Ragusa, M.; Di Pietro, C.; Caltabiano, R.; Magro, G.; Peschillo, S.; Purrello, M.; et al. Peritumoral Microenvironment in High-Grade Gliomas: From FLAIRectomy to Microglia-Glioma Cross-Talk. Brain Sci. 2021, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Stavrinou, P.; Lipke, K.; Bauer, E.K.; Ceccon, G.; Werner, J.M.; Neumaier, B.; Fink, G.R.; Shah, N.J.; Langen, K.J.; et al. FET PET reveals considerable spatial differences in tumour burden compared to conventional MRI in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müther, M.; Koch, R.; Weckesser, M.; Sporns, P.; Schwindt, W.; Stummer, W. 5-Aminolevulinic Acid Fluorescence-Guided Resection of 18F-FET-PET Positive Tumor Beyond Gadolinium Enhancing Tumor Improves Survival in Glioblastoma. Neurosurgery 2019, 85, E1020–E1029. [Google Scholar] [CrossRef] [Green Version]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; La Fougère, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18 F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 540–557. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Nishikawa, M.; Suehiro, S.; Matsumoto, S.; Ozaki, S.; Fukushima, M.; Kurata, M.; et al. Met-PET uptake index for total tumor resection: Identification of 11 C-methionine uptake index as a goal for total tumor resection including infiltrating tumor cells in glioblastoma. Neurosurg. Rev. 2021, 44, 587–597. [Google Scholar] [CrossRef]
- Pirotte, B.; Goldman, S.; Massager, N.; David, P.; Wikler, D.; Lipszyc, M.; Salmon, I.; Brotchi, J.; Levivier, M. Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J. Neurosurg. 2004, 101, 476–483. [Google Scholar] [CrossRef]
- Stockhammer, F.; Thomale, U.W.; Plotkin, M.; Hartmann, C.; Von Deimling, A. Association between fluorine-18–labeled fluorodeoxyglucose uptake and 1p and 19q loss of heterozygosity in World Health Organization Grade II gliomas. J. Neurosurg. 2007, 106, 633–637. [Google Scholar] [CrossRef]
- Stockhammer, F.; Plotkin, M.; Amthauer, H.; Landeghem, F.K.H.; Woiciechowsky, C. Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J. Neurooncol. 2008, 88, 205–210. [Google Scholar] [CrossRef]
- Weber, M.A.; Henze, M.; Tüttenberg, J.; Stieltjes, B.; Meissner, M.; Zimmer, F.; Burkholder, I.; Kroll, A.; Combs, S.E.; Vogt-Schaden, M.; et al. Biopsy targeting gliomas: Do functional imaging techniques identify similar target areas? Investig. Radiol. 2010, 45, 755–768. [Google Scholar] [CrossRef]
- Kunz, M.; Thon, N.; Eigenbrod, S.; Hartmann, C.; Egensperger, R.; Herms, J.; Geisler, J.; La Fougere, C.; Lutz, J.; Linn, J.; et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 2011, 13, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Floeth, F.W.; Sabel, M.; Ewelt, C.; Stummer, W.; Felsberg, J.; Reifenberger, G.; Steiger, H.J.; Stoffels, G.; Coenen, H.H.; Langen, K.J. Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 731–741. [Google Scholar] [CrossRef]
- Ewelt, C.; Floeth, F.W.; Felsberg, J.; Steiger, H.J.; Sabel, M.; Langen, K.J.; Stoffels, G.; Stummer, W. Finding the anaplastic focus in diffuse gliomas: The value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011, 113, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Kinoshita, M.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Hashimoto, N.; Yoshimine, T. 11C-methionine uptake and intraoperative 5-aminolevulinic acid-induced fluorescence as separate index markers of cell density in glioma: A stereotactic image-histological analysis. Cancer 2012, 118, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro Oncol. 2013, 15, 1058–1067. [Google Scholar] [CrossRef]
