Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinically Approved Agents for BNCT
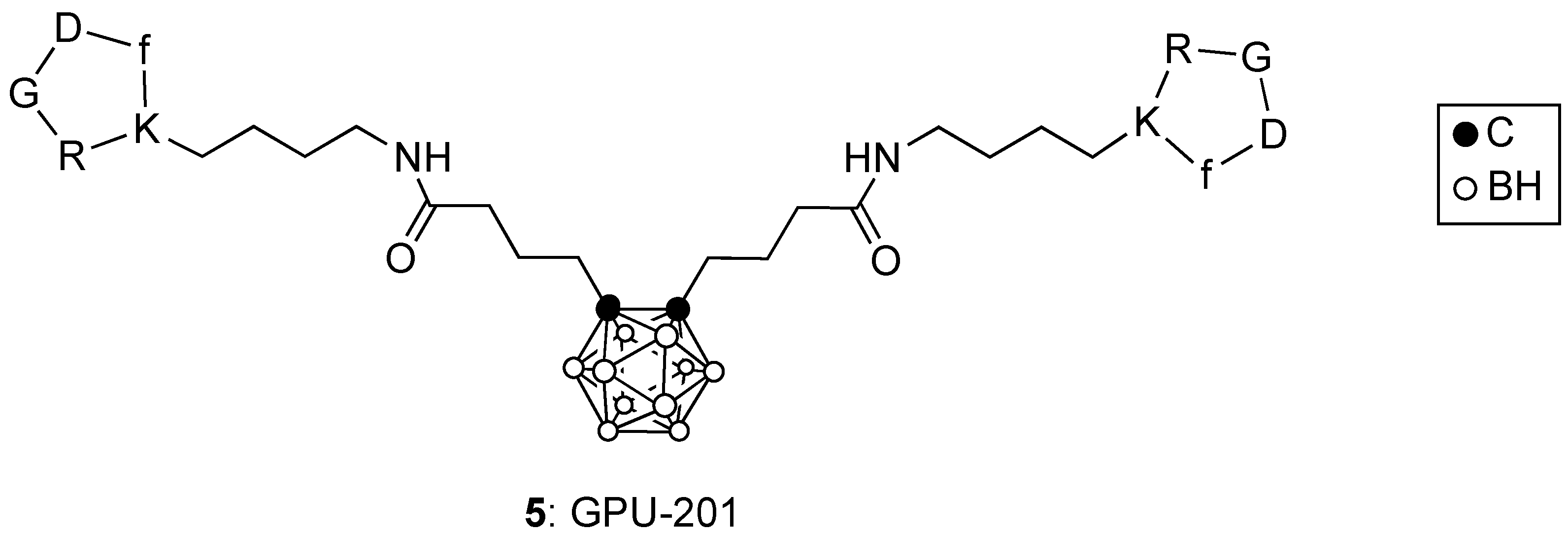
3. Boronated Peptides and Monoclonal Antibodies
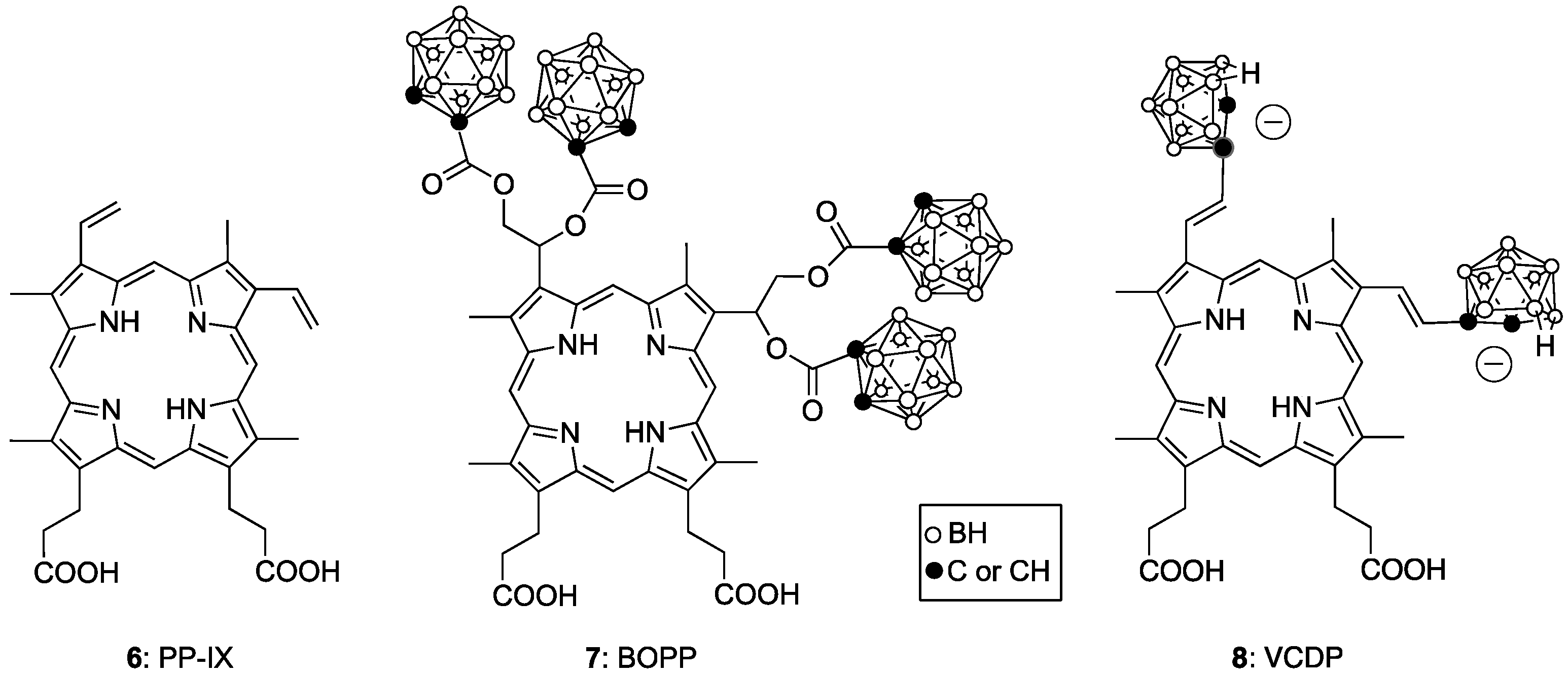
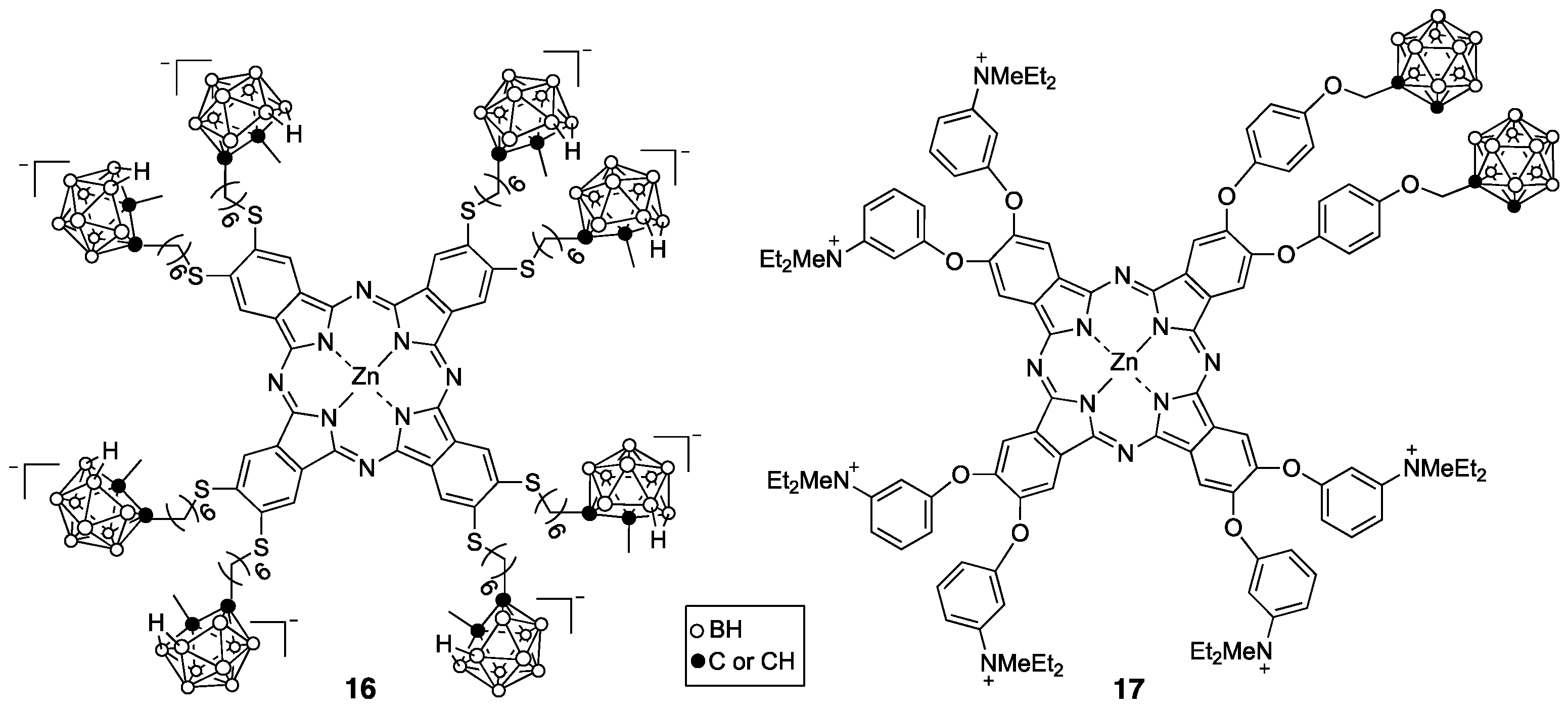
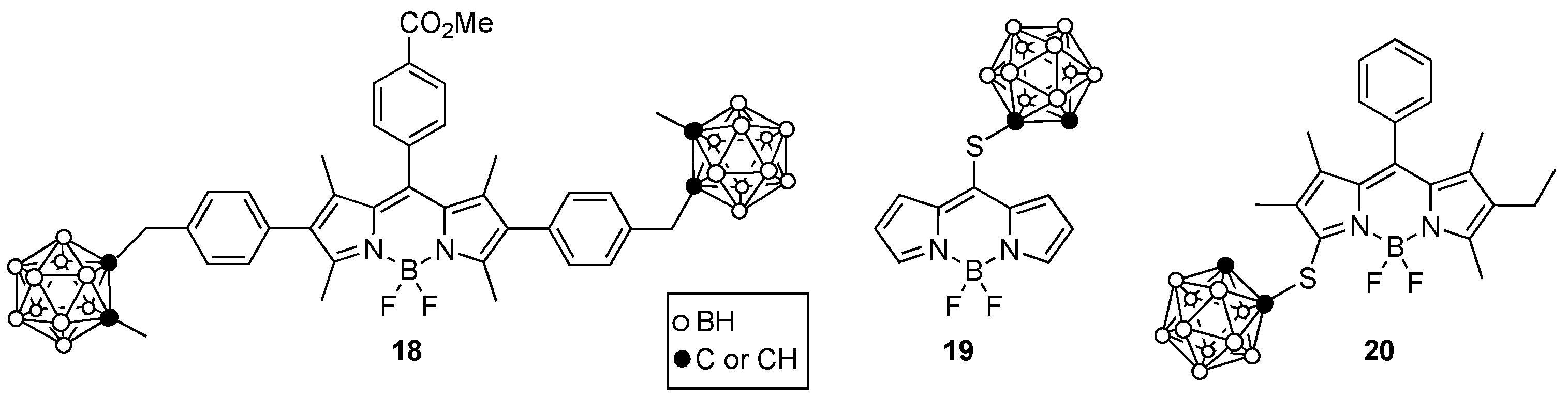
4. Porphyrin Derivatives for BNCT
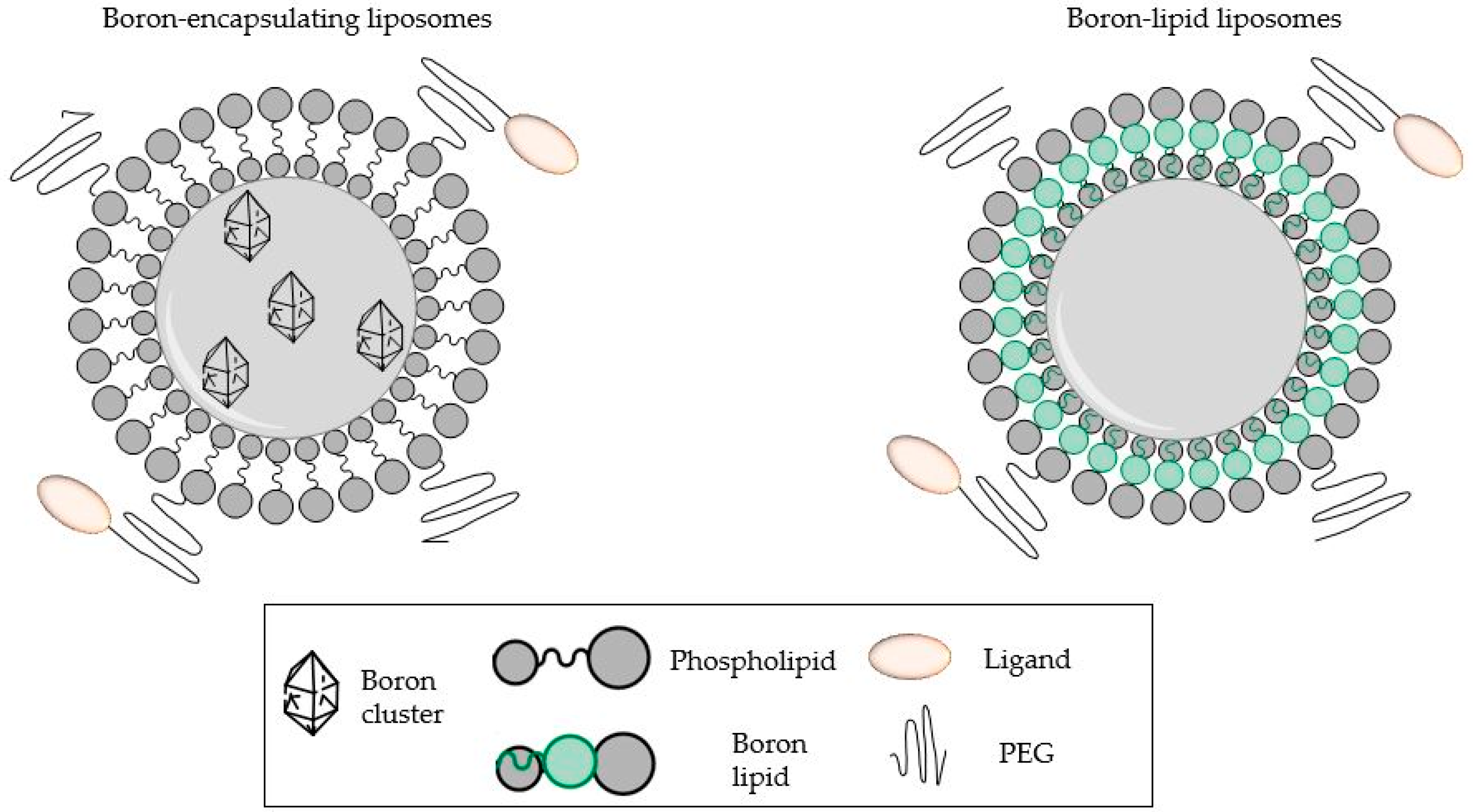
5. Boron-Containing Liposomes
6. Boron-Containing Nanoparticles
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The Chemistry of Neutron Capture Therapy. Chem. Rev. 1998, 98, 2389–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, R.F.; Vicente, M.H.; Harling, O.K.; Kiger, W.S., 3rd; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head-and-Neck Cancer: Final Analysis of a Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Hopewell, J.; Gorlia, T.; Pellettieri, L.; Giusti, V.; H-Stenstam, B.; Sköld, K. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Appl. Radiat. Isot. 2011, 69, 1737–1740. [Google Scholar] [CrossRef]
- Moss, R.L. Critical review, with an optimistic outlook, on Boron Neutron Capture Therapy (BNCT). Appl. Radiat. Isot. 2014, 88, 2–11. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. 2018, 38, 36–37. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Sibrian, V.; Vicente, M.G.H. Boron Tumor-Delivery for BNCT: Recent developments and perspectives. In Boron Science: New Technologies & Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 203–232. ISBN 978-1-4398-2662-1. [Google Scholar]
- Nakamura, H.; Kirihata, M. Boron Compounds: New Candidates for Boron Carriers in BNCT. In Neutron Capture Therapy; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–116. ISBN 978-3-642-31333-2. [Google Scholar]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef] [Green Version]
- Xuan, S.; Vicente, M.G.H. Recent development of Boron Delivery Agents for Boron Neutron Capture Therapy. In Medicinal Chemistry of Boron Compounds; Vinas, C., Hey-Hawkins, Eds.; Wiley Publishers: Hoboken, NJ, USA, 2019; pp. 298–342. [Google Scholar]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [Green Version]
- Couto, M.; Alamón, C.; Nievas, S.; Perona, M.; Dagrosa, M.A.; Teixidor, F.; Cabral, P.; Viñas, C.; Cerecetto, H. Bimodal Therapeutic Agents Against Glioblastoma, One of the Most Lethal Forms of Cancer. Chem. A Eur. J. 2020, 26, 14335–14340. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.G.; Marques, F.; Robalo, M.P.; Fontrodona, X.; Garcia, M.H.; Crich, S.G.; Viñas, C.; Valente, A. Ruthenium carboranyl complexes with 2,2′-bipyridine derivatives for potential bimodal therapy application. RSC Adv. 2020, 10, 16266–16276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, N.; Hirano, F.; Temma, T. Evaluation of 3-Borono-l-Phenylalanine as a Water-Soluble Boron Neutron Capture Therapy Agent. Pharmaceutics 2022, 14, 1106. [Google Scholar] [CrossRef] [PubMed]
- Bonjoch, J.; Drew, M.G.B.; González, A.; Greco, F.; Jawaid, S.; Osborn, H.M.I.; Williams, N.A.O.; Yaqoob, P. Synthesis and Evaluation of Novel Boron-Containing Complexes of Potential Use for the Selective Treatment of Malignant Melanoma. J. Med. Chem. 2008, 51, 6604–6608. [Google Scholar] [CrossRef]
