Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies?
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. The Human Microbiota
1.2. The Gut Microbiome
1.3. Pancreatic Ductal Adenocarcinoma
1.4. Changes in the Gut Microbiome in PDAC Patients
1.5. Pancreatic Exocrine Insufficiency in Pancreatic Cancer
1.6. Pancreatic Enzyme Replacement Therapy
1.7. Pancreatic Exocrine Insufficiency and the Intestinal Microbiome
2. The Interaction between Pancreatic Exocrine Function and Gut Microbiome
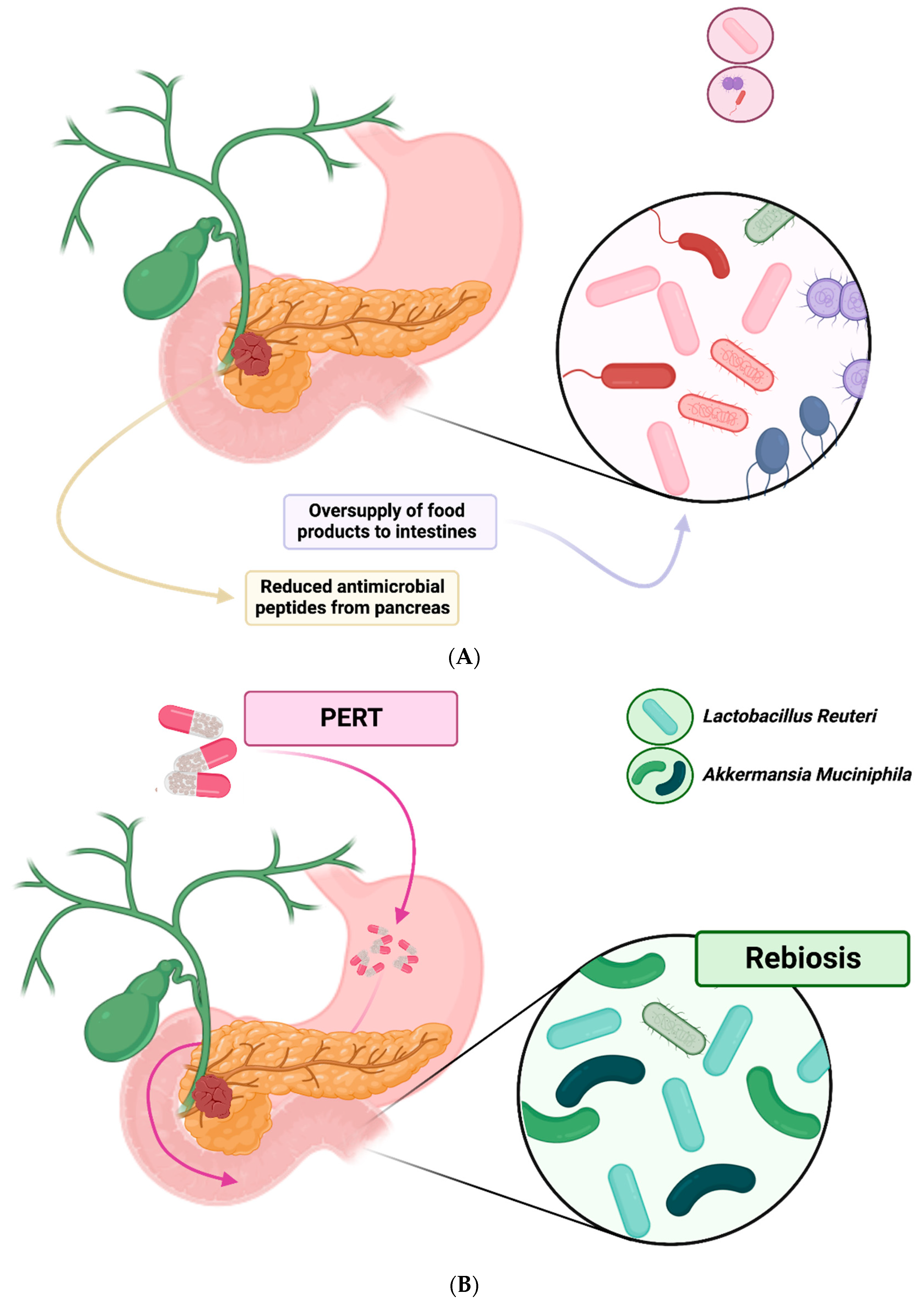
2.1. Effects of Pancreatic Exocrine Insufficiency on the Gut Microbiome
2.2. Pancreatic Regulation of Gut Microbiome
2.3. Gut Microbiome Changes from Pancreatic Exocrine Insufficiency in Different Disease States
2.4. Effects of Pancreatic Enzyme Replacement on the Gut Microbiome
3. Effects of the Intestinal Microbiome in Pancreatic Cancer Patients
3.1. Intestinal Microbiome and Clinical Outcomes in Pancreatic Cancer
3.2. Extraintestinal Effects of the Gut Microbiome in Pancreatic Disease
3.3. Tumour Microbiome and Immune Infiltration
4. Diagnostic and Therapeutic Applications for Greater Understanding of the Gut Microbiome in Pancreatic Cancer Patients
4.1. Use of Intestinal Microbiome Testing for Diagnosis of Pancreatic Exocrine Insufficiency
4.2. Titration of Pancreatic Enzyme Replacement Therapy
4.3. Positive Effects of Pancreatic Enzyme Replacement Therapies on the Intestinal Microbiome
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lederberg, J.; Mccray, A.T. COMMENTARY ‘Ome Sweet’ Omics-A Genealogical Treasury of Words. Science 2001, 15, 8. [Google Scholar]
- Marchesi, J.R.; Ravel, J. The vocabulary of microbiome research: A proposal. Microbiome 2015, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; Ianiro, G.; Iqbal, T.H. The gut microbiome: What every gastroenterologist needs to know. Frontline Gastroenterol. 2021, 12, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Jarvie, T.; Hattori, M. Our second genome-human metagenome: How next-generation sequencer changes our life through microbiology. Adv. Microb. Physiol. 2013, 62, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Nie, L. Integrating multiple “omics” analysis for microbial biology: Application and methodologies. Microbiology 2010, 156, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Medini, D.; Serruto, D.; Parkhill, J.; Relman, D.A.; Donati, C.; Moxon, R.; Falkow, S.; Rappuoli, R. Microbiology in the post-genomic era. Nat. Rev. Microbiol. 2008, 6, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Kyrpides, N.C. Fifteen years of microbial genomics: Meeting the challenges and fulfilling the dream. Nat. Biotechnol. 2009, 27, 627–632. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20. [Google Scholar] [CrossRef]
- Lepage, P.; Hösler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef]