- Beppu, T.; Sasaki, T.; Terasaki, K.; Saura, H.; Mtsuura, H.; Ogasawara, K.; Sasaki, M.; Ehara, S.; Iwata, R.; Takai, Y. High-uptake areas on positron emission tomography with the hypoxic radiotracer 18F-FRP170 in glioblastomas include regions retaining proliferative activity under hypoxia. Ann. Nucl. Med. 2015, 29, 336. [Google Scholar] [CrossRef] [Green Version]
- Karlberg, A.; Berntsen, E.M.; Johansen, H.; Skjulsvik, A.J.; Reinertsen, I.; Dai, H.Y.; Xiao, Y.; Rivaz, H.; Borghammer, P.; Solheim, O.; et al. 18F-FACBC PET/MRI in Diagnostic Assessment and Neurosurgery of Gliomas. Clin. Nucl. Med. 2019, 44, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, P.; Zanotti-Fregonara, P.; Eimer, S.; Gimbert, E.; Monteil, P.; Penchet, G.; Lamare, F.; Perez, P.; Vimont, D.; Ledure, S.; et al. Combining 3′-Deoxy-3′-[18F] fluorothymidine and MRI increases the sensitivity of glioma volume detection. Nucl. Med. Commun. 2019, 40, 1066–1071. [Google Scholar] [CrossRef]
- Ponisio, M.R.; McConathy, J.E.; Dahiya, S.M.; Miller-Thomas, M.M.; Rich, K.M.; Salter, A.; Wang, Q.; LaMontagne, P.J.; Guzmán Pérez-Carrillo, G.J.; Benzinger, T.L.S. Dynamic 18 F-FDOPA-PET/MRI for the preoperative evaluation of gliomas: Correlation with stereotactic histopathology. Neuro -Oncol. Pract. 2020, 7, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Verburg, N.; Koopman, T.; Yaqub, M.M.; Hoekstra, O.S.; Lammertsma, A.A.; Barkhof, F.; Pouwels, P.J.W.; Reijneveld, J.C.; Heimans, J.J.; Rozemuller, A.J.M.; et al. Improved detection of diffuse glioma infiltration with imaging combinations: A diagnostic accuracy study. Neuro Oncol. 2020, 22, 412. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Hirose, Y.; Miyake, K.; Arakawa, Y.; Kagawa, N.; Nariai, T.; Narita, Y.; Nishikawa, R.; Tsuyuguchi, N.; Fukami, T.; et al. Determining the extent of tumor resection at surgical planning with 18F-fluciclovine PET/CT in patients with suspected glioma: Multicenter phase III trials. Ann. Nucl. Med. 2021, 35, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.W.; Zilles, K.; Coenen, H.H.; Langen, K.J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirotte, B.J.M.; Levivier, M.; Goldman, S.; Massager, N.; Wikler, D.; Dewitte, O.; Bruneau, M.; Rorive, S.; David, P.; Brotchi, J. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: A survival analysis in 66 consecutive patients. Neurosurgery 2009, 64, 471–481. [Google Scholar] [CrossRef]
- Ort, J.; Hamou, H.A.; Kernbach, J.M.; Hakvoort, K.; Blume, C.; Lohmann, P.; Galldiks, N.; Heiland, D.H.; Mottaghy, F.M.; Clusmann, H.; et al. 18 F-FET-PET-guided gross total resection improves overall survival in patients with WHO grade III/IV glioma: Moving towards a multimodal imaging-guided resection. J. Neurooncol. 2021, 155, 71–80. [Google Scholar] [CrossRef]
- Kracht, L.W.; Miletic, H.; Busch, S.; Jacobs, A.H.; Voges, J.; Hoevels, M.; Klein, J.C.; Herholz, K.; Heiss, W.D. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: Local comparison with stereotactic histopathology. Clin. Cancer Res. 2004, 10, 7163–7170. [Google Scholar] [CrossRef] [Green Version]