- Barth, R.F.; Kabalka, G.W.; Yang, W.; Huo, T.; Nakkula, R.J.; Shaikh, A.L.; Haider, S.A.; Chandra, S. Evaluation of unnatural cyclic amino acids as boron delivery agents for treatment of melanomas and gliomas. Appl. Radiat. Isot. 2014, 88, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.-I.; Matsui, H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.-I.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Beck-Sickinger, A.G. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Pept. Sci. 2020, 112, e24171. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Kimura, S.; Masunaga, S.-I.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorganic Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, S.-I.; Kimura, S.; Harada, T.; Okuda, K.; Sakurai, Y.; Tanaka, H.; Suzuki, M.; Kondo, N.; Maruhashi, A.; Nagasawa, H.; et al. Evaluating the Usefulness of a Novel 10B-Carrier Conjugated With Cyclic RGD Peptide in Boron Neutron Capture Therapy. World J. Oncol. 2012, 3, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Nishimura, K.; Okada, S.; Sato, S.; Suzuki, M.; Takata, T.; Nakamura, H. Cyclic RGD-Functionalized closo-Dodecaborate Albumin Conjugates as Integrin Targeting Boron Carriers for Neutron Capture Therapy. Mol. Pharm. 2020, 17, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Worm, D.J.; Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. Selective Neuropeptide Y Conjugates with Maximized Carborane Loading as Promising Boron Delivery Agents for Boron Neutron Capture Therapy. J. Med. Chem. 2019, 63, 2358–2371. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Lerchen, H.-G.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A Selective Carborane-Functionalized Gastrin-Releasing Peptide Receptor Agonist as Boron Delivery Agent for Boron Neutron Capture Therapy. J. Org. Chem. 2019, 85, 1446–1457. [Google Scholar] [CrossRef]
- Yang, W.; Wu, G.; Barth, R.F.; Swindall, M.R.; Bandyopadhyaya, A.K.; Tjarks, W.; Tordoff, K.; Moeschberger, M.; Sferra, T.J.; Binns, P.J.; et al. Molecular Targeting and Treatment of Composite EGFR and EGFRvIII-Positive Gliomas Using Boronated Monoclonal Antibodies. Clin. Cancer Res. 2008, 14, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Barth, R.; Wu, G.; Tjarks, W.; Binns, P.; Riley, K. Boron neutron capture therapy of EGFR or EGFRvIII positive gliomas using either boronated monoclonal antibodies or epidermal growth factor as molecular targeting agents. Appl. Radiat. Isot. 2009, 67, S328–S331. [Google Scholar] [CrossRef]
- Pandey Ravindra, K.; Zheng, G. Porphyrins as Photosensitizers in Photodynamic Therapy. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 157–230. [Google Scholar]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic Therapy (PDT) for Lung Cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef] [Green Version]
- El-Zaria, M.E.; Ban, H.S.; Nakamura, H. Boron-Containing Protoporphyrin IX Derivatives and Their Modification for Boron Neutron Capture Therapy: Synthesis, Characterization, and Comparative In Vitro Toxicity Evaluation. Chem. A Eur. J. 2010, 16, 1543–1552. [Google Scholar] [CrossRef]
- Ozawa, T.; Afzal, J.; Lamborn, K.R.; Bollen, A.W.; Bauer, W.; Koo, M.-S.; Kahl, S.B.; Deen, D.F. Toxicity, biodistribution, and convection-enhanced delivery of the boronated porphyrin BOPP in the 9L intracerebral rat glioma model. Int. J. Radiat. Oncol. 2005, 63, 247–252. [Google Scholar] [CrossRef]
- Miura, M.; Gabel, D.; Oenbrink, G.; Fairchild, R.G. Preparation of carboranyl porphyrins for boron neutron capture therapy. Tetrahedron Lett. 1990, 31, 2247–2250. [Google Scholar] [CrossRef]
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A. Outcome of mTHPC Mediated Photodynamic Therapy is Primarily Determined by the Vascular Response. Photochem. Photobiol. 2005, 81, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, H.M.; Slatkin, D.N.; Micca, P.L.; Miura, M. Microlocalization of lipophilic porphyrins: Non-toxic enhancers of boron neutron-capture therapy. Int. J. Radiat. Biol. 2013, 89, 611–617. [Google Scholar] [CrossRef]
