The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Sarcopenia
The Assessment of Sarcopenia
| Males | Females | |
|---|---|---|
| Martin et al., 2013 [47] | <53 cm2/m2 (BMI ≥ 25 kg/m2) | <41 cm2/m2 |
| <43 cm2/m2 (BMI < 25 kg/m2) | ||
| Prado et al., 2008 [48] | <52.4 cm2/m2 | <38.5 cm2/m2 |
| Age (Years) | Males | Females |
|---|---|---|
| ≥80 | <7.78 ± 0.88 kg/m2 | <6.45 ± 0.78 kg/m2 |
| 70–79 | <8.22 ± 0.82 kg/m2 | <6.60 ± 0.76 kg/m2 |
| 60–69 | <8.53 ± 0.79 kg/m2 | <6.66 ± 0.64 kg/m2 |
| 50–59 | <8.77 ± 0.79 kg/m2 | <6.84 ± 0.72 kg/m2 |
| 40–49 | <8.96 ± 0.93 kg/m2 | <6.82 ± 0.68 kg/m2 |
| 30–39 | <8.92 ± 0.95 kg/m2 | <6.83 ± 0.74 kg/m2 |
| 20–29 | <8.67 ± 0.90 kg/m2 | <6.84 ± 0.80 kg/m2 |
| Handgrip Strength | ||
|---|---|---|
| Males | Females | |
| Chen et al., 2020 [23] | <28 kg | <18 kg |
| Cruz-Jentoft et al., 2019 [21] | <27 kg | <16 kg |
| Chair-Stand-Test | ||
| Males | Females | |
| Chen et al., 2020 [23] | ≥12 s | ≥12 s |
| Gait Speed | ||
| Males | Females | |
| Cruz-Jentoft et al., 2019 [21] | ≤0.8 m/s | ≤0.8 m/s |
3. Sarcopenic Obesity
The Assessment of Sarcopenic Obesity
4. The Prognostic Value of Sarcopenia and Sarcopenic Obesity
5. Toxicity of Cancer Treatments in Sarcopenic and Sarcopenic Obese Patients
6. The Role of Exercise in Sarcopenia
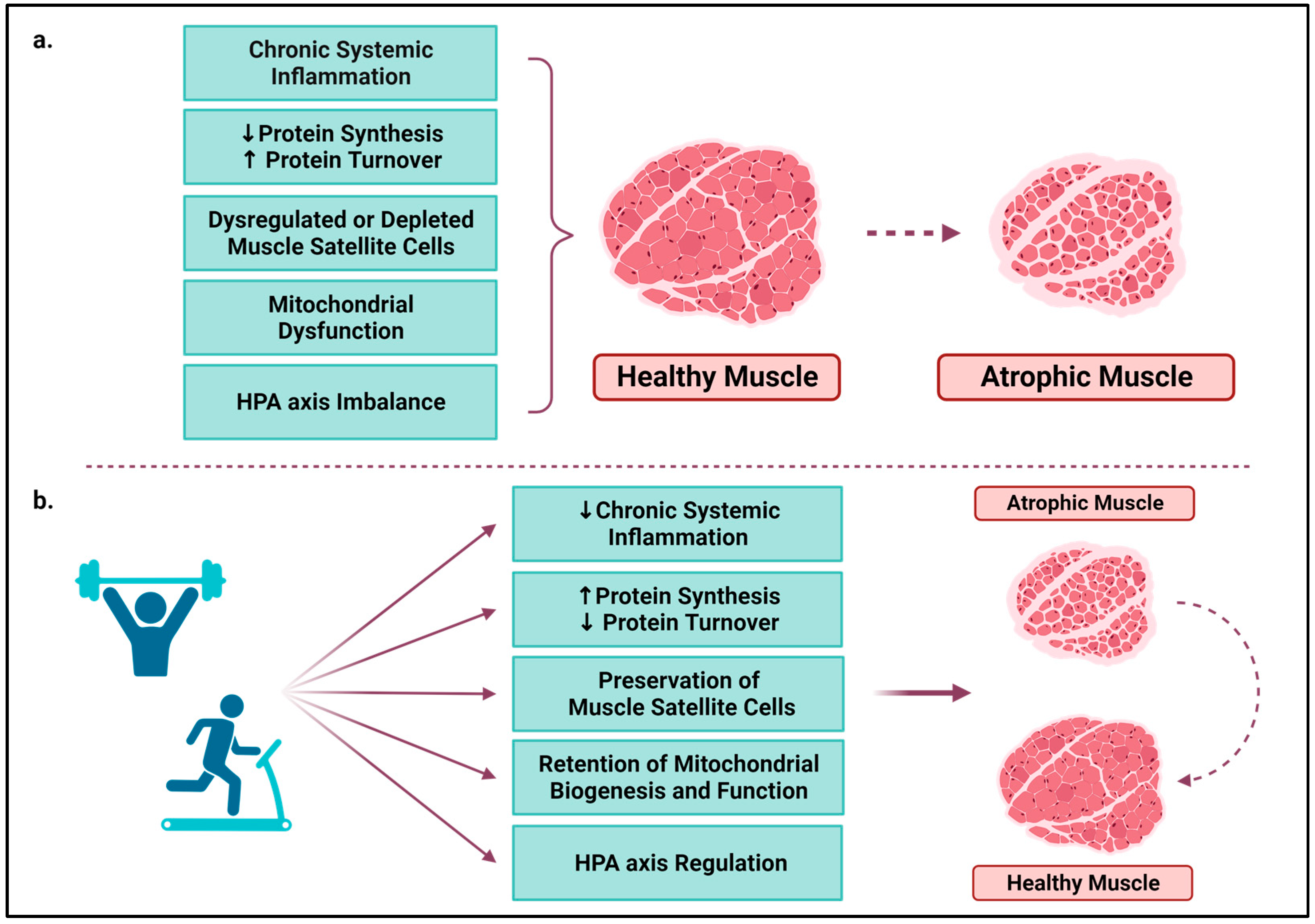
6.1. Chronic Systemic Inflammation
6.2. Protein Synthesis and Turnover
6.3. Muscle Satellite Cells
6.4. Mitochondria
6.5. HPA Axis
| Author (Year) | Type of Research | Cancer Site | Sex | Type of Exercise | Exercise Characteristics | Intervention Period | Counteracting Mechanism |
|---|---|---|---|---|---|---|---|
| Mader et al. (2022) [105] | Preclinical | Breast | Female | Aerobic | Voluntary wheel running | 4 weeks | ↓Chronic systemic inflammation and ↑Mitochondrial biogenesis and function |
| Aoi et al. (2010) [106] | Preclinical | Colon | N/A | Aerobic | Treadmill; 3 times/week; 30 min; 20 m/min | 6 weeks | ↓Chronic systemic inflammation |
| Alves de Lima et al. (2020) [107] | Preclinical | Lewis lung | N/A | Aerobic | Treadmill; 5 times/week; 40–60 min; 60% maximum speed | 2 or 3 weeks | ↓Chronic systemic inflammation ↑Protein synthesis |
| Schmidt et al. (2016) [108] | Clinical | Breast | Female | Resistance exercise | 2 times/week; 8 machine-based exercises; 3 sets × 8–12 reps; 60–80% of 1-RM | 12 weeks | ↓Chronic systemic inflammation |
| Hiensch et al. (2021) [109] | Clinical | Breast | Female | HIIT and resistance exercise | 2 times/week; 8 resistance exercises (2–3 sets × 8–12 reps; 70–80% of 1-RM) followed by 3 × 3 min bouts of HIIT | 16 weeks | ↓Chronic systemic inflammation |
| Schauer et al. (2021) [110] | Clinical | Breast, prostate, or colorectal | Both | Group A: HIIT and resistance exercise; Group B: low-to-moderate aerobic and resistance exercise | Group A: 2 times/week; 2 min intervals at 80–90% HRR and 2 min active rest; 40–80 min weekly and 3 × 6 RM sets to 3 × 10 RM sets; Group B: 2 times/week; 10 min intervals at 40–50% HRR; 150 min weekly and 3 × 12 reps at 50% of 6 RM to 3 × 20 reps at 50% of 6 RM | 6 months | ↓Chronic systemic inflammation |
| Khamoui et al. (2016) [112] | Preclinical | Colon | Female | Group A: Aerobic; Group B: Resistance exercise | Group A: motorised wheels; 5 times/week; 60 min at 5–6.5 m/min; Group B: 3 times/week; 5 × 3 reps at 50% of body weight followed by 10% increases bi-weekly | 11 weeks | ↑Protein synthesis |
| Sato et al. (2019) [118] | Preclinical | Colorectal | Female | Eccentric (high-frequency electric stimulation) | 10 sets × 6 reps, ~22 min | Acute or 2 weeks | ↑Protein synthesis |
| Hardee et al. (2020) [119] | Preclinical | Colorectal | Male | eccentric (high-frequency electric stimulation) | 10 sets × 6 reps every 48 h | 2 weeks | ↑Protein synthesis and ↑Mitochondrial Function |
| Pigna et al. (2016) [120] | Preclinical | Colon | Female | Aerobic | Voluntary wheel running | 19 days | ↑Protein synthesis |
| Coletti et al. (2016) [121] | Preclinical | Colon | Female | Aerobic | Voluntary wheel running | 20 days | ↑Protein synthesis and muscle satellite cells preservation |
| Møller et al. (2019) [122] | Clinical | Breast, head, and neck, rectal or sarcoma | Female | Aerobic and resistance exercise | 3 times/week; 90 min; aerobic on ergometer bicycle and 6 resistance exercises | 10 week | ↑Protein synthesis and ↑Mitochondrial Function |
| Mijwel et al. (2018) [127] | Clinical | Breast | Female | HIIT and resistance exercise | 2 times/week; 9 resistance exercises (2–3 sets × 8–12 reps; 70–80% of 1-RM) followed by 3 × 3 min bouts of HIIT (cycling) at 16–18 Borg scale | 16 weeks | ↑Muscle satellite cells and mitochondrial function |
| Ballarò et al. (2019) [134] | Preclinical | Colon | Both | Aerobic | 3 days out of 4; 11 m/min for 45 min; wheel running | 2 weeks | ↑Protein synthesis and ↑Mitochondrial function |
| Dieli-Conwright et al. (2021) [135] | Clinical | Breast | Female | Aerobic and resistance exercise | 3 times/week; aerobic at 65–80% HRmax; 30–50 min and 3 sets × 10 reps; 8 resistance exercises; 60% or 80% 1RM) | 16 weeks | ↑Mitochondrial function |
| Toohey et al. (2020) [141] | Clinical | Breast | Female | HIIT | 3 times/week; 7 intervals × 30 s cycling (maximum effort) with 2 min active rest between intervals | 12 weeks | HPA axis regulation |
| Evans et al. (2019) [142] | Clinical | Breast | Female | Aerobic | 30 min cycling at 60% VO2peak | Acute | HPA axis regulation |
7. Exercise Interventions after the Onset of Sarcopenia in Cancer Patients