- Torii, K.; Tsuyuguchi, N.; Kawabe, J.; Sunada, I.; Hara, M.; Shiomi, S. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann. Nucl. Med. 2005, 19, 677–683. [Google Scholar] [CrossRef]
- Nojiri, T.; Nariai, T.; Aoyagi, M.; Senda, M.; Ishii, K.; Ishiwata, K.; Ohno, K. Contributions of biological tumor parameters to the incorporation rate of L: -[methyl-(11)C] methionine into astrocytomas and oligodendrogliomas. J. Neurooncol. 2009, 93, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Okita, Y.; Kinoshita, M.; Goto, T.; Kagawa, N.; Kishima, H.; Shimosegawa, E.; Hatazawa, J.; Hashimoto, N.; Yoshimine, T. (11)C-methionine uptake correlates with tumor cell density rather than with microvessel density in glioma: A stereotactic image-histology comparison. Neuroimage 2010, 49, 2977–2982. [Google Scholar] [CrossRef]
- Roberts, D.W.; Valdés, P.A.; Harris, B.T.; Fontaine, K.M.; Hartov, A.; Fan, X.; Ji, S.; Lollis, S.S.; Pogue, B.W.; Leblond, F.; et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: Relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J. Neurosurg. 2011, 114, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunet, V.; Pomoni, A.; Hottinger, A.; Nicod-Lalonde, M.; Prior, J.O. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: Systematic review and meta-analysis. Neuro Oncol. 2015, 18, 426–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, P.; Mayer, S.; Herz, M.; Wester, H.-J.; Schwaiger, M.; Senekowitsch-Schmidtke, R. Investigation of Transport Mechanism and Uptake Kinetics of O-(2-[18F]Fluoroethyl)-L-Tyrosine In Vitro and In Vivo. J. Nucl. Med. 1999, 40, 1367–1373. [Google Scholar] [PubMed]
- Okubo, S.; Zhen, H.N.; Kawai, N.; Nishiyama, Y.; Haba, R.; Tamiya, T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J. Neurooncol. 2010, 99, 217–225. [Google Scholar] [CrossRef]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J. Neurooncol. 2013, 111, 11–18. [Google Scholar] [CrossRef]
- Juhász, C.; Dwivedi, S.; Kamson, D.O.; Michelhaugh, S.K.; Mittal, S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol. Imaging 2014, 13, 7290.2014.00015. [Google Scholar] [CrossRef]
- Morana, G.; Piccardo, A.; Milanaccio, C.; Puntoni, M.; Nozza, P.; Cama, A.; Zefiro, D.; Cabria, M.; Rossi, A.; Garrè, M.L. Value of 18F-3,4-dihydroxyphenylalanine PET/MR image fusion in pediatric supratentorial infiltrative astrocytomas: A prospective pilot study. J. Nucl. Med. 2014, 55, 718–723. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.; Dowson, N.; Puttick, S.; Gal, Y.; Thomas, P.; Fay, M.; Smith, J.; Rose, S. Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl. Med. Biol. 2015, 42, 788–795. [Google Scholar] [CrossRef] [Green Version]
- Morana, G.; Piccardo, A.; Tortora, D.; Puntoni, M.; Severino, M.; Nozza, P.; Ravegnani, M.; Consales, A.; Mascelli, S.; Raso, A.; et al. Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2084–2093. [Google Scholar] [CrossRef]
- Zaragori, T.; Ginet, M.; Marie, P.Y.; Roch, V.; Grignon, R.; Gauchotte, G.; Rech, F.; Blonski, M.; Lamiral, Z.; Taillandier, L.; et al. Use of static and dynamic [18 F]-F-DOPA PET parameters for detecting patients with glioma recurrence or progression. EJNMMI Res. 2020, 10, 56. [Google Scholar] [CrossRef]
- Somme, F.; Bender, L.; Namer, I.J.; Noël, G.; Bund, C. Usefulness of 18 F-FDOPA PET for the management of primary brain tumors: A systematic review of the literature. Cancer Imaging 2020, 20, 70. [Google Scholar] [CrossRef]