- Fabris, C.; Vicente, M.H.; Hao, E.; Friso, E.; Borsetto, L.; Jori, G.; Miotto, G.; Colautti, P.; Moro, D.; Esposito, J.; et al. Tumour-localizing and -photosensitising properties of meso-tetra(4-nido-carboranylphenyl)porphyrin (H2TCP). J. Photochem. Photobiol. B: Biol. 2007, 89, 131–138. [Google Scholar] [CrossRef]
- Jori, G.; Soncin, M.; Friso, E.; Vicente, M.; Hao, E.; Miotto, G.; Colautti, P.; Moro, D.; Esposito, J.; Rosi, G.; et al. A novel boronated-porphyrin as a radio-sensitizing agent for boron neutron capture therapy of tumours: In vitro and in vivo studies. Appl. Radiat. Isot. 2009, 67, S321–S324. [Google Scholar] [CrossRef]
- Gottumukkala, V.; Luguya, R.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and cellular studies of an octa-anionic 5,10,15,20-tetra[3,5-(nido-carboranylmethyl)phenyl]porphyrin (H2OCP) for application in BNCT. Bioorganic Med. Chem. 2005, 13, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Sibrian-Vazquez, M.; Serem, W.; Garno, J.C.; Fronczek, F.R.; Vicente, M.G.H. Synthesis, Aggregation and Cellular Investigations of Porphyrin–Cobaltacarborane Conjugates. Chem. A Eur. J. 2007, 13, 9035–9042. [Google Scholar] [CrossRef]
- Bhupathiraju, N.D.K.; Gottumukkala, V.; Hao, E.; Hu, X.; Fronczek, F.R.; Baker, D.G.; Wakamatsu, N.; Vicente, M.G.H. Synthesis and toxicity of cobaltabisdicarbollide-containing porphyrins of high boron content. J. Porphyrins Phthalocyanines 2011, 15, 973–983. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Hao, E.; Jensen, T.J.; Vicente, M.G.H. Enhanced Cellular Uptake with a Cobaltacarborane−Porphyrin−HIV-1 Tat 48−60 Conjugate. Bioconjugate Chem. 2006, 17, 928–934. [Google Scholar] [CrossRef]
- Bhupathiraju, N.V.S.D.K.; Hu, X.; Zhou, Z.; Fronczek, F.R.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Vicente, M.G.H. Synthesis and in Vitro Evaluation of BBB Permeability, Tumor Cell Uptake, and Cytotoxicity of a Series of Carboranylporphyrin Conjugates. J. Med. Chem. 2014, 57, 6718–6728. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, R.; Kawabata, S.; Tanaka, H.; Sakurai, Y.; Suzuki, M.; Ono, K.; Miyatake, S.-I.; Kuroiwa, T.; Hao, E.; Vicente, M.G.H. Tetrakis(p-Carboranylthio-Tetrafluorophenyl)Chlorin (TPFC): Application for Photodynamic Therapy and Boron Neutron Capture Therapy. J. Pharm. Sci. 2015, 104, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushpan, S.K.; Venkatraman, S.; Anand, V.G.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T.K. Porphyrins in Photodynamic Therapy—A Search for Ideal Photosensitizers. Curr. Med. Chem. Agents 2002, 2, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Loewen, G.M.; Pandey, R.; Bellnier, D.; Henderson, B.; Dougherty, T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg. Med. 2006, 38, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, Z.; Luck, D.; Beckers, J.; Brun, P.-H.; Wilson, B.C.; Scherz, A.; Salomon, Y.; Hetzel, F.W. Preclinical Studies in Normal Canine Prostate of a Novel Palladium-Bacteriopheophorbide (WST09) Photosensitizer for Photodynamic Therapy of Prostate Cancer. Photochem. Photobiol. 2007, 76, 438–445. [Google Scholar] [CrossRef]
- Mironov, A.F. Chemical Transformations of Chlorophyll a and Possible Areas for Application of Its Derivatives. Russ. J. Gen. Chem. 2019, 89, 1952–1983. [Google Scholar] [CrossRef]