- Dicksved, J.; Halfvarson, J.; Rosenquist, M.; Järnerot, G.; Tysk, C.; Apajalahti, J.; Engstrand, L.; Jansson, J.K. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. ISME J. 2008, 2, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; Mullish, B.H.; Quraishi, M.N.; Acharjee, A.; Williams, H.R.T.; Iqbal, T.; Hart, A.L.; Marchesi, J.R. The application of omics techniques to understand the role of the gut microbiota in inflammatory bowel disease. Ther. Adv. Gastroenterol. 2019, 12, 1756284818822250. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Osborne, L.S.; Marchesi, J.R.; Mcdonald, J.A. The implementation of omics technologies in cancer microbiome research. Ecancermedicalscience 2018, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.L.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered gut microbiome composition by appendectomy contributes to colorectal cancer. Oncogene 2022, 42, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2022, 164, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP Rep. 2019, 1, 214. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut microbiome and liver diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Nagata, N.; Nishijima, S.; Kojima, Y.; Hisada, Y.; Imbe, K.; Miyoshi-Akiyama, T.; Suda, W.; Kimura, M.; Aoki, R.; Sekine, K.; et al. Metagenomic Identification of Microbial Signatures Predicting Pancreatic Cancer from a Multinational Study. Gastroenterology 2022, 163, 222–238. [Google Scholar] [CrossRef]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 2022, 71, 1359–1372. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 53–64. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Langheinrich, M.; Wirtz, S.; Kneis, B.; Gittler, M.M.; Tyc, O.; Schierwagen, R.; Brunner, M.; Krautz, C.; Weber, G.F.; Pilarsky, C.; et al. Microbiome Patterns in Matched Bile, Duodenal, Pancreatic Tumor Tissue, Drainage, and Stool Samples: Association with Preoperative Stenting and Postoperative Pancreatic Fistula Development. J. Clin. Med. 2020, 9, 2785. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Fernández-del Castillo, C.; Hackert, T.; Hayasaki, A.; Katz, M.H.G.; Kim, S.W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hagio, M.; Naito, Z.; Ishiwata, T. Clinicopathological features of 30 autopsy cases of pancreatic carcinoma. J. Nippon Med. Sch. 2012, 79, 459–467. [Google Scholar] [CrossRef]
- Mierke, F.; Hempel, S.; Distler, M.; Aust, D.E.; Saeger, H.D.; Weitz, J.; Welsch, T. Impact of Portal Vein Involvement from Pancreatic Cancer on Metastatic Pattern After Surgical Resection. Ann. Surg. Oncol. 2016, 23, 730–736. [Google Scholar] [CrossRef]
- Bissolati, M.; Sandri, M.T.; Burtulo, G.; Zorzino, L.; Balzano, G.; Braga, M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015, 36, 991–996. [Google Scholar] [CrossRef]
- Conroy, T.; Galais, M.-P.; Raoul, J.-L.; Bouché, O.; Gourgou-Bourgade, S.; Douillard, J.-Y.; Etienne, P.-L.; Boige, V.; Martel-Lafay, I.; Michel, P.; et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014, 15, 305–314. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Behrman, S.W.; Benson, A.B.; Casper, E.S.; Chiorean, E.G.; Chung, V.; Cohen, S.J.; Czito, B.; Engebretson, A.; et al. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2014, 12, 1083–1093. [Google Scholar] [CrossRef]
- Bellon, E.; Gebauer, F.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M. Pancreatic cancer and liver metastases: State of the art. Updates Surg. 2016, 68, 247–251. [Google Scholar] [CrossRef]
- Seufferlein, T.; Bachet, J.B.; Van cutsem, E.; Rougier, P. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii33–vii40. [Google Scholar] [CrossRef] [PubMed]