- Sipos, D.; László, Z.; Tóth, Z.; Kovács, P.; Tollár, J.; Gulybán, A.; Lakosi, F.; Repa, I.; Kovács, A. Additional Value of 18F-FDOPA Amino Acid Analog Radiotracer to Irradiation Planning Process of Patients With Glioblastoma Multiforme. Front. Oncol. 2021, 11, 699360. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.; Le Reste, P.J.; Metais, A.; Chaboub, N.; Devillers, A.; Saint-Jalmes, H.; Le Jeune, F.; Palard-Novello, X. Additive Value of Dynamic FDOPA PET/CT for Glioma Grading. Front. Med. 2021, 8, 705996. [Google Scholar] [CrossRef] [PubMed]
- Grosu, A.L.; Astner, S.T.; Riedel, E.; Nieder, C.; Wiedenmann, N.; Heinemann, F.; Schwaiger, M.; Molls, M.; Wester, H.J.; Weber, W.A. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.A.; Wester, H.J.; Grosu, A.L.; Herz, M.; Dzewas, B.; Feldmann, H.J.; Molls, M.; Stöcklin, G.; Schwaiger, M. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: Initial results of a comparative study. Eur. J. Nucl. Med. 2000, 27, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Becherer, A.; Karanikas, G.; Szabó, M.; Zettinig, G.; Asenbaum, S.; Marosi, C.; Henk, C.; Wunderbaldinger, P.; Czech, T.; Wadsak, W.; et al. Brain tumour imaging with PET: A comparison between [18F]fluorodopa and [11C]methionine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T.; Narayanan, T.K.; Jain, V.; Mukherjee, J.; Mantil, J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol. Imaging Biol. 2008, 10, 1–18. [Google Scholar] [CrossRef]
- Pöpperl, G.; Kreth, F.W.; Mehrkens, J.H.; Herms, J.; Seelos, K.; Koch, W.; Gildehaus, F.J.; Kretzschmar, H.A.; Tonn, J.C.; Tatsch, K. FET PET for the evaluation of untreated gliomas: Correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1933–1942. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Y.; Nie, J.; Chen, F.; Ma, X. Diagnostic and grading accuracy of 18 F-FDOPA PET and PET/CT in patients with gliomas: A systematic review and meta-analysis. BMC Cancer 2019, 19, 767. [Google Scholar] [CrossRef] [Green Version]
- Verger, A.; Stoffels, G.; Bauer, E.K.; Lohmann, P.; Blau, T.; Fink, G.R.; Neumaier, B.; Shah, N.J.; Langen, K.J.; Galldiks, N. Static and dynamic 18 F-FET PET for the characterization of gliomas defined by IDH and 1p/19q status. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 443–451. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Alexiou, G.A.; Fotopoulos, A.D.; Jabbour, P.; Kyritsis, A.P.; Sioka, C. Performance of 18F-FDG, 11C-Methionine, and 18F-FET PET for Glioma Grading: A Meta-analysis. Clin. Nucl. Med. 2019, 44, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Tovi, M.; Hartman, M.; Lilja, A.; Ericsson, A. MR imaging in cerebral gliomas: Tissue component analysis in correlation with histopathology of whole-brain specimens. Acta Radiol. 1994, 35, 495–505. [Google Scholar] [CrossRef]