| Author (Year) and Type of Research | Participants (=n) | Age and Sex | Type of Exercise | Intervention Period | Supervision | Nutrition Therapy | Main Outcomes |
|---|---|---|---|---|---|---|---|
| Adams et al. (2016) [46] randomised control trial | Breast cancer patients during chemotherapy (R.E.= 66, A.E. = 64, U.C= 70) | 25.0–78.0 Females | R.E. (2 sets × 8–12 reps; 9 exercises, 60–70% 1RM)—A.E. (15 min 60% VO2peak and 45 min VO2peak) | 3 times/week for a total of 9–24 weeks (median: 17 weeks) | Yes | No | R.E. reversed sarcopenia and dynapenia: ↑SMI, ↑Μuscle strength (upper and lower extremities), ↑QoL,↑fatigue, ↑anaemia |
| Dieli-Conwright et al. (2018) [145] randomised control trial | Breast cancer survivors (COMB. EX. = 50, U.C. = 50) | 53 ± 10.4 Females | COMB. EX. (A.E.: 65–80% HRmax; 30–50 min and R.E.: 3 sets × 10 reps; 8 exercises; 60% or 80% 1RM) | 3 times/week for a total of 16 weeks | Yes | No | COMB. EX. attenuated the sarcopenic obese phenotype: ↑ASMI, ↓ΒΜΙ, ↓Hip circumference, ↑Lean mass, ↓Fat mass, ↓Trunk fat |
| Dawson et al. (2018) [143] pilot randomised control trial | Prostate cancer patients on ADT (R.E. = 7, R.E and PRO = 6, PRO = 10, STR = 9) | 67.5 ± 8.7 Males | R.E. (3 sets; 10 exercises) Weeks 1–2: 60% 1RM, 15 repetitions; weeks 3–4: 65–67% 1RM, 15–12 repetitions;weeks 5–6: 70% 1RM, 12–10 repetitions; weeks 7–8:75% 1RM, 10–8 repetitions; weeks 9–10: 80% 1RM, 10–8 repetitions; weeks 11–12: 83% 1RM, 8 repetitions | 3 times/week for a total of 12 weeks | Yes | Yes | R.E improved sarcopenia prevalence: ↑Lean mass, ↓%body fat, ↑Muscle strength, ↑QoL; Protein supplementation did not offer additional benefits in improving body composition |
| Koya et al. (2019) [144] retrospective case–control study | Patients with hepatocellular carcinoma during chemoembolization period (COMB. EX. = 102, U.C. = 107) | 60.0–91.0 Females/ males: 74/135 | COMB. EX.: median 2.5 metabolic equivalents/20–40 min/day (R.E.: 3 sets × 10 reps; 60% 1RM or body weight and A.E.:10–15 min; 11–13 Borg scale and stretching and balance training) | Daily for 5–10 days (median: 7.5 days) | YES | YES | COMB. EX. improved clinical appearance: ↑SMI, ↓Δcreatinine, ↓ΔeGFR |
| Yamamoto et al. (2017) [146] pilot uncontrolled trial | Patients with gastric cancer during pre-operation period (COMB.EX. = 22) | 75 ± 5 Females/ males: 12/10 | COMB. EX.: handgrip(10 kg) 20 times/day; walking > 7500 steps/day; R.E.: 3 sets × 10 reps; 40–60% 1RM; 3 exercises | Daily for 7–26 days (median: 16 days) | NO | YES | COMB. EX.: ↑Handgrip, tendency to ↑SMI and ↑Gait speed |
| Moug et al. (2020) [147] randomised control trial | Patients with colorectal cancer during neoadjuvant chemoradiotherapy (EX. = 20, U.C = 24) | 66.8 ± 9.6 Both sexes (64% males) | ΕΧ.: progressively increasing walking using pedometers | Daily for ≥13 weeks (median: 14 weeks) | NO | NO | EX improved sarcopenia-related parameters: EX.: 65% of patients ↑Muscle mass and 35% of patients ↓Muscle mass; U.C: 67% of patients ↓Muscle mass and 33% of patients ↑Muscle mass |
| Delrieu et al. (2021) [148] uncontrolled trial | Metastatic breast cancer survivors (EX. = 49) | 54.9 ± 10.4 Females | ΕΧ.: progressively increasing walking using activity trackers | Daily for 6 months | NO | NO | EX improved physical performance but not body composition: ~CSA; ~lean mass; ~skeletal muscle radiodensity; ~SMI; ↑Aerobic capacity; ↑Muscle strength |
8. The Role of Myokines in the Reversal of Cancer-Related Sarcopenia
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Garlanda, C.; Allavena, P. Molecular Pathways and Targets in Cancer-Related Inflammation. Ann. Med. 2010, 42, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.A.; Agostinetto, E.; Perachino, M.; Del Mastro, L.; de Azambuja, E.; Vaz-Luis, I.; Partridge, A.H.; Lambertini, M. Evidence-Based Approaches for the Management of Side-Effects of Adjuvant Endocrine Therapy in Patients with Breast Cancer. Lancet Oncol. 2021, 22, e303–e313. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.M.; Chua, W.; Charles, K.A.; Clarke, S.J. Inflammation and Cancer: Causes and Consequences. Clin. Pharmacol. Ther. 2010, 87, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of Cancer Treatment by Chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Bentzen, S.M. Preventing or Reducing Late Side Effects of Radiation Therapy: Radiobiology Meets Molecular Pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef]
- George, A.; McLachlan, J.; Tunariu, N.; Della Pepa, C.; Migali, C.; Gore, M.; Kaye, S.; Banerjee, S. The Role of Hormonal Therapy in Patients with Relapsed High-Grade Ovarian Carcinoma: A Retrospective Series of Tamoxifen and Letrozole. BMC Cancer 2017, 17, 456. [Google Scholar] [CrossRef]