- Golovina, V.G.; Rychkov, N.G.; Ol’shevskaya, A.V.; Zaitsev, V.A.; Kalinin, N.V.; Kuzmin, A.V.; Shtil, A.A. Differential Binding Preference of Methylpheophorbide a and its Diboronated derivatives to Albumin and low density Lipoproteins. Anti Cancer Agents Med. Chem. 2013, 13, 639–646. [Google Scholar] [CrossRef]
- Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Chestnova, A.V.; Feofanov, A.; Lobanova, I.A.; Sivaev, I.B.; Bregadze, V.I.; Mironov, A.F. Synthesis of cobalt bis(dicarbollide) conjugates with natural chlorins by the Sonogashira reaction. Russ. Chem. Bull. 2010, 59, 219–224. [Google Scholar] [CrossRef]
- Volovetsky, A.B.; Sukhov, V.S.; Balalaeva, I.V.; Dudenkova, V.V.; Shilyagina, N.Y.; Feofanov, V.; Efremenko, A.V.; Grin, M.A.; Mironov, A.F.; Sivaev, I.B.; et al. Pharmacokinetics of Chlorin e6-Cobalt Bis(Dicarbollide) Conjugate in Balb/c Mice with Engrafted Carcinoma. Int. J. Mol. Sci. 2017, 18, 2556. [Google Scholar] [CrossRef] [Green Version]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [Green Version]
- Nar, I.; Bortolussi, S.; Postuma, I.; Atsay, A.; Berksun, E.; Viola, E.; Ferrari, C.; Cansolino, L.; Ricciardi, G.; Donzello, M.P.; et al. A Phthalocyanine-ortho-Carborane Conjugate for Boron Neutron Capture Therapy: Synthesis, Physicochemical Properties, and in vitro Tests. Chempluschem 2019, 84, 345–351. [Google Scholar] [CrossRef]
- Pietrangeli, D.; Rosa, A.; Pepe, A.; Altieri, S.; Bortolussi, S.; Postuma, I.; Protti, N.; Ferrari, C.; Cansolino, L.; Clerici, A.M.; et al. Water-soluble carboranyl-phthalocyanines for BNCT. Synthesis, characterization, and in vitro tests of the Zn(ii)-nido-carboranyl-hexylthiophthalocyanine. Dalton Trans. 2015, 44, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, D.; Ricciardi, G. Neutral and polyanionic carboranylporphyrazines: Synthesis and physico-chemical properties. Appl. Radiat. Isot. 2009, 67, S97–S100. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, T.-P.; Li, D.; Leamer, L.; Shan, H.; Li, Z.; Gabbaï, F.P.; Conti, P.S. Lewis Acid-Assisted Isotopic 18F-19F Exchange in BODIPY Dyes: Facile Generation of Positron Emission Tomography/Fluorescence Dual Modality Agents for Tumor Imaging. Theranostics 2013, 3, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, J.H.; Wang, H.; Bhupathiraju, N.D.K.; Fronczek, F.R.; Smith, K.M.; Vicente, M.G.H. Synthesis and properties of a series of carboranyl-BODIPYs. J. Organomet. Chem. 2015, 798, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, S.; Zhao, N.; Zhou, Z.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and in Vitro Studies of a Series of Carborane-Containing Boron Dipyrromethenes (BODIPYs). J. Med. Chem. 2016, 59, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Eloy, J.O.; de Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Olusanya, T.O.; Calabrese, G.; Fatouros, D.G.; Tsibouklis, J.; Smith, J.R. Liposome formulations of o-carborane for the boron neutron capture therapy of cancer. Biophys. Chem. 2019, 247, 25–33. [Google Scholar] [CrossRef]
- Nakamura, H. Liposomal Boron Delivery for Neutron Capture Therapy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 465, pp. 179–208. ISBN 978-0-12-381379-4. [Google Scholar]
- Takeuchi, I.; Kanno, Y.; Uchiro, H.; Makino, K. Polyborane-encapsulated PEGylated Liposomes Prepared Using Post-insertion Technique for Boron Neutron Capture Therapy. J. Oleo Sci. 2019, 68, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Ye, L.; Zhang, X.; Cui, W.; Lou, J.; Nagai, T.; Hou, X. Transport of nerve growth factor encapsulated into liposomes across the blood–brain barrier: In vitro and in vivo studies. J. Control. Release 2005, 105, 106–119. [Google Scholar] [CrossRef]