- Ginsberg, L.E.; Fuller, G.N.; Hashmi, M.; Leeds, N.E.; Schomer, D.F. The significance of lack of MR contrast enhancement of supratentorial brain tumors in adults: Histopathological evaluation of a series. Surg. Neurol. 1998, 49, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Eidel, O.; Burth, S.; Neumann, J.O.; Kieslich, P.J.; Sahm, F.; Jungk, C.; Kickingereder, P.; Bickelhaupt, S.; Mundiyanapurath, S.; Bäumer, P.; et al. Tumor Infiltration in Enhancing and Non-Enhancing Parts of Glioblastoma: A Correlation with Histopathology. PLoS ONE 2017, 12, e0169292. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, M.; Arita, H.; Okita, Y.; Kagawa, N.; Kishima, H.; Hashimoto, N.; Tanaka, H.; Watanabe, Y.; Shimosegawa, E.; Hatazawa, J.; et al. Comparison of diffusion tensor imaging and 11 C-methionine positron emission tomography for reliable prediction of tumor cell density in gliomas. J. Neurosurg. 2016, 125, 1136–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; He, M.Z.; Li, T.; Yang, X. MRI combined with PET-CT of different tracers to improve the accuracy of glioma diagnosis: A systematic review and meta-analysis. Neurosurg. Rev. 2019, 42, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Zhang-Yin, J.T.; Girard, A.; Bertaux, M. What Does PET Imaging Bring to Neuro-Oncology in 2022? A Review. Cancers 2022, 14, 879. [Google Scholar] [CrossRef]
- Kinoshita, M.; Goto, T.; Arita, H.; Okita, Y.; Isohashi, K.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Shimosegawa, E.; Saitoh, Y.; et al. Imaging 18F-fluorodeoxy glucose/ 11C-methionine uptake decoupling for identification of tumor cell infiltration in peritumoral brain edema. J. Neurooncol. 2012, 106, 417–425. [Google Scholar] [CrossRef]
- Kinoshita, M.; Arita, H.; Goto, T.; Okita, Y.; Isohashi, K.; Watabe, T.; Kagawa, N.; Fujimoto, Y.; Kishima, H.; Shimosegawa, E.; et al. A novel PET index, 18F-FDG-11C-methionine uptake decoupling score, reflects glioma cell infiltration. J. Nucl. Med. 2012, 53, 1701–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, M.; Uchikoshi, M.; Tateishi, S.; Miyazaki, S.; Sakai, M.; Ozaki, T.; Asai, K.; Fujita, Y.; Matsuhashi, T.; Kanemura, Y.; et al. Magnetic Resonance Relaxometry for Tumor Cell Density Imaging for Glioma: An Exploratory Study via 11 C-Methionine PET and Its Validation via Stereotactic Tissue Sampling. Cancers 2021, 13, 4067. [Google Scholar] [CrossRef]
- Grosu, A.L.; Weber, W.A.; Riedel, E.; Jeremic, B.; Nieder, C.; Franz, M.; Gumprecht, H.; Jaeger, R.; Schwaiger, M.; Molls, M. L-(methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Miwa, K.; Tanaka, O.; Shinoda, J.; Nishibori, H.; Tsuge, Y.; Yano, H.; Iwama, T.; Hayashi, S.; Hoshi, H.; et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.; Goldman, S.; Dewitte, O.; Massager, N.; Wikler, D.; Lefranc, F.; Taib, N.O.B.; Rorive, S.; David, P.; Brotchi, J.; et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: A report of 103 consecutive procedures. J. Neurosurg. 2006, 104, 238–253. [Google Scholar] [CrossRef]
- Dunet, V.; Rossier, C.; Buck, A.; Stupp, R.; Prior, J.O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: A systematic review and Metaanalysis. J. Nucl. Med. 2012, 53, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapa, C.; Linsenmann, T.; Monoranu, C.M.; Samnick, S.; Buck, A.K.; Bluemel, C.; Czernin, J.; Kessler, A.F.; Homola, G.A.; Ernestus, R.I.; et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J. Nucl. Med. 2014, 55, 1611–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Combs, S.E.; Leotta, K.; Afshar-Oromieh, A.; Rieken, S.; Debus, J.; Haberkorn, U.; Giesel, F.L. Intra-individual comparison of 18F-FET and 18F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014, 16, 434. [Google Scholar] [CrossRef] [Green Version]