- Merz, V.; Mangiameli, D.; Zecchetto, C.; Quinzii, A.; Pietrobono, S.; Messina, C.; Casalino, S.; Gaule, M.; Pesoni, C.; Vitale, P.; et al. Predictive Biomarkers for a Personalized Approach in Resectable Pancreatic Cancer. Front. Surg. 2022, 9, 866173. [Google Scholar] [CrossRef] [PubMed]
- Daamen, L.A.; Groot, V.P.; Besselink, M.G.; Bosscha, K.; Busch, O.R.; Cirkel, G.A.; van Dam, R.M.; Festen, S.; Groot Koerkamp, B.; Haj Mohammad, N.; et al. Detection, Treatment, and Survival of Pancreatic Cancer Recurrence in the Netherlands. Ann. Surg. 2020, 275, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Sinn, M.; Bahra, M.; Liersch, T.; Gellert, K.; Messmann, H.; Bechstein, W.; Waldschmidt, D.; Jacobasch, L.; Wilhelm, M.; Rau, B.M.; et al. CONKO-005: Adjuvant Chemotherapy with Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J. Clin. Oncol. 2017, 35, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Dominguez-Muñoz, J.E. Diagnosis and treatment of pancreatic exocrine insufficiency. Curr. Opin. Gastroenterol. 2018, 34, 349–354. [Google Scholar] [CrossRef]
- Hartman, V.; Roeyen, E.; Bracke, B.; Huysentruyt, F.; De Gendt, S.; Chapelle, T.; Ysebaert, D.; Hendrikx, B.; Roeyen, G. Prevalence of pancreatic exocrine insufficiency after pancreatic surgery measured by 13C mixed triglyceride breath test: A prospective cohort study. Pancreatology 2023, 23, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.S.J.; Molenaar, I.Q.; Besselink, M.G.; Van Eijck, C.H.; Rinkes, I.H.B.; Van Santvoort, H.C. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer a systematic review. Pancreas 2016, 45, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Powell-Brett, S.; de Liguori Carino, N.; Roberts, K. Understanding pancreatic exocrine insufficiency and replacement therapy in pancreatic cancer. Eur. J. Surg. Oncol. 2021, 47, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chirletti Professor, P.; Peparini, N.; Caronna, R.; Papini, F.; Vietri Professor, F.; Gualdi, G. Monitoring Fibrosis of the Pancreatic Remnant after a Pancreaticoduodenectomy with Dynamic MRI: Are the Results Independent of the Adopted Reconstructive Technique? J. Surg. Res. 2010, 164, e49–e52. [Google Scholar] [CrossRef]
- Zaccari, P.; Ribichini, E.; Stigliano, S.; Serrao, G.; Scalese, G.; Caronna, R.; Chirletti, P.; Severi, C. Occurrence of Pathological Abdominal Fat Distribution After Pancreaticoduodenectomy at Long-term Follow-up: A Single-Center Experience. Pancreas 2021, 50, E15–E16. [Google Scholar] [CrossRef]
- Johnson, C.D.; Arbuckle, R.; Bonner, N.; Connett, G.; Dominguez-Munoz, E.; Levy, P.; Staab, D.; Williamson, N.; Lerch, M.M. Qualitative Assessment of the Symptoms and Impact of Pancreatic Exocrine Insufficiency (PEI) to Inform the Development of a Patient-Reported Outcome (PRO) Instrument. Patient 2017, 10, 615–628. [Google Scholar] [CrossRef]
- Johnson, C.D.; Williamson, N.; Janssen-van Solingen, G.; Arbuckle, R.; Johnson, C.; Simpson, S.; Staab, D.; Dominguez-Munoz, E.; Levy, P.; Connett, G.; et al. Psychometric evaluation of a patient-reported outcome measure in pancreatic exocrine insufficiency (PEI). Pancreatology 2019, 19, 182–190. [Google Scholar] [CrossRef]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran Cao, H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and Metabolic Derangements in Pancreatic Cancer and Pancreatic Resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef]