- Liu, D.; Guo, P.; McCarthy, C.; Wang, B.; Tao, Y.; Auguste, D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018, 9, 2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Khan, M.A.; Burgess, D.J. Predicting hydrophilic drug encapsulation inside unilamellar liposomes. Int. J. Pharm. 2012, 423, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Guan, L.; Kane, R.R.; Kasamatsu, H.; Hawthorne, M.F. Toward a cancer therapy with boron-rich oligomeric phosphate diesters that target the cell nucleus. Proc. Natl. Acad. Sci. USA 1999, 96, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagie, H.; Yanagawa, M.; Morishita, Y.; Shinohara, A.; Dewi, N.; Nonaka, Y.; Furuya, Y.; Mizumachi, R.; Murata, Y.; Nakamura, H.; et al. Suppression of Tumor Growth in a Rabbit Hepatic Cancer Model by Boron Neutron Capture Therapy With Liposomal Boron Delivery Systems. In Vivo 2021, 35, 3125–3135. [Google Scholar] [CrossRef]
- Ishida, O.; Maruyama, K.; Tanahashi, H.; Iwatsuru, M.; Sasaki, K.; Eriguchi, M.; Yanagie, H. Liposomes Bearing Polyethyleneglycol-Coupled Transferrin with Intracellular Targeting Property to the Solid Tumors In Vivo. Pharm. Res. 2001, 18, 1042–1048. [Google Scholar] [CrossRef]
- Kanygin, V.; Zaboronok, A.; Taskaeva, I.; Zavjalov, E.; Mukhamadiyarov, R.; Kichigin, A.; Kasatova, A.; Razumov, I.; Sibirtsev, R.; Mathis, B.J. In Vitro and In Vivo Evaluation of Fluorescently Labeled Borocaptate-Containing Liposomes. J. Fluoresc. 2021, 31, 73–83. [Google Scholar] [CrossRef]
- Lozano, N.; Al-Ahmady, Z.S.; Beziere, N.S.; Ntziachristos, V.; Kostarelos, K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int. J. Pharm. 2015, 482, 2–10. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Zavjalov, E.; Zaboronok, A.; Kanygin, V.; Kasatova, A.; Kichigin, A.; Mukhamadiyarov, R.; Razumov, I.; Sycheva, T.; Mathis, B.J.; Maezono, S.E.B.; et al. Accelerator-based boron neutron capture therapy for malignant glioma: A pilot neutron irradiation study using boron phenylalanine, sodium borocaptate and liposomal borocaptate with a heterotopic U87 glioblastoma model in SCID mice. Int. J. Radiat. Biol. 2020, 96, 868–878. [Google Scholar] [CrossRef]
- Shimizu, K.; Oku, N. Liposomes Conjugated with a Pilot Molecule. In Cancer Drug Delivery Systems Based on the Tumor Microenvironment; Matsumura, Y., Tarin, D., Eds.; Springer: Tokyo, Japan, 2019; pp. 187–216. ISBN 978-4-431-56878-0. [Google Scholar]
- Kullberg, E.B.; Wei, Q.; Capala, J.; Giusti, V.; Malmström, P.-U.; Gedda, L. EGF-receptor targeted liposomes with boronated acridine: Growth inhibition of cultured glioma cells after neutron irradiation. Int. J. Radiat. Biol. 2005, 81, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Kullberg, E.B.; Gedda, L. Trastuzumab-conjugated boron-containing liposomes for tumor-cell targeting; development and cellular studies. Int. J. Oncol. 2003, 23, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Koltover, I.; Salditt, T.; Rädler, J.O.; Safinya, C.R. An Inverted Hexagonal Phase of Cationic Liposome-DNA Complexes Related to DNA Release and Delivery. Science 1998, 281, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Ristori, S.; Oberdisse, J.; Grillo, I.; Donati, A.; Spalla, O. Structural Characterization of Cationic Liposomes Loaded with Sugar-Based Carboranes. Biophys. J. 2005, 88, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, S.; Balzi, M.; Bortolussi, S.; Bruschi, P.; Ciani, L.; Clerici, A.M.; Faraoni, P.; Ferrari, C.; Gadan, M.A.; Panza, L.; et al. Carborane Derivatives Loaded into Liposomes as Efficient Delivery Systems for Boron Neutron Capture Therapy. J. Med. Chem. 2009, 52, 7829–7835. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Q.; Lu, C.; Xiao, H.; Guo, Z.; Duan, D.; Zhang, Z.; Liu, T.; Liu, Z. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nat. Commun. 2022, 13, 2143. [Google Scholar] [CrossRef]