- Morana, G.; Puntoni, M.; Garrè, M.L.; Massollo, M.; Lopci, E.; Naseri, M.; Severino, M.; Tortora, D.; Rossi, A.; Piccardo, A. Ability of (18)F-DOPA PET/CT and fused (18)F-DOPA PET/MRI to assess striatal involvement in paediatric glioma. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1664–1672. [Google Scholar] [CrossRef]
- Stockhammer, F.; Misch, M.; Horn, P.; Koch, A.; Fonyuy, N.; Plotkin, M. Association of F18-fluoro-ethyl-tyrosin uptake and 5-aminolevulinic acid-induced fluorescence in gliomas. Acta Neurochir. 2009, 151, 1377–1383. [Google Scholar] [CrossRef]
- Díez Valle, R.; Tejada Solis, S.; Idoate Gastearena, M.A.; García De Eulate, R.; Domínguez Echávarri, P.; Aristu Mendiroz, J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: Volumetric analysis of extent of resection in single-center experience. J. Neurooncol. 2011, 102, 105–113. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Barone, D.G.; Bryant, A.; Vale, L.; Bulbeck, H.; Lawrie, T.A.; Hart, M.G.; Watts, C. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst. Rev. 2018, 2018, CD013630. [Google Scholar] [CrossRef]
- Hansen, R.W.; Pedersen, C.B.; Halle, B.; Korshoej, A.R.; Schulz, M.K.; Kristensen, B.W.; Poulsen, F.R. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: A single-center retrospective study. J. Neurosurg. 2019, 133, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Della Puppa, A.; Munari, M.; Gardiman, M.P.; Volpin, F. Combined Fluorescence Using 5-Aminolevulinic Acid and Fluorescein Sodium at Glioblastoma Border: Intraoperative Findings and Histopathologic Data About 3 Newly Diagnosed Consecutive Cases. World Neurosurg. 2019, 122, e856–e863. [Google Scholar] [CrossRef] [PubMed]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Perna, G.D.; Melcarne, A.; Altieri, R.; La Rocca, G.; Sabatino, G.; et al. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Zeppa, P.; De Marco, R.; Monticelli, M.; Massara, A.; Bianconi, A.; Di Perna, G.; Greco Crasto, S.; Cofano, F.; Melcarne, A.; Lanotte, M.M.; et al. Fluorescence-Guided Surgery in Glioblastoma: 5-ALA, SF or Both? Differences between Fluorescent Dyes in 99 Consecutive Cases. Brain Sci 2022, 12, 555. [Google Scholar] [CrossRef]
- Tuncer, M.S.; Salvati, L.F.; Grittner, U.; Hardt, J.; Schilling, R.; Bährend, I.; Silva, L.L.; Fekonja, L.S.; Faust, K.; Vajkoczy, P.; et al. Towards a tractography-based risk stratification model for language area associated gliomas. NeuroImage Clin. 2021, 29, 102541. [Google Scholar] [CrossRef] [PubMed]
- Salvati, L.F.; De Marco, R.; Palmieri, G.; Minardi, M.; Massara, A.; Pesaresi, A.; Cagetti, B.; Melcarne, A.; Garbossa, D. The Relevant Role of Navigated Tractography in Speech Eloquent Area Glioma Surgery: Single Center Experience. Brain Sci. 2021, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, P.; Neitzert, L.; Mammi, M.; Monticelli, M.; Altieri, R.; Castaldo, M.; Cofano, F.; Borrè, A.; Zenga, F.; Melcarne, A.; et al. How Reliable Are Volumetric Techniques for High-Grade Gliomas? A Comparison Study of Different Available Tools. Neurosurgery 2020, 87, E672–E679. [Google Scholar] [CrossRef]
- Altieri, R.; Raimondo, S.; Tiddia, C.; Sammarco, D.; Cofano, F.; Zeppa, P.; Monticelli, M.; Melcarne, A.; Junemann, C.; Zenga, F.; et al. Glioma surgery: From preservation of motor skills to conservation of cognitive functions. J. Clin. Neurosci. 2019, 70, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Eyüpoglu, I.Y.; Hore, N.; Merkel, A.; Buslei, R.; Buchfelder, M.; Savaskan, N.; Eyüpoglu, I.Y.; Hore, N.; Merkel, A.; Buslei, R.; et al. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 2016, 7, 25755–25768. [Google Scholar] [CrossRef] [PubMed]