- Gheorghe, G.S.; Hodorogea, A.S.; Ciobanu, A.; Nanea, I.T.; Gheorghe, A.C.D. Androgen Deprivation Therapy, Hypogonadism and Cardiovascular Toxicity in Men with Advanced Prostate Cancer. Curr. Oncol. 2021, 28, 3331–3346. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Di Meglio, A.; Pistilli, B.; Gbenou, A.S.; El-Mouhebb, M.; Dauchy, S.; Charles, C.; Joly, F.; Everhard, S.; Lambertini, M.; et al. Differential Impact of Endocrine Therapy and Chemotherapy on Quality of Life of Breast Cancer Survivors: A Prospective Patient-Reported Outcomes Analysis. Ann. Oncol. 2019, 30, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Morishita, S. Prevalence of Sarcopenia in Cancer Patients: Review and Future Directions. Int. J. Phys. Med. Rehabil. 2016, 4, 1000342. [Google Scholar] [CrossRef]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships among Body Mass Index, Longitudinal Body Composition Alterations, and Survival in Patients with Locally Advanced Pancreatic Cancer Receiving Chemoradiation: A Pilot Study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Tan, B.H.; Cui, H.; Bhalla, A.; Fearon, K.C.H.; Parsons, S.L.; Catton, J.A.; Lobo, D.N. Marked Changes in Body Composition Following Neoadjuvant Chemotherapy for Oesophagogastric Cancer. Clin. Nutr. 2012, 31, 74–77. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Parsons, H.; Warneke, C.L.; Pulivarthi, K.; Litton, J.K.; Dev, R.; Palla, S.L.; Brewster, A.; Bruera, E. The Relationship between Body Composition and Response to Neoadjuvant Chemotherapy in Women with Operable Breast Cancer. Oncologist 2012, 17, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lima, I.S.F.; Baracos, V.E.; Bies, R.R.; McCargar, L.J.; Reiman, T.; Mackey, J.R.; Kuzma, M.; Damaraju, V.L.; Sawyer, M.B. An Exploratory Study of Body Composition as a Determinant of Epirubicin Pharmacokinetics and Toxicity. Cancer Chemother. Pharmacol. 2011, 67, 93–101. [Google Scholar] [CrossRef]
- Yang, M.; Shen, Y.; Tan, L.; Li, W. Prognostic Value of Sarcopenia in Lung Cancer. Chest 2019, 156, 101–111. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
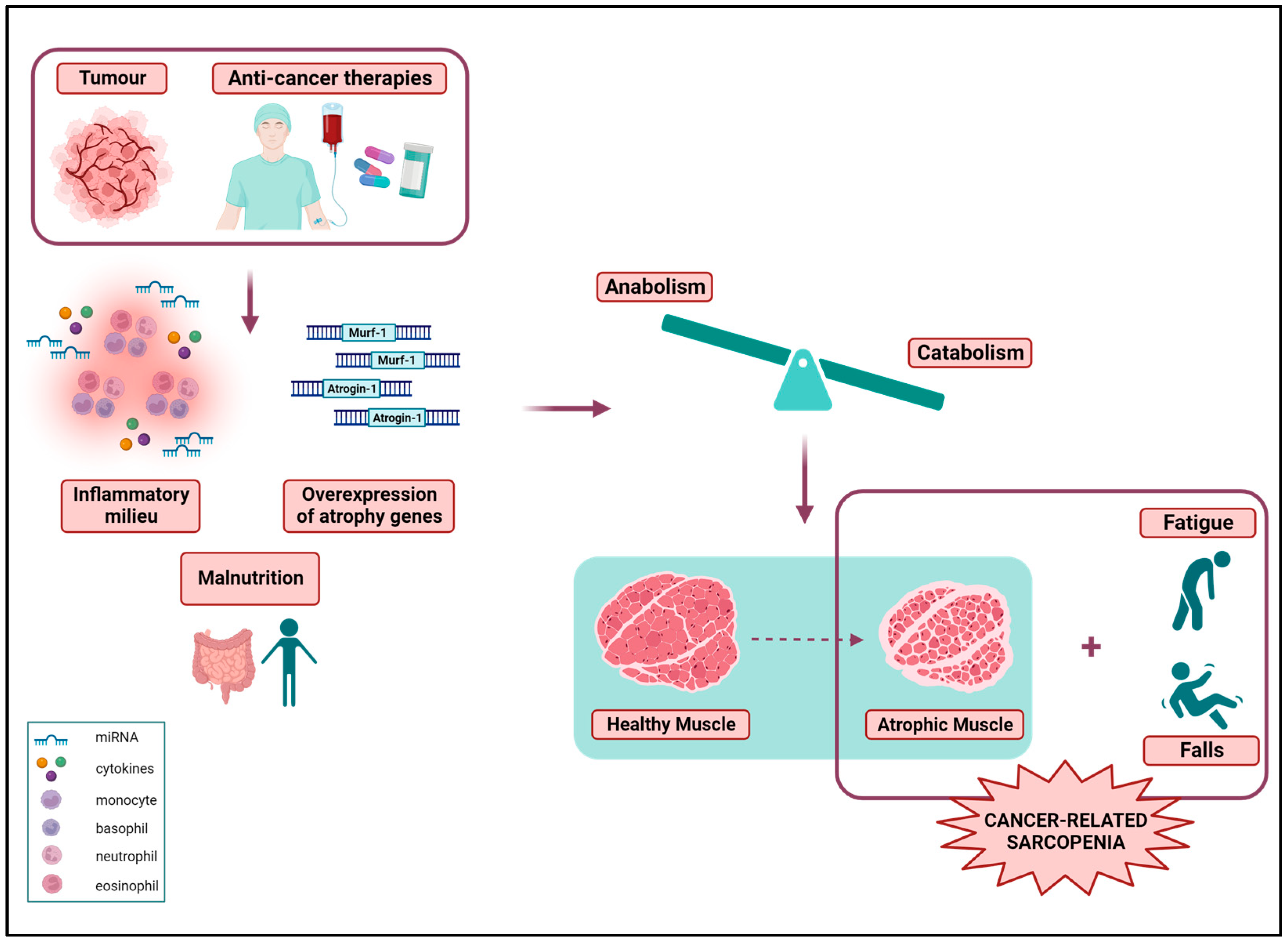
- Peixoto Da Silva, S.; Santos, J.M.O.; Costa ESilva, M.P.; Gil Da Costa, R.M.; Medeiros, R. Cancer Cachexia and Its Pathophysiology: Links with Sarcopenia, Anorexia and Asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Saeteaw, M.; Sanguanboonyaphong, P.; Yoodee, J.; Craft, K.; Sawangjit, R.; Ngamphaiboon, N.; Shantavasinkul, P.C.; Subongkot, S.; Chaiyakunapruk, N. Efficacy and Safety of Pharmacological Cachexia Interventions: Systematic Review and Network Meta-Analysis. BMJ Support. Palliat. Care 2021, 11, 75–85. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle Wasting in Disease: Molecular Mechanisms and Promising Therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Li, C.; Wu, Q.; Li, Z.; Wang, Z.; Tu, Y.; Chen, C.; Sun, S.; Sun, S. Exosomal microRNAs in Cancer-Related Sarcopenia: Tumor-Derived Exosomal microRNAs in Muscle Atrophy. Exp. Biol. Med. 2021, 246, 1156–1166. [Google Scholar] [CrossRef]
- Philippou, A.; Xanthis, D.; Chryssanthopοulos, C.; Maridaki, M.; Koutsilieris, M. Heart Failure–Induced Skeletal Muscle Wasting. Curr. Heart Fail. Rep. 2020, 17, 299–308. [Google Scholar] [CrossRef]
- Tanganelli, F.; Meinke, P.; Hofmeister, F.; Jarmusch, S.; Baber, L.; Mehaffey, S.; Hintze, S.; Ferrari, U.; Neuerburg, C.; Kammerlander, C.; et al. Type-2 Muscle Fiber Atrophy Is Associated with Sarcopenia in Elderly Men with Hip Fracture. Exp. Gerontol. 2021, 144, 111171. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- He, W.A.; Berardi, E.; Cardillo, V.M.; Acharyya, S.; Aulino, P.; Thomas-Ahner, J.; Wang, J.; Bloomston, M.; Muscarella, P.; Nau, P.; et al. NF-κB-mediated Pax7 Dysregulation in the Muscle Microenvironment Promotes Cancer Cachexia. J. Clin. Investig. 2013, 123, 4821–4835. [Google Scholar] [CrossRef]
- Fontes-Oliveira, C.C.; Busquets, S.; Toledo, M.; Penna, F.; Aylwin, M.P.; Sirisi, S.; Silva, A.P.; Orpí, M.; García, A.; Sette, A.; et al. Mitochondrial and Sarcoplasmic Reticulum Abnormalities in Cancer Cachexia: Altered Energetic Efficiency? Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 2770–2778. [Google Scholar] [CrossRef]
- Shum, A.M.Y.; Mahendradatta, T.; Taylor, R.J.; Painter, A.B.; Moore, M.M.; Tsoli, M.; Tan, T.C.; Clarke, S.J.; Robertson, G.R.; Polly, P. Disruption of MEF2C Signaling and Loss of Sarcomeric and Mitochondrial Integrity in Cancer-Induced Skeletal Muscle Wasting. Aging 2012, 4, 133–143. [Google Scholar] [CrossRef]
- Kiss, N.; Loeliger, J.; Findlay, M.; Isenring, E.; Baguley, B.J.; Boltong, A.; Butler, A.; Deftereos, I.; Eisenhuth, M.; Fraser, S.F.; et al. Clinical Oncology Society of Australia: Position Statement on cancer-related Malnutrition and Sarcopenia. Nutr. Diet. 2020, 77, 416–425. [Google Scholar] [CrossRef]
- Marshall, K.M.; Loeliger, J.; Nolte, L.; Kelaart, A.; Kiss, N.K. Prevalence of Malnutrition and Impact on Clinical Outcomes in Cancer Services: A Comparison of Two Time Points. Clin. Nutr. 2019, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Johal, J.; Han, C.Y.; Joseph, R.; Munn, Z.; Agbejule, O.A.; Crawford-Williams, F.; Wallen, M.P.; Chan, R.J.; Hart, N.H. Dietary Supplements in People with Metastatic Cancer Who Are Experiencing Malnutrition, Cachexia, Sarcopenia, and Frailty: A Scoping Review. Nutrients 2022, 14, 2642. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Schmitz, K.H.; Berger, N.A. Sarcopenia in Aging, Obesity, and Cancer. Transl. Cancer Res. TCR 2020, 9, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic Obesity and Myosteatosis Are Associated with Higher Mortality in Patients with Cirrhosis. J. Cachexia Sarcopenia Muscle 2016, 7, 126–135. [Google Scholar] [CrossRef]
- Albano, D.; Messina, C.; Vitale, J.; Sconfienza, L.M. Imaging of Sarcopenia: Old Evidence and New Insights. Eur. Radiol. 2020, 30, 2199–2208. [Google Scholar] [CrossRef]
- De Nardi, P.; Giani, A.; Maggi, G.; Braga, M. Relation between Skeletal Muscle Volume and Prognosis in Rectal Cancer Patients Undergoing Neoadjuvant Therapy. World J. Gastrointest. Oncol. 2022, 14, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Lee, E.J.; Chung, H.S.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, K.M. Comparisons of Three Different Methods for Defining Sarcopenia: An Aspect of Cardiometabolic Risk. Sci. Rep. 2017, 7, 6491. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Spiteri, T.; Cochrane, J.L.; Newton, R.U. Segmental Musculoskeletal Examinations Using Dual-Energy X-Ray Absorptiometry (DXA): Positioning and Analysis Considerations. J. Sports Sci. Med. 2015, 14, 620–626. [Google Scholar] [PubMed]