- Ailuno, G.; Balboni, A.; Caviglioli, G.; Lai, F.; Barbieri, F.; Dellacasagrande, I.; Florio, T.; Baldassari, S. Boron Vehiculating Nanosystems for Neutron Capture Therapy in Cancer Treatment. Cells 2022, 11, 4029. [Google Scholar] [CrossRef]
- Sumitani, S.; Oishi, M.; Nagasaki, Y. Carborane confined nanoparticles for boron neutron capture therapy: Improved stability, blood circulation time and tumor accumulation. React. Funct. Polym. 2011, 71, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Uspenskii, S.A.; Khaptakhanova, P.A.; Zaboronok, A.A.; Kurkin, T.S.; Volkova, O.Y.; Mechetina, L.V.; Taranin, A.V.; Kanygin, V.V.; Akira, M.; Taskaev, S.Y. Elemental Boron Nanoparticles: Production by Ultrasonication in Aqueous Medium and Application in Boron Neutron Capture Therapy. Dokl. Chem. 2020, 491, 45–48. [Google Scholar] [CrossRef]
- Zaboronok, A.; Khaptakhanova, P.; Uspenskii, S.; Bekarevich, R.; Mechetina, L.; Volkova, O.; Mathis, B.J.; Kanygin, V.; Ishikawa, E.; Kasatova, A.; et al. Polymer-Stabilized Elemental Boron Nanoparticles for Boron Neutron Capture Therapy: Initial Irradiation Experiments. Pharmaceutics 2022, 14, 761. [Google Scholar] [CrossRef] [PubMed]
- Kaniowski, D.; Ebenryter-Olbińska, K.; Kulik, K.; Suwara, J.; Cypryk, W.; Jakóbik-Kolon, A.; Leśnikowski, Z.; Nawrot, B. Composites of Nucleic Acids and Boron Clusters (C2B10H12) as Functional Nanoparticles for Downregulation of EGFR Oncogene in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 4863. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioran, A.M.; Teixidor, F.; Krpetić, Ž.; Brust, M.; Viñas, C. Preparation and characterization of Au nanoparticles capped with mercaptocarboranyl clusters. Dalton Trans. 2014, 43, 5054–5061. [Google Scholar] [CrossRef]
- Sawant, R.R.; Torchilin, V.P. Multifunctional nanocarriers and intracellular drug delivery. Curr. Opin. Solid State Mater. Sci. 2012, 16, 269–275. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-J.; Chang, W.-Y.; Hsieh, C.-Y.; Wu, C.-C.; Chen, H.-S.; Hsu, H.-J.; Yang, A.-S.; Hsu, M.-H.; Kuo, W.-Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Ye, J.; Li, Z.; Jiang, H.; Yan, H.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I.; Wang, X. Carborane Derivative Conjugated with Gold Nanoclusters for Targeted Cancer Cell Imaging. Biomacromolecules 2017, 18, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Pyshnaya, I.A.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Poletaeva, J.; Zavjalov, E.L.; Silnikov, V.N.; Ryabchikova, E.I.; Godovikova, T.S. Rational Design of Albumin Theranostic Conjugates for Gold Nanoparticles Anticancer Drugs: Where the Seed Meets the Soil? Biomedicines 2021, 9, 74. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hsieh, H.-H.; Chang, T.-Y.; Lin, J.-J.; Wu, C.-C.; Hsu, M.-H.; Lin, M.-C.; Peng, S.-L. Development of MRI-Detectable Boron-Containing Gold Nanoparticle-Encapsulated Biodegradable Polymeric Matrix for Boron Neutron Capture Therapy (BNCT). Int. J. Mol. Sci. 2021, 22, 8050. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gómez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossío, U.; Llop, J. Gold Nanoparticles as Boron Carriers for Boron Neutron Capture Therapy: Synthesis, Radiolabelling and In Vivo Evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef] [Green Version]