- Pala, A.; Reske, S.N.; Eberhardt, N.; Scheuerle, A.; König, R.; Schmitz, B.; Beer, A.J.; Wirtz, C.R.; Coburger, J. Diagnostic accuracy of intraoperative perfusion-weighted MRI and 5-aminolevulinic acid in relation to contrast-enhanced intraoperative MRI and 11 C-methionine positron emission tomography in resection of glioblastoma: A prospective study. Neurosurg. Rev. 2019, 42, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Is Supratotal Resection of Glioblastoma in Noneloquent Areas Possible? World Neurosurg. 2014, 82, e101–e103. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Surgery for malignant brain gliomas: Fluorescence-guided resection or functional-based resection? Front. Surg. 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigiotto, L.; Annicchiarico, L.; Corsini, F.; Vitali, L.; Falchi, R.; Dalpiaz, C.; Rozzanigo, U.; Barbareschi, M.; Avesani, P.; Papagno, C.; et al. Effects of supra-total resection in neurocognitive and oncological outcome of high-grade gliomas comparing asleep and awake surgery. J. Neurooncol. 2020, 148, 97–108. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year | No. of Pts | No. of Recurrence | Study Type | PET Radiotracer | PET Use | Glioma Grade (WHO 2021) |
|---|---|---|---|---|---|---|
| Pirotte et al., 2004 [29] | 32 | 0 | Retrospective | Both 18F-FDG and Met | Comparison of distribution, extent, and relative contributions of 18F-FDG and Met | 2–4 |
| Pauleit et al., 2005 [44] | 28 | 0 | Prospective | FET | FET-PET combined with MRI imaging to improve distinction between cellular glioma tissue and unspecific peritumoral brain tissue | 1–4 |
| Stockhammer et al., 2007 [30] | 25 | 4 | Retrospective | FDG | Prediction of LOH 1p/19q in grade II gliomas | 2, 3 |
| Stockhammer et al., 2008 * [31] | 22 | 9 | Prospective | FET | Histological evaluation of surgically collected tissue in FET-PET-positive areas of non-contrast-enhancing lesions | 2, 3 |
| Weber et al., 2010 [32] | 61 1 | 0 | Retrospective | FLT and FDG | Multiparametric evaluation and functional imaging comparison | 2–4 |
| Kunz et al., 2010 [33] | 55 | 0 | Prospective | FET | Correlation between dynamic PET parameters and histopathological characteristics, metabolic and molecular signatures | 2–4 |
| Floeth et al., 2011 * [34] | 30 2 | 4 | Prospective | FET | Correlation between FET-PET uptake and intraoperative 5-ALA fluorescence | 2, 3 |
| Ewelt et al., 2011 * [35] | 30 2 | NA | Prospective | FET | Relationship between FET-PET uptake, contrast-enhanced areas at MRI, and 5-ALA fluorescence intraoperatively | 2, 3 |
| Arita et al., 2012 * [36] | 11 | NA | Prospective | Met | Correlation between Met-PET uptake and intraoperative 5-ALA fluorescence | 2–4 |
| Pafundi et al., 2013 [37] | 10 | 2 | Prospective | 18FDOPA | Histopathological differences between CE-MRI areas and 18FDOPA areas and correlation of pathological characteristics with PET uptake and use in RT planning | 2–4 |
| Beppu et al., 2015 * [38] | 13 | 0 | Prospective | FRP170 | Comparison of FRP170-PET uptake areas with histological findings | 4 |