- Lindkvist, B.; Phillips, M.E.; Domínguez-Muñoz, J.E. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology 2015, 15, 589–597. [Google Scholar] [CrossRef]
- Shintakuya, R.; Uemura, K.; Murakami, Y.; Kondo, N.; Nakagawa, N.; Urabe, K.; Okano, K.; Awai, K.; Higaki, T.; Sueda, T. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology 2017, 17, 70–75. [Google Scholar] [CrossRef]
- de la Iglesia, D.; Vallejo-Senra, N.; López-López, A.; Iglesias-Garcia, J.; Lariño-Noia, J.; Nieto-García, L.; Domínguez-Muñoz, J.E. Pancreatic exocrine insufficiency and cardiovascular risk in patients with chronic pancreatitis: A prospective, longitudinal cohort study. J. Gastroenterol. Hepatol. 2019, 34, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.M.; Cahen, D.L.; Koch, A.D.; Braat, H.; Poley, J.W.; Kuipers, E.J.; Bruno, M.J. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology 2013, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Skipworth, R.J.E.; Stewart, G.D.; Dejong, C.H.C.; Preston, T.; Fearon, K.C.H. Pathophysiology of cancer cachexia: Much more than host-tumour interaction? Clin. Nutr. 2007, 26, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.H.; Baracos, V.E. Cachexia in pancreatic cancer: New treatment options and measures of success. HPB 2010, 12, 323. [Google Scholar] [CrossRef]
- Partelli, S.; Frulloni, L.; Minniti, C.; Bassi, C.; Barugola, G.; D’Onofrio, M.; Crippa, S.; Falconi, M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig. Liver Dis. 2012, 44, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, H.D.; van Berkel, L.; Tseng, D.S.J.; Monninkhof, E.M.; van Santvoort, H.C.; Hagendoorn, J.; Borel Rinkes, I.H.M.; Lips, I.M.; Intven, M.; Molenaar, I.Q. Long-term health-related quality of life after pancreatic resection for malignancy in patients with and without severe postoperative complications. HPB 2018, 20, 188–195. [Google Scholar] [CrossRef]
- Landers, A.; Brown, H.; Strother, M. The effectiveness of pancreatic enzyme replacement therapy for malabsorption in advanced pancreatic cancer, a pilot study. Palliat. Care Res. Treat. 2019, 12, 1178224218825270. [Google Scholar] [CrossRef]
- Seiler, C.M.; Izbicki, J.; Varga-Szabõ, L.; Czakõ, L.; Fiõk, J.; Sperti, C.; Lerch, M.M.; Pezzilli, R.; Vasileva, G.; Pap, Á.; et al. Randomised clinical trial: A 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment. Pharmacol. Ther. 2013, 37, 691–702. [Google Scholar] [CrossRef]
- Roberts, K.J.; Bannister, C.A.; Schrem, H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology 2019, 19, 114–121. [Google Scholar] [CrossRef]
- Roberts, K.J.; Schrem, H.; Hodson, J.; Angelico, R.; Dasari, B.V.M.; Coldham, C.A.; Marudanayagam, R.; Sutcliffe, R.P.; Muiesan, P.; Isaac, J.; et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB 2017, 19, 859–867. [Google Scholar] [CrossRef]
- Pancreatic Cancer in Adults: Diagnosis and Management NICE Guideline. 2018. Available online: www.nice.org.uk/guidance/ng85 (accessed on 3 January 2023).