- Pulagam, K.R.; Henriksen-Lacey, M.; Uribe, K.B.; Renero-Lecuna, C.; Kumar, J.; Charalampopoulou, A.; Facoetti, A.; Protti, N.; Gómez-Vallejo, V.; Baz, Z.; et al. In Vivo Evaluation of Multifunctional Gold Nanorods for Boron Neutron Capture and Photothermal Therapies. ACS Appl. Mater. Interfaces 2021, 13, 49589–49601. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Muro, S.; Stepanov, P.Y.; Weinberg, I.N. Open challenges in magnetic drug targeting. WIREs Nanomed. Nanobiotechnology 2015, 7, 446–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemons, T.D.; Kerr, R.H.; Joos, A. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. In Comprehensive Nanoscience and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–210. ISBN 978-0-12-812296-9. [Google Scholar]
- Torresan, V.; Guadagnini, A.; Badocco, D.; Pastore, P.; Medina, G.A.M.; van Raap, M.B.F.; Postuma, I.; Bortolussi, S.; Bekić, M.; Čolić, M.; et al. Biocompatible Iron–Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv. Health Mater. 2021, 10, e2001632. [Google Scholar] [CrossRef]
- Malik, A.; Butt, T.T.; Zahid, S.; Zahid, F.; Waquar, S.; Rasool, M.; Qazi, M.H.; Qazi, A.M. Use of Magnetic Nanoparticles as Targeted Therapy: Theranostic Approach to Treat and Diagnose Cancer. J. Nanotechnol. 2017, 2017, 1098765. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Cui, B.; Bu, Y.; Wang, Y. Preparation of flower-dewdrops Fe3O4/carbon-SiO2 microsphere for microwave-triggered drug delivery. J. Alloys Compd. 2019, 775, 826–835. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Gawali, S.L.; Barick, B.; Barick, K.; Babu, P.; Pandey, B.; Priyadarsini, K.; Hassan, P. PEG mediated shape-selective synthesis of cubic Fe3O4 nanoparticles for cancer therapeutics. J. Alloys Compd. 2018, 737, 347–355. [Google Scholar] [CrossRef]
- Korolkov, I.; Ludzik, K.; Kozlovskiy, A.; Fadeev, M.; Shumskaya, A.; Gorin, Y.; Marciniak, B.; Jazdzewska, M.; Chudoba, D.; Kontek, R.; et al. Carboranes immobilization on Fe3O4 nanocomposites for targeted delivery. Mater. Today Commun. 2020, 24, 101247. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, J.; Qiu, X.; Li, N.; Zhu, Y.; Yan, L.; Li, W.; Huang, X.; Liang, K.; et al. Carborane based mesoporous nanoparticles as a potential agent for BNCT. Mater. Chem. Front. 2021, 5, 2771–2776. [Google Scholar] [CrossRef]
- Vares, G.; Jallet, V.; Matsumoto, Y.; Rentier, C.; Takayama, K.; Sasaki, T.; Hayashi, Y.; Kumada, H.; Sugawara, H. Functionalized mesoporous silica nanoparticles for innovative boron-neutron capture therapy of resistant cancers. Nanomed. Nanotechnol. Biol. Med. 2020, 27, 102195. [Google Scholar] [CrossRef]
- Kuthala, N.; Vankayala, R.; Li, Y.; Chiang, C.; Hwang, K.C. Engineering Novel Targeted Boron-10-Enriched Theranostic Nanomedicine to Combat against Murine Brain Tumors via MR Imaging-Guided Boron Neutron Capture Therapy. Adv. Mater. 2017, 29, 1700850. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential Applications of Carbon Nanotubes. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties, and Applications; Topics in applied physics; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-72864-1. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Zhu, Y.; Hosmane, N. Nanostructured Boron Compounds for Boron Neutron Capture Therapy (BNCT) in cancer treatment. In Boron-Based Compounds; Hey-Hawkins, E., Teixidor, C.V., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 371–388. ISBN 978-1-119-27560-2. [Google Scholar]