- Gould, H.; Brennan, S.L.; Kotowicz, M.A.; Nicholson, G.C.; Pasco, J.A. Total and Appendicular Lean Mass Reference Ranges for Australian Men and Women: The Geelong Osteoporosis Study. Calcif. Tissue Int. 2014, 94, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Wiegert, E.V.M.; de Oliveira, L.C.; Calixto-Lima, L.; Chaves, G.V.; Silva Lopes, M.S.; Peres, W.A.F. New Cancer Cachexia Staging System for Use in Clinical Practice. Nutrition 2021, 90, 111271. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, A.; Steurer-Stey, C.; Lana, K.D.; Zoller, M.; Turk, A.J.; Suter, P.; Puhan, M.A. Population-Based Reference Values for the 1-Min Sit-to-Stand Test. Int. J. Public Health 2013, 58, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of Resistance and Aerobic Exercise on Sarcopenia and Dynapenia in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Multicenter Randomized Controlled Trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.; McCargar, L.; Murphy, R.; Ghosh, S.; Sawyer, M.; Baracos, V. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.; Boirie, Y.; Busetto, L.; Cederholm, T.; Dicker, D.; Toplak, H.; Van Gossum, A.; Yumuk, V.; Vettor, R. Sarcopenic Obesity: Time to Meet the Challenge. Obes. Facts 2018, 11, 294–305. [Google Scholar] [CrossRef]
- Hadjispyrou, S.; Giannopoulos, A.; Philippou, A.; Theos, A. Mitochondrial Dysfunction and Sarcopenic Obesity: The Role of Exercise. J. Clin. Med. 2023, 12, 5628. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic Obesity: Hidden Muscle Wasting and Its Impact for Survival and Complications of Cancer Therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Cushen, S.J.; Power, D.G.; Murphy, K.P.; McDermott, R.; Griffin, B.T.; Lim, M.; Daly, L.; MacEneaney, P.; O’Sullivan, K.; Prado, C.M.; et al. Impact of Body Composition Parameters on Clinical Outcomes in Patients with Metastatic Castrate-Resistant Prostate Cancer Treated with Docetaxel. Clin. Nutr. ESPEN 2016, 13, e39–e45. [Google Scholar] [CrossRef]
- Rollins, K.E.; Tewari, N.; Ackner, A.; Awwad, A.; Madhusudan, S.; Macdonald, I.A.; Fearon, K.C.H.; Lobo, D.N. The Impact of Sarcopenia and Myosteatosis on Outcomes of Unresectable Pancreatic Cancer or Distal Cholangiocarcinoma. Clin. Nutr. 2016, 35, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Anandavadivelan, P.; Brismar, T.B.; Nilsson, M.; Johar, A.M.; Martin, L. Sarcopenic Obesity: A Probable Risk Factor for Dose Limiting Toxicity during Neo-Adjuvant Chemotherapy in Oesophageal Cancer Patients. Clin. Nutr. 2016, 35, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Kenyon, A.J.; Eberle, P.; Skye, A.; Kraus, W.E. Preventing Sarcopenic Obesity among Breast Cancer Patients Who Receive Adjuvant Chemotherapy: Results of a Feasibility Study. Clin. Exerc. Physiol. 2002, 4, 44. [Google Scholar] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.P.; Lieffers, J.; Ghosh, S.; Belch, A.; Chua, N.S.; Fontaine, A.; Sangha, R.; Turner, R.A.; Baracos, V.E.; Sawyer, M.B. Skeletal Muscle Density Is an Independent Predictor of Diffuse Large B-cell Lymphoma Outcomes Treated with Rituximab-based Chemoimmunotherapy. J. Cachexia Sarcopenia Muscle 2017, 8, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; De Van Der Schueren, M.A.E.; Den Braver, N.R.; Berkhof, J.; Langius, J.A.E.; Verheul, H.M.W. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.-Y.; Ling, H.H.; Ng, S.-H.; Wang, C.-H.; Chang, P.-H.; Chou, W.-C.; Chen, F.-P.; Lin, Y.-C. Role of the Appendicular Skeletal Muscle Index for Predicting the Recurrence-Free Survival of Head and Neck Cancer. Diagnostics 2021, 11, 309. [Google Scholar] [CrossRef]
- Chung, E.; Lee, H.S.; Cho, E.-S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Prognostic Significance of Sarcopenia and Skeletal Muscle Mass Change during Preoperative Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Nutr. 2020, 39, 820–828. [Google Scholar] [CrossRef]
- Kim, E.Y.; Jun, K.H.; Kim, S.Y.; Chin, H.M. Body Mass Index and Skeletal Muscle Index Are Useful Prognostic Factors for Overall Survival after Gastrectomy for Gastric Cancer: Retrospective Cohort Study. Medicine 2020, 99, e23363. [Google Scholar] [CrossRef]
- Gruber, E.S.; Jomrich, G.; Tamandl, D.; Gnant, M.; Schindl, M.; Sahora, K. Sarcopenia and Sarcopenic Obesity Are Independent Adverse Prognostic Factors in Resectable Pancreatic Ductal Adenocarcinoma. PLoS ONE 2019, 14, e0215915. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Okamoto, T.; Fujishita, T.; Katsura, M.; Akamine, T.; Takamori, S.; Morodomi, Y.; Tagawa, T.; Shoji, F.; Maehara, Y. Clinical Implications of Sarcopenia in Patients Undergoing Complete Resection for Early Non-Small Cell Lung Cancer. Lung Cancer 2016, 101, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Shoji, F.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; Toyokawa, G.; Okamoto, T.; Maehara, Y. Relationship Between Preoperative Sarcopenia Status and Immuno-Nutritional Parameters in Patients with Early-Stage Non-Small Cell Lung Cancer. Anticancer Res. 2017, 37, 6997–7003. [Google Scholar] [CrossRef] [PubMed]