- Phillips, M.E.; Hopper, A.D.; Leeds, J.S.; Roberts, K.J.; McGeeney, L.; Duggan, S.N.; Kumar, R. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021, 8, e000643. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, Y.S.; Han, Y.; Kwon, W.; Kim, S.W.; Han, H.S.; Yoon, D.S.; Park, J.S.; Park, S.J.; Han, S.S.; et al. Effects of pancreatic enzyme replacement therapy on body weight and nutritional assessments after pancreatoduodenectomy in a randomized trial. Clin. Gastroenterol. Hepatol. 2020, 18, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.J.; Haverkort, E.B.; Tijssen, G.P.; Tytgat, G.N.J.; Van Leeuwen, D.J. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998, 42, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Peddi, P.F.; Ding, K.; Chen, L.; Thomas, D.; Wang, J.; Lockhart, A.C.; Tan, B.; Wang-Gillam, A. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J. Transl. Med. 2013, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bülow, R.; Kühn, J.P.; Franke, A.; Heinsen, F.A.; Pietzner, M.; Nauck, M.; Völker, U.; et al. Impaired Exocrine Pancreatic Function Associates With Changes in Intestinal Microbiota Composition and Diversity. Gastroenterology 2019, 156, 1010–1015. [Google Scholar] [CrossRef]
- Frost, F.; Weiss, F.U.; Sendler, M.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Völzke, H.; Lamprecht, G.; et al. The Gut Microbiome in Patients With Chronic Pancreatitis Is Characterized by Significant Dysbiosis and Overgrowth by Opportunistic Pathogens. Clin. Transl. Gastroenterol. 2020, 11, e00232. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Trespi, E.; Ferrieri, A. Intestinal bacterial overgrowth during chronic pancreatitis. Curr. Med. Res. Opin. 1999, 15, 47–52. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, M.; Kashyap, P.C.; Chia, N.; Tran, N.H.; McWilliams, R.R.; Bekaii-Saab, T.S.; Ma, W.W. The role of microbiome in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 777. [Google Scholar] [CrossRef]
- Sammallahti, H.; Kokkola, A.; Rezasoltani, S.; Ghanbari, R.; Aghdaei, H.A.; Knuutila, S.; Puolakkainen, P.; Sarhadi, V.K. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12978. [Google Scholar] [CrossRef] [PubMed]
- Merali, N.; Chouari, T.; Kayani, K.; Rayner, C.J.; Jiménez, J.I.; Krell, J.; Giovannetti, E.; Bagwan, I.; Relph, K.; Rockall, T.A.; et al. A Comprehensive Review of the Current and Future Role of the Microbiome in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S. The Exocrine Pancreas; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Nicetto, D.; McCarthy, R.L.; Zaret, K.S. Epigenetic Mechanisms in Liver and Pancreas Generation and Regeneration. Epigenetics Regen. 2019, 11, 231–257. [Google Scholar] [CrossRef]
- Struyvenberg, M.R.; Martin, C.R.; Freedman, S.D. Practical guide to exocrine pancreatic insufficiency—Breaking the myths. BMC Med. 2017, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Dominici, R.; Franzini, C. Fecal elastase-1 as a test for pancreatic function: A review. Clin. Chem. Lab. Med. 2002, 40, 325–332. [Google Scholar] [CrossRef]
- Anaizi, A.; Hart, P.A.; Conwell, D.L. Diagnosing Chronic Pancreatitis. Dig. Dis. Sci. 2017, 62, 1713. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Mark, Z.; Haspel, J.; Ben-Ari, G.; Dreznik, Z.; Mirelman, D.; Tadmor, A. Antibacterial Activity of the Pancreatic Fluid. Gastroenterology 1985, 88, 927–932. [Google Scholar] [CrossRef]
- Pierzynowski, S.G.; Sharma, P.; Sobczyk, J.; Garwacki, S.; Barej, W.; Westrom, B. Comparative study of antibacterial activity of pancreatic juice in six mammalian species. Pancreas 1993, 8, 546–550. [Google Scholar] [CrossRef]