- Golberg, D.; Bando, Y.; Tang, C.C.; Zhi, C.Y. Boron Nitride Nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate Functionalized Boron Nitride Nanotubes and their Selective Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res. Lett. 2009, 4, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Ciofani, G.; Del Turco, S.; Genchi, G.G.; D’alessandro, D.; Basta, G.; Mattoli, V. Transferrin-conjugated boron nitride nanotubes: Protein grafting, characterization, and interaction with human endothelial cells. Int. J. Pharm. 2012, 436, 444–453. [Google Scholar] [CrossRef]
- Nakamura, H.; Koganei, H.; Miyoshi, T.; Sakurai, Y.; Ono, K.; Suzuki, M. Antitumor effect of boron nitride nanotubes in combination with thermal neutron irradiation on BNCT. Bioorganic Med. Chem. Lett. 2015, 25, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Achilli, C.; Grandi, S.; Ciana, A.; Guidetti, G.F.; Malara, A.; Abbonante, V.; Cansolino, L.; Tomasi, C.; Balduini, A.; Fagnoni, M.; et al. Biocompatibility of functionalized boron phosphate (BPO4) nanoparticles for boron neutron capture therapy (BNCT) application. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 589–597. [Google Scholar] [CrossRef]
- Kuthala, N.; Shanmugam, M.; Yao, C.-L.; Chiang, C.-S.; Hwang, K.C. One step synthesis of 10B-enriched 10BPO4 nanoparticles for effective boron neutron capture therapeutic treatment of recurrent head-and-neck tumor. Biomaterials 2022, 290, 121861. [Google Scholar] [CrossRef]
- Chiang, C.-W.; Chien, Y.-C.; Yu, W.-J.; Ho, C.-Y.; Wang, C.-Y.; Wang, T.-W.; Chiang, C.-S.; Keng, P.-Y. Polymer-Coated Nanoparticles for Therapeutic and Diagnostic Non-10B Enriched Polymer-Coated Boron Carbon Oxynitride (BCNO) Nanoparticles as Potent BNCT Drug. Nanomaterials 2021, 11, 2936. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Hsu, Y.-T.; Chiang, C.-W.; Keng, P.Y.; Wang, T.-W. Investigating the electrostatic complexation of BCNO nanoparticles with a stimuli-responsive double hydrophilic graft copolymer. Giant 2023, 14, 100162. [Google Scholar] [CrossRef]
- Shao, M.; Lopes, D.; Lopes, J.; Yousefiasl, S.; Macário-Soares, A.; Peixoto, D.; Ferreira-Faria, I.; Veiga, F.; Conde, J.; Huang, Y.; et al. Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter 2023, 6, 761–799. [Google Scholar] [CrossRef]
- Li, J.; Kong, J.; Ma, S.; Li, J.; Mao, M.; Chen, K.; Chen, Z.; Zhang, J.; Chang, Y.; Yuan, H.; et al. Exosome-Coated 10 B Carbon Dots for Precise Boron Neutron Capture Therapy in a Mouse Model of Glioma In Situ. Adv. Funct. Mater. 2021, 31, 2100969. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, Z.; Li, J.; Chen, K.; Liang, H.; Lv, L.; Chang, Y.; Liu, S.; Yang, W.; et al. Multifunctional high boron content MOFs nano-co-crystals for precise boron neutron capture therapy for brain glioma in situ. Nano Today 2022, 45, 101558. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oloo, S.O.; Smith, K.M.; Vicente, M.d.G.H. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers 2023, 15, 3277. https://doi.org/10.3390/cancers15133277
Oloo SO, Smith KM, Vicente MdGH. Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers. 2023; 15(13):3277. https://doi.org/10.3390/cancers15133277
Chicago/Turabian StyleOloo, Sebastian O., Kevin M. Smith, and Maria da Graça H. Vicente. 2023. "Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers" Cancers 15, no. 13: 3277. https://doi.org/10.3390/cancers15133277
APA StyleOloo, S. O., Smith, K. M., & Vicente, M. d. G. H. (2023). Multi-Functional Boron-Delivery Agents for Boron Neutron Capture Therapy of Cancers. Cancers, 15(13), 3277. https://doi.org/10.3390/cancers15133277