- Song, E.J.; Lee, C.W.; Jung, S.-Y.; Kim, B.N.; Lee, K.S.; Lee, S.; Kang, H.-S.; Park, I.H.; Lee, M.H.; Kim, Y.J.; et al. Prognostic Impact of Skeletal Muscle Volume Derived from Cross-Sectional Computed Tomography Images in Breast Cancer. Breast Cancer Res. Treat. 2018, 172, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; Hari, P.; Janz, S.; Stolley, M.; D’Souza, A. Prevalence and Significance of Sarcopenia in Multiple Myeloma Patients Undergoing Autologous Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2021, 56, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Xiao, M.; Berano Teh, J.; Lee, B.; Chang, H.A.; Mascarenhas, K.; Lee, S.; Iukuridze, A.; Xie, J.J.; Scott, J.M.; et al. Impact of Sarcopenia on Adverse Outcomes After Allogeneic Hematopoietic Cell Transplantation. J. Natl. Cancer Inst. 2019, 111, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. 1916. Nutrition 1989, 5, 303–311; discussion 312–313. [Google Scholar]
- Kurk, S.; Peeters, P.; Stellato, R.; Dorresteijn, B.; De Jong, P.; Jourdan, M.; Creemers, G.; Erdkamp, F.; De Jongh, F.; Kint, P.; et al. Skeletal Muscle Mass Loss and Dose-limiting Toxicities in Metastatic Colorectal Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 803–813. [Google Scholar] [CrossRef]
- Ali, R.; Baracos, V.E.; Sawyer, M.B.; Bianchi, L.; Roberts, S.; Assenat, E.; Mollevi, C.; Senesse, P. Lean Body Mass as an Independent Determinant of Dose-limiting Toxicity and Neuropathy in Patients with Colon Cancer Treated with FOLFOX Regimens. Cancer Med. 2016, 5, 607–616. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Williams, G.R.; Nyrop, K.A.; Popuri, K.; Choi, S.K.; Muss, H.B. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane–Based Chemotherapy for Early-Stage Breast Cancer. Clin. Cancer Res. 2017, 23, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.L.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia Is Associated with Toxicity in Patients Undergoing Neo-Adjuvant Chemotherapy for Oesophago-Gastric Cancer. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; De Graeff, A.; Smid, E.J.; De Bree, R.; Pothen, A.J. Low Skeletal Muscle Mass Is a Predictive Factor for Chemotherapy Dose-Limiting Toxicity in Patients with Locally Advanced Head and Neck Cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a Predictor of Prognosis in Patients Following Hepatectomy for Hepatocellular Carcinoma. Br. J. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Huiskamp, L.F.J.; Chargi, N.; Devriese, L.A.; May, A.M.; Huitema, A.D.R.; De Bree, R. The Predictive Value of Low Skeletal Muscle Mass Assessed on Cross-Sectional Imaging for Anti-Cancer Drug Toxicity: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3780. [Google Scholar] [CrossRef]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body Composition and Sarcopenia: The next-Generation of Personalized Oncology and Pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Roubenoff, R.; Kehayias, J.J. The Meaning and Measurement of Lean Body Mass. Nutr. Rev. 1991, 49, 163–175. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.-D. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Jain, R.; Yee, J.-L.; Shaikh, T.; Au, C.; Handorf, E.; Meyer, J.E.; Dotan, E. Treatment-Related Toxicity and Outcomes in Older versus Younger Patients with Esophageal Cancer Treated with Neoadjuvant Chemoradiation. J. Geriatr. Oncol. 2020, 11, 668–674. [Google Scholar] [CrossRef]
- Jung, H.-W.; Kim, J.W.; Kim, J.-Y.; Kim, S.-W.; Yang, H.K.; Lee, J.W.; Lee, K.-W.; Kim, D.-W.; Kang, S.-B.; Kim, K.; et al. Effect of Muscle Mass on Toxicity and Survival in Patients with Colon Cancer Undergoing Adjuvant Chemotherapy. Support. Care Cancer 2015, 23, 687–694. [Google Scholar] [CrossRef]
- Lanic, H.; Kraut-Tauzia, J.; Modzelewski, R.; Clatot, F.; Mareschal, S.; Picquenot, J.M.; Stamatoullas, A.; Leprêtre, S.; Tilly, H.; Jardin, F. Sarcopenia Is an Independent Prognostic Factor in Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with Immunochemotherapy. Leuk. Lymphoma 2014, 55, 817–823. [Google Scholar] [CrossRef]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a Disease of Old Age: Changing Mutational and Microenvironmental Landscapes. Br. J. Cancer 2020, 122, 943–952. Available online: https://www.nature.com/articles/s41416-019-0721-1 (accessed on 16 October 2023). [CrossRef] [PubMed]
- Gusella, M.; Toso, S.; Ferrazzi, E.; Ferrari, M.; Padrini, R. Relationships between Body Composition Parameters and Fluorouracil Pharmacokinetics. Br. J. Clin. Pharmacol. 2002, 54, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Drami, I.; Pring, E.T.; Gould, L.; Malietzis, G.; Naghibi, M.; Athanasiou, T.; Glynne-Jones, R.; Jenkins, J.T. Body Composition and Dose-Limiting Toxicity in Colorectal Cancer Chemotherapy Treatment; a Systematic Review of the Literature. Could Muscle Mass Be the New Body Surface Area in Chemotherapy Dosing? Clin. Oncol. 2021, 33, e540–e552. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Chen, A.; Ha, V.; Chambers, C.; Eurich, D.T.; McCall, M.; Sawyer, M.B. An Exploratory Study of Body Composition as a Predictor of Dose-Limiting Toxicity in Metastatic Pancreatic Cancer Treated with Gemcitabine plus Nab-Paclitaxel. Clin. Nutr. 2021, 40, 4888–4892. [Google Scholar] [CrossRef] [PubMed]
- Philippou, A.; Papadopetraki, A.; Maridaki, M.; Koutsilieris, M. Εxercise as Complementary Therapy for Cancer Patients during and after Treatment. In Sports Medicine; 2020; Volume 1, Chapter 1; pp. 1–24. Available online: https://openaccessebooks.com/sports-medicine/exercise-as-complementary-therapy-for-cancer-patients-during-and-after-treatment.pdf (accessed on 16 October 2023).