- Ahuja, M.; Schwartz, D.M.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017, 25, 635–646. [Google Scholar] [CrossRef]
- Bassi, C.; Fontana, R.; Vesentini, S.; Cavallini, G.; Marchiori, L.; Falconi, M.; Corra, S.; Pederzoli, P. Antibacterial and mezlocillin-enhancing activity of pure human pancreatic fluid. Int. J. Pancreatol. 1991, 10, 293–297. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Meng, Y.T.; Xu, J.J.; Fang, X.; Zhao, J.L.; Zhou, W.; Zhao, J.; Han, J.C.; Zhang, L.; Wang, K.X.; et al. Altered diversity and composition of gut microbiota in Chinese patients with chronic pancreatitis. Pancreatology 2020, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The Microbiota and Cancer Cachexia. Int. J. Mol. Sci. 2019, 20, 6267. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Villani, A.; Potenza, A.; Favaro, E.; Finocchiaro, C.; Perri, F.; Pazienza, V. Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. Int. J. Mol. Sci. 2023, 24, 1849. [Google Scholar] [CrossRef]
- Ritz, S.; Hahn, D.; Wami, H.T.; Tegelkamp, K.; Dobrindt, U.; Schnekenburger, J. Gut microbiome as a response marker for pancreatic enzyme replacement therapy in a porcine model of exocrine pancreas insufficiency. Microb. Cell Fact. 2020, 19, 221. [Google Scholar] [CrossRef]
- Nishiyama, H.; Nagai, T.; Kudo, M.; Okazaki, Y.; Azuma, Y.; Watanabe, T.; Goto, S.; Ogata, H.; Sakurai, T. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem. Biophys. Res. Commun. 2017, 495, 273–279. [Google Scholar] [CrossRef]
- Isaiah, A.; Parambeth, J.C.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017, 45, 50–58. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Guo, S.; Mei, Z.; Liao, H.; Dong, H.; Wu, K.; Ye, H.; Zhang, Y.; Zhu, Y.; et al. Tumor microbiome contributes to an aggressive phenotype in the basal-like subtype of pancreatic cancer. Commun. Biol. 2021, 4, 1019. [Google Scholar] [CrossRef]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important Metabolic Pathways and Biological Processes Expressed by Chicken Cecal Microbiota. Appl. Environ. Microbiol. 2015, 82, 1569–1576. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Takada, T.; Kurakawa, T.; Tsuji, H.; Nomoto, K. Fusicatenibacter saccharivorans gen. nov., sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Ménard, S.; Candalh, C.; Bambou, J.C.; Terpend, K.; Cerf-Bensussan, N.; Heyman, M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004, 53, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.D.; Bullman, S. The tumour-associated microbiome. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 347–348. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y. Intratumor microbiome in cancer progression: Current developments, challenges and future trends. Biomark. Res. 2022, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, S12–S16. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Sayin, S.; Rosener, B.; Li, C.G.; Ho, B.; Ponomarova, O.; Ward, D.V.; Walhout, A.J.M.; Mitchell, A. Evolved bacterial resistance to the chemotherapy gemcitabine modulates its efficacy in co-cultured cancer cells. Elife 2023, 12, 1–23. [Google Scholar] [CrossRef]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schönlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168–174. [Google Scholar] [CrossRef]
- Fulop, D.J.; Zylberberg, H.M.; Wu, Y.L.; Aronson, A.; Labiner, A.J.; Wisnivesky, J.; Cohen, D.J.; Sigel, K.M.; Lucas, A.L. Association of Antibiotic Receipt with Survival Among Patients with Metastatic Pancreatic Ductal Adenocarcinoma Receiving Chemotherapy. JAMA Netw. Open 2023, 6, e234254. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef]