| Karlberg et al., 2019 * [39] | 11 | 3 | Prospective | F-FACBC | Diagnostic value of 18F-FACBC PET/MRI in distinguishing between low-grade and high-grade gliomas and its use in guiding surgical resection | 2–4 |
| Fernandez et al., 2019 * [40] | 13 | 0 | Prospective | FLT | Assessment of the added value of PET imaging integration to MRI in detecting tumoral tissue and correlation of PET uptake to tumor proliferation and grading | 4 |
| Ponisio et al., 2020 [41] | 10 | 4 | Prospective | 18FDOPA | A better assessment of tumor volume and surgical margins and correlation of 18FDOPA-PET/MRI imaging with grade, histopathology, and molecular markers | 2–4 |
| Verburg et al., 2020 * [42] | 20 | 0 | Prospective | FET | Assessment of best imaging studies’ (both FET-PET imaging and different MRI sequences) combination to detect glioma infiltration in enhancing and nonenhancing glioma | 2–4 |
| Wakabayashi et al., 2021 * [43] | 45 | 0 | Multicenter, nonrandomized, open-label phase III clinical trial | 18F-fluciclovine | Assessment of diagnostic accuracy of 18F-fluciclovine and useful in determining the extent of resection | 2–4 |
| Authors, Year | No. of All Pts with HGG/No. of Primary HGG | Sex (All Pts) | Age (Mean; Range) | PET Radiotracers | PET Resection (y/n) | EOR | EOR Improvement (y/n) | PFS (Mean and Range) | OS (Mean and Range) | Limits |
|---|---|---|---|---|---|---|---|---|---|---|
| Pirotte et al., 2009 1 [45] | 66/31 | 23♀; 43♂ | 6–70 | FDG (n. 9), Met (n. 22) | y | 10/31 PET-STR 21/31 PET-GTR | y | NA NA | 32.8 (NA) | 3 children were included in the study; although the difference in overall survival was statistically significant, 32.6 or 32.4, compared with 17.6 months (χ2 = 20.231 (df, 1); p = 0.0001; median survival: R = 5.714; hazard ratio, 0.532), they did not report the OS for each group considered in the comparison |
| Inoue et al., 2021 [28] | 10 | 3♀; 7♂ | 66.4 y (38–79) | Met | y | 8/10 CE-GTR 1 2/10 CE-STR | NA | 17.5 mos (2.1–65) | 26.4 mos (6–65) | |
| Ort et al., 2021; [46] | 30/16 | 11♀; 19♂ | 59.9 y (53–63 | FET | NA | 12/20 PET-GTR 8/20 PET-STR | y | NA NA | 15.1 13.6 | Impossible to extrapolate the use of PET imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, R.; Pesaresi, A.; Bianconi, A.; Zotta, M.; Deandreis, D.; Morana, G.; Zeppa, P.; Melcarne, A.; Garbossa, D.; Cofano, F. A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective. Cancers 2023, 15, 90. https://doi.org/10.3390/cancers15010090
De Marco R, Pesaresi A, Bianconi A, Zotta M, Deandreis D, Morana G, Zeppa P, Melcarne A, Garbossa D, Cofano F. A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective. Cancers. 2023; 15(1):90. https://doi.org/10.3390/cancers15010090
Chicago/Turabian StyleDe Marco, Raffaele, Alessandro Pesaresi, Andrea Bianconi, Michela Zotta, Désirée Deandreis, Giovanni Morana, Pietro Zeppa, Antonio Melcarne, Diego Garbossa, and Fabio Cofano. 2023. "A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective" Cancers 15, no. 1: 90. https://doi.org/10.3390/cancers15010090
APA StyleDe Marco, R., Pesaresi, A., Bianconi, A., Zotta, M., Deandreis, D., Morana, G., Zeppa, P., Melcarne, A., Garbossa, D., & Cofano, F. (2023). A Systematic Review of Amino Acid PET Imaging in Adult-Type High-Grade Glioma Surgery: A Neurosurgeon’s Perspective. Cancers, 15(1), 90. https://doi.org/10.3390/cancers15010090