- Maridaki, M.; Papadopetraki, A.; Karagianni, H.; Koutsilieris, M.; Philippou, A. The Assessment and Relationship Between Quality of Life and Physical Activity Levels in Greek Breast Cancer Female Patients under Chemotherapy. Sports 2020, 8, 32. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Stout, N.L.; Maitin-Shepard, M.; Campbell, A.; Schwartz, A.L.; Grimmett, C.; Meyerhardt, J.A.; Sokolof, J.M. Moving through Cancer: Setting the Agenda to Make Exercise Standard in Oncology Practice. Cancer 2021, 127, 476–484. [Google Scholar] [CrossRef]
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417. [Google Scholar] [CrossRef]
- Andrioti, A.; Papadopetraki, A.; Maridaki, M.; Philippou, A. The Effect of a Home-Based Tele-Exercise Training Program on the Quality of Life and Physical Performance in Breast Cancer Survivors. Sports 2023, 11, 102. [Google Scholar] [CrossRef]
- Stefani, L.; Galanti, G.; Klika, R. Clinical Implementation of Exercise Guidelines for Cancer Patients: Adaptation of ACSM’s Guidelines to the Italian Model. J. Funct. Morphol. Kinesiol. 2017, 2, 4. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical Activity and Exercise as Countermeasures to Physical Frailty and Sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Sim, M.; Taaffe, D.R.; Galvão, D.A.; Spry, N.; Kraemer, W.J.; Häkkinen, K.; Newton, R.U. Exercise Medicine for Cancer Cachexia: Targeted Exercise to Counteract Mechanisms and Treatment Side Effects. J. Cancer Res. Clin. Oncol. 2022, 148, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.E.; Eiken, O.; Miklavcic, L.; Mekjavic, I.B. Hip, Thigh and Calf Muscle Atrophy and Bone Loss after 5-Week Bedrest Inactivity. Eur. J. Appl. Physiol. 2007, 99, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Wall, B.T.; van de Valk, B.; Holloway, T.M.; Holloway, G.P.; Chabowski, A.; Goossens, G.H.; van Loon, L.J.C. One Week of Bed Rest Leads to Substantial Muscle Atrophy and Induces Whole-Body Insulin Resistance in the Absence of Skeletal Muscle Lipid Accumulation. Diabetes 2016, 65, 2862–2875. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J.; Frost, H.M.; Schiessl, H.; Ohshima, H.; Alkner, B.; Tesch, P.; Felsenberg, D. Muscle Atrophy and Bone Loss after 90 Days’ Bed Rest and the Effects of Flywheel Resistive Exercise and Pamidronate: Results from the LTBR Study. Bone 2005, 36, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Marusic, U.; Narici, M.; Simunic, B.; Pisot, R.; Ritzmann, R. Nonuniform Loss of Muscle Strength and Atrophy during Bed Rest: A Systematic Review. J. Appl. Physiol. 2021, 131, 194–206. [Google Scholar] [CrossRef]
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M.; et al. Re-Establishing the Comprehension of Phytomedicine and Nanomedicine in Inflammation-Mediated Cancer Signaling. Semin. Cancer Biol. 2022, 86, 1086–1104. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted Skeletal Muscle Mitochondrial Dynamics, Mitophagy, and Biogenesis during Cancer Cachexia: A Role for Inflammation. Oxidative Med. Cell. Longev. 2017, 2017, e3292087. [Google Scholar] [CrossRef]
- Miller, A.; McLeod, L.; Alhayyani, S.; Szczepny, A.; Watkins, D.N.; Chen, W.; Enriori, P.; Ferlin, W.; Ruwanpura, S.; Jenkins, B.J. Blockade of the IL-6 Trans-Signalling/STAT3 Axis Suppresses Cachexia in Kras-Induced Lung Adenocarcinoma. Oncogene 2017, 36, 3059–3066. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal Muscle Atrophy: From Mechanisms to Treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Mangano, G.D.; Fouani, M.; D’Amico, D.; Di Felice, V.; Barone, R. Cancer-Related Cachexia: The Vicious Circle between Inflammatory Cytokines, Skeletal Muscle, Lipid Metabolism and the Possible Role of Physical Training. Int. J. Mol. Sci. 2022, 23, 3004. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.; Chaillou, T.; Alves, E.S.; Jude, B.; Cheng, A.J.; Kenne, E.; Mijwel, S.; Kurzejamska, E.; Vincent, C.T.; Rundqvist, H.; et al. Exercise Reduces Intramuscular Stress and Counteracts Muscle Weakness in Mice with Breast Cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Naito, Y.; Takagi, T.; Kokura, S.; Mizushima, K.; Takanami, Y.; Kawai, Y.; Tanimura, Y.; Hung, L.P.; Koyama, R.; et al. Regular Exercise Reduces Colon Tumorigenesis Associated with Suppression of iNOS. Biochem. Biophys. Res. Commun. 2010, 399, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Alves de Lima, E., Jr.; de Souza Teixeira, A.A.; Biondo, L.A.; Diniz, T.A.; Silveira, L.S.; Coletti, D.; Busquets Rius, S.; Rosa Neto, J.C. Exercise Reduces the Resumption of Tumor Growth and Proteolytic Pathways in the Skeletal Muscle of Mice Following Chemotherapy. Cancers 2020, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Meynköhn, A.; Habermann, N.; Wiskemann, J.; Oelmann, J.; Hof, H.; Wessels, S.; Klassen, O.; Debus, J.; Potthoff, K.; et al. Resistance Exercise and Inflammation in Breast Cancer Patients Undergoing Adjuvant Radiation Therapy: Mediation Analysis From a Randomized, Controlled Intervention Trial. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hiensch, A.E.; Mijwel, S.; Bargiela, D.; Wengström, Y.; May, A.M.; Rundqvist, H. Inflammation Mediates Exercise Effects on Fatigue in Patients with Breast Cancer. Med. Sci. Sports Exerc. 2021, 53, 496–504. [Google Scholar] [CrossRef]
- Schauer, T.; Mazzoni, A.-S.; Henriksson, A.; Demmelmaie, I. Exercise Intensity and Markers of Inflammation during and after (Neo-) Adjuvant Cancer Treatment. Endocr.-Relat. Cancer 2021, 28, 191–201. Available online: https://erc.bioscientifica.com/view/journals/erc/28/3/ERC-20-0507.xml (accessed on 16 October 2023). [CrossRef]
- Rosa-Caldwell, M.E.; Fix, D.K.; Washington, T.A.; Greene, N.P. Muscle Alterations in the Development and Progression of Cancer-Induced Muscle Atrophy: A Review. J. Appl. Physiol. 2020, 128, 25–41. [Google Scholar] [CrossRef]
- Khamoui, A.V.; Park, B.-S.; Kim, D.-H.; Yeh, M.-C.; Oh, S.-L.; Elam, M.L.; Jo, E.; Arjmandi, B.H.; Salazar, G.; Grant, S.C.; et al. Aerobic and Resistance Training Dependent Skeletal Muscle Plasticity in the Colon-26 Murine Model of Cancer Cachexia. Metab. Clin. Exp. 2016, 65, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Philippou, A.; Maridaki, M.; Halapas, A.; Koutsilieris, M. The Role of the Insulin-like Growth Factor 1 (IGF-1) in Skeletal Muscle Physiology. In Vivo 2007, 21, 45–54. [Google Scholar] [PubMed]
- Philippou, A.; Halapas, A.; Maridaki, M.; Koutsilieris, M. Type I Insulin-like Growth Factor Receptor Signaling in Skeletal Muscle Regeneration and Hypertrophy. J. Musculoskelet. Neuronal Interact. 2007, 7, 208–218. [Google Scholar]