- Daley, D. The role of the microbiome in pancreatic oncogenesis. Int. Immunol. 2022, 34, 447–454. [Google Scholar] [CrossRef]
- Benini, L.; Amodio, A.; Campagnola, P.; Agugiaro, F.; Cristofori, C.; Micciolo, R.; Magro, A.; Gabbrielli, A.; Cabrini, G.; Moser, L.; et al. Fecal elastase-1 is useful in the detection of steatorrhea in patients with pancreatic diseases but not after pancreatic resection. Pancreatology 2013, 13, 38–42. [Google Scholar] [CrossRef]
- Halloran, C.M.; Cox, T.F.; Chauhan, S.; Raraty, M.G.T.; Sutton, R.; Neoptolemos, J.P.; Ghaneh, P. Partial Pancreatic Resection for Pancreatic Malignancy Is Associated with Sustained Pancreatic Exocrine Failure and Reduced Quality of Life: A Prospective Study. Pancreatology 2011, 11, 535–545. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.; Nieto, L.; Pancreas, M.V. Development and diagnostic accuracy of a breath test for pancreatic exocrine insufficiency in chronic pancreatitis. Pancreas 2016, 45, 241–247. [Google Scholar] [CrossRef]
- Powell-Brett, S.; Hall, L.; Edwards, M.; Roberts, K. A systematic review and meta-analysis of the accuracy and methodology of the 13C mixed triglyceride breath test for the evaluation of pancreatic function. Pancreatology 2023, 23, 283–293. [Google Scholar] [CrossRef]
- Halle-Smith, J.M.; Hall, L.A.; Powell-Brett, S.F.; Merali, N.; Frampton, A.; Roberts, K.J. Realising the therapeutic potential of the human microbiota in metastatic pancreatic ductal adenocarcinoma. Clin. Surg. Oncol. 2023, 2, 100020. [Google Scholar] [CrossRef]

| Exocrine Pancreatic Product | Example | Source Pancreatic Cell Type | Function | Effect on Gut Microbiome |
|---|---|---|---|---|
| Bicarbonate rich fluid | - | Duct epithelial cell | Carry pancreatic enzymes into small intestine. Optimise pH for enzyme action. | sMRCP indicates negligible role |
| Digestive enzymes | Lipase, protease, amylase | Acinar cell | Digest food products | Oversupply of food products leads to significant changes especially complex carbohydrates. Inhibition in canine models did not affect composition of microbiome. |
| Antimicrobial peptides | CAMP and CRAMP | Acinar cell | Bacteriostatic properties | Murine studies indicate a regulatory role for the gut microbiome. Human studies remain inconclusive due to sampling issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halle-Smith, J.M.; Hall, L.A.; Powell-Brett, S.F.; Merali, N.; Frampton, A.E.; Beggs, A.D.; Moss, P.; Roberts, K.J. Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies? Cancers 2023, 15, 5140. https://doi.org/10.3390/cancers15215140
Halle-Smith JM, Hall LA, Powell-Brett SF, Merali N, Frampton AE, Beggs AD, Moss P, Roberts KJ. Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies? Cancers. 2023; 15(21):5140. https://doi.org/10.3390/cancers15215140
Chicago/Turabian StyleHalle-Smith, James M., Lewis A. Hall, Sarah F. Powell-Brett, Nabeel Merali, Adam E. Frampton, Andrew D. Beggs, Paul Moss, and Keith J. Roberts. 2023. "Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies?" Cancers 15, no. 21: 5140. https://doi.org/10.3390/cancers15215140
APA StyleHalle-Smith, J. M., Hall, L. A., Powell-Brett, S. F., Merali, N., Frampton, A. E., Beggs, A. D., Moss, P., & Roberts, K. J. (2023). Pancreatic Exocrine Insufficiency and the Gut Microbiome in Pancreatic Cancer: A Target for Future Diagnostic Tests and Therapies? Cancers, 15(21), 5140. https://doi.org/10.3390/cancers15215140