- Philippou, A.; Papageorgiou, E.; Bogdanis, G.; Halapas, A.; Sourla, A.; Maridaki, M.; Pissimissis, N.; Koutsilieris, M. Expression of IGF-1 Isoforms after Exercise-Induced Muscle Damage in Humans: Characterization of the MGF E Peptide Actions in Vitro. In Vivo 2009, 23, 567–575. [Google Scholar]
- Philippou, A.; Barton, E.R. Optimizing IGF-I for Skeletal Muscle Therapeutics. Growth Horm. IGF Res. 2014, 24, 157–163. [Google Scholar] [CrossRef]
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I Signaling in Muscle Bone Interactions. Bone 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Gao, S.; Puppa, M.J.; Kostek, M.C.; Wilson, L.B.; Carson, J.A. High-Frequency Stimulation on Skeletal Muscle Maintenance in Female Cachectic Mice. Med. Sci. Sports Exerc. 2019, 51, 1828–1837. [Google Scholar] [CrossRef]
- Hardee, J.P.; Fix, D.K.; Koh, H.-J.; Wang, X.; Goldsmith, E.C.; Carson, J.A. Repeated Eccentric Contractions Positively Regulate Muscle Oxidative Metabolism and Protein Synthesis during Cancer Cachexia in Mice. J. Appl. Physiol. 2020, 128, 1666–1676. [Google Scholar] [CrossRef]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef]
- Coletti, D.; Aulino, P.; Pigna, E.; Barteri, F.; Moresi, V.; Annibali, D.; Adamo, S.; Berardi, E. Spontaneous Physical Activity Downregulates Pax7 in Cancer Cachexia. Stem Cells Int. 2016, 2016, 6729268. [Google Scholar] [CrossRef]
- Møller, A.B.; Lønbro, S.; Farup, J.; Voss, T.S.; Rittig, N.; Wang, J.; Højris, I.; Mikkelsen, U.R.; Jessen, N. Molecular and Cellular Adaptations to Exercise Training in Skeletal Muscle from Cancer Patients Treated with Chemotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 1449–1460. [Google Scholar] [CrossRef]
- Feige, P.; Brun, C.E.; Ritso, M.; Rudnicki, M.A. Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease. Cell Stem Cell 2018, 23, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Tsitkanou, S.; Murach, K.A.; Washington, T.A.; Greene, N.P. Exercise Counteracts the Deleterious Effects of Cancer Cachexia. Cancers 2022, 14, 2512. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and Sarcopenia: A Focus on Circulating Inflammatory Cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef] [PubMed]
- D’Lugos, A.C.; Fry, C.S.; Ormsby, J.C.; Sweeney, K.R.; Brightwell, C.R.; Hale, T.M.; Gonzales, R.J.; Angadi, S.S.; Carroll, C.C.; Dickinson, J.M. Chronic Doxorubicin Administration Impacts Satellite Cell and Capillary Abundance in a Muscle-specific Manner. Physiol. Rep. 2019, 7, e14052. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Cardinale, D.A.; Norrbom, J.; Chapman, M.; Ivarsson, N.; Wengström, Y.; Sundberg, C.J.; Rundqvist, H. Exercise Training during Chemotherapy Preserves Skeletal Muscle Fiber Area, Capillarization, and Mitochondrial Content in Patients with Breast Cancer. FASEB J. 2018, 32, 5495–5505. [Google Scholar] [CrossRef]
- Nilsen, T.S.; Thorsen, L.; Fosså, S.D.; Wiig, M.; Kirkegaard, C.; Skovlund, E.; Benestad, H.B.; Raastad, T. Effects of Strength Training on Muscle Cellular Outcomes in Prostate Cancer Patients on Androgen Deprivation Therapy. Scand. Med. Sci. Sports 2016, 26, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.F.; Schjerling, P.; Andersen, J.L.; Daugaard, G.; Rørth, M.; Mackey, A.L. Muscle Satellite Cell Content and mRNA Signaling in Germ Cell Cancer Patients—Effects of Chemotherapy and Resistance Training. Acta Oncol. 2016, 55, 1246–1250. Available online: https://www.tandfonline.com/doi/full/10.3109/0284186X.2016.1170200 (accessed on 16 October 2023). [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial Degeneration Precedes the Development of Muscle Atrophy in Progression of Cancer Cachexia in Tumour-bearing Mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Hureau, T.J.; Pagano, A.F. Skeletal Muscle Deconditioning in Breast Cancer Patients Undergoing Chemotherapy: Current Knowledge and Insights From Other Cancers. Front. Cell Dev. Biol. 2021, 9, 719643. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2021.719643/full (accessed on 16 October 2023). [CrossRef]
- Chatzinikita, E.; Maridaki, M.; Palikaras, K.; Koutsilieris, M.; Philippou, A. The Role of Mitophagy in Skeletal Muscle Damage and Regeneration. Cells 2023, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.E.; Stanton, D.A.; Rellick, S.; Geldenhuys, W.; Pistilli, E.E. Breast Cancer-Associated Skeletal Muscle Mitochondrial Dysfunction and Lipid Accumulation Is Reversed by PPARG. Am. J. Physiol. Cell Physiol. 2021, 320, C577–C590. [Google Scholar] [CrossRef] [PubMed]
- Ballarò, R.; Beltrà, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate Exercise in Mice Improves Cancer plus Chemotherapy-Induced Muscle Wasting and Mitochondrial Alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Sami, N.; Norris, M.K.; Wan, J.; Kumagai, H.; Kim, S.-J.; Cohen, P. Effect of Aerobic and Resistance Exercise on the Mitochondrial Peptide MOTS-c in Hispanic and Non-Hispanic White Breast Cancer Survivors. Sci. Rep. 2021, 11, 16916. [Google Scholar] [CrossRef]
- Ruggiero, C.; Lalli, E. Impact of ACTH Signaling on Transcriptional Regulation of Steroidogenic Genes. Front. Endocrinol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The Regulation of Muscle Mass by Endogenous Glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.L.; Kleckner, I.R.; Jatoi, A.; Schwarz, E.M.; Dunne, R.F. The Role of Systemic Inflammation in Cancer-Associated Muscle Wasting and Rationale for Exercise as a Therapeutic Intervention. JCSM Clin. Rep. 2018, 3, e00065. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and Mental Health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Rebello, C.J.; Axelrod, C.L.; Reynolds, C.F.; Greenway, F.L.; Kirwan, J.P. Exercise as a Moderator of Persistent Neuroendocrine Symptoms of COVID-19. Exerc. Sport Sci. Rev. 2022, 50, 65–72. [Google Scholar] [CrossRef]
- Toohey, K.; Pumpa, K.; McKune, A.; Cooke, J.; Welvaert, M.; Northey, J.; Quinlan, C.; Semple, S. The Impact of High-Intensity Interval Training Exercise on Breast Cancer Survivors: A Pilot Study to Explore Fitness, Cardiac Regulation and Biomarkers of the Stress Systems. BMC Cancer 2020, 20, 787. [Google Scholar] [CrossRef]
- Evans, E.S.; Little, J.; McNeill, K.T.; Poole, C.; Bailey, S.P. Hypothalamic-Pituitary-Adrenal Axis Responses to an Acute Bout of Moderate Intensity Aerobic Exercise in Breast Cancer Survivors. FASEB J. 2019, 33, 534.3. [Google Scholar] [CrossRef]
- Dawson, J.K.; Dorff, T.B.; Todd Schroeder, E.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of Resistance Training on Body Composition and Metabolic Syndrome Variables during Androgen Deprivation Therapy for Prostate Cancer: A Pilot Randomized Controlled Trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Hirota, K.; Bekki, M.; Goto, E.; Yamada, M.; Sugimoto, M.; Hayashi, S.; Goshima, N.; et al. Effects of In-hospital Exercise on Sarcopenia in Hepatoma Patients Who Underwent Transcatheter Arterial Chemoembolization. J. Gastroenterol. Hepatol. 2019, 34, 580–588. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nagatsuma, Y.; Fukuda, Y.; Hirao, M.; Nishikawa, K.; Miyamoto, A.; Ikeda, M.; Nakamori, S.; Sekimoto, M.; Fujitani, K.; et al. Effectiveness of a Preoperative Exercise and Nutritional Support Program for Elderly Sarcopenic Patients with Gastric Cancer. Gastric Cancer 2017, 20, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Moug, S.J.; Barry, S.J.E.; Maguire, S.; Johns, N.; Dolan, D.; Steele, R.J.C.; Buchan, C.; Mackay, G.; Anderson, A.S.; Mutrie, N. Does Prehabilitation Modify Muscle Mass in Patients with Rectal Cancer Undergoing Neoadjuvant Therapy? A Subanalysis from the REx Randomised Controlled Trial. Tech. Coloproctol. 2020, 24, 959–964. [Google Scholar] [CrossRef]
- Delrieu, L.; Martin, A.; Touillaud, M.; Pérol, O.; Morelle, M.; Febvey-Combes, O.; Freyssenet, D.; Friedenreich, C.; Dufresne, A.; Bachelot, T.; et al. Sarcopenia and Serum Biomarkers of Oxidative Stress after a 6-Month Physical Activity Intervention in Women with Metastatic Breast Cancer: Results from the ABLE Feasibility Trial. Breast Cancer Res. Treat. 2021, 188, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Di Sipio, T.; Schmitz, K.; Hayes, S. Is Unsupervised Exercise Following Breast Cancer Safe for All Women? Int. J. Phys. Med. Rehabil. 2014, 2, 1971–1978. [Google Scholar]
- Arrieta, H.; Astrugue, C.; Regueme, S.; Durrieu, J.; Maillard, A.; Rieger, A.; Terrebonne, E.; Laurent, C.; Maget, B.; Servent, V.; et al. Effects of a Physical Activity Programme to Prevent Physical Performance Decline in Onco-geriatric Patients: A Randomized Multicentre Trial. J. Cachexia Sarcopenia Muscle 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Papadopetraki, A.; Maridaki, M.; Zagouri, F.; Dimopoulos, M.-A.; Koutsilieris, M.; Philippou, A. Physical Exercise Restrains Cancer Progression through Muscle-Derived Factors. Cancers 2022, 14, 1892. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Uchida, M.C.; Marzetti, E. If My Muscle Could Talk: Myokines as a Biomarker of Frailty. Exp. Gerontol. 2019, 127, 110715. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Flato, U.A.P.; Tofano, R.J.; de Alvares Goulart, R.; Guiguer, E.L.; Detregiachi, C.R.P.; Buchaim, D.V.; Araújo, A.C.; Buchaim, R.L.; Reina, F.T.R.; et al. Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications. Int. J. Mol. Sci. 2020, 21, 3607. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Graca, F.A.; Rai, M.; Hunt, L.C.; Stephan, A.; Wang, Y.-D.; Gordon, B.; Wang, R.; Quarato, G.; Xu, B.; Fan, Y.; et al. The Myokine Fibcd1 Is an Endogenous Determinant of Myofiber Size and Mitigates Cancer-Induced Myofiber Atrophy. Nat. Commun. 2022, 13, 2370. [Google Scholar] [CrossRef] [PubMed]
- Seaberg, B.; Henslee, G.; Wang, S.; Paez-Colasante, X.; Landreth, G.E.; Rimer, M. Muscle-Derived Extracellular Signal-Regulated Kinases 1 and 2 Are Required for the Maintenance of Adult Myofibers and Their Neuromuscular Junctions. Mol. Cell. Biol. 2015, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Aykul, S.; Martinez-Hackert, E. Transforming Growth Factor-β Family Ligands Can Function as Antagonists by Competing for Type II Receptor Binding. J. Biol. Chem. 2016, 291, 10792–10804. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/Activin Pathway Antagonism: Molecular Basis and Therapeutic Potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef]
- Shabani, R.; Izaddoust, F. Effects of Aerobic Training, Resistance Training, or Both on Circulating Irisin and Myostatin in Untrained Women. Acta Gymnica 2018, 48, 47–55. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, Y.; Jung, J.; Lee, J.; Chang, Y.; Lee, S.P.; Park, B.-C.; Wolfe, R.R.; Choi, C.S.; et al. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients 2021, 13, 1508. [Google Scholar] [CrossRef]
- Vernerová, L.; Horváthová, V.; Kropáčková, T.; Vokurková, M.; Klein, M.; Tomčík, M.; Oreská, S.; Špiritović, M.; Štorkánová, H.; Heřmánková, B.; et al. Alterations in Activin A–Myostatin–Follistatin System Associate with Disease Activity in Inflammatory Myopathies. Rheumatology 2020, 59, 2491–2501. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Summermatter, S.; Jourdain, M.; Melly, S.; Minetti, G.C.; Lach-Trifilieff, E. ActRII Blockade Protects Mice from Cancer Cachexia and Prolongs Survival in the Presence of Anti-Cancer Treatments. Skelet. Muscle 2016, 6, 26. [Google Scholar] [CrossRef]
- Busquets, S.; Toledo, M.; Orpí, M.; Massa, D.; Porta, M.; Capdevila, E.; Padilla, N.; Frailis, V.; López-Soriano, F.J.; Han, H.Q.; et al. Myostatin Blockage Using actRIIB Antagonism in Mice Bearing the Lewis Lung Carcinoma Results in the Improvement of Muscle Wasting and Physical Performance. J. Cachexia Sarcopenia Muscle 2012, 3, 37–43. [Google Scholar] [CrossRef]
- Benny Klimek, M.E.; Aydogdu, T.; Link, M.J.; Pons, M.; Koniaris, L.G.; Zimmers, T.A. Acute Inhibition of Myostatin-Family Proteins Preserves Skeletal Muscle in Mouse Models of Cancer Cachexia. Biochem. Biophys. Res. Commun. 2010, 391, 1548–1554. [Google Scholar] [CrossRef]
- Lee, S.-J. Targeting the Myostatin Signaling Pathway to Treat Muscle Loss and Metabolic Dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Baccam, A.; Benoni-Sviercovich, A.; Rocchi, M.; Moresi, V.; Seelaender, M.; Li, Z.; Adamo, S.; Xue, Z.; Coletti, D. The Mechanical Stimulation of Myotubes Counteracts the Effects of Tumor-Derived Factors Through the Modulation of the Activin/Follistatin Ratio. Front. Physiol. 2019, 10, 401. [Google Scholar] [CrossRef]
- Loumaye, A.; De Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; Van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.-P. Role of Activin A and Myostatin in Human Cancer Cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Kogita, A.; Sakamoto, H.; Hayashi, H.; Terashima, M.; de Velasco, M.A.; Sakai, K.; Fujita, Y.; Tomida, S.; Kitano, M.; et al. Activin Signal Promotes Cancer Progression and Is Involved in Cachexia in a Subset of Pancreatic Cancer. Cancer Lett. 2015, 356, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, A.; De Barsy, M.; Nachit, M.; Lause, P.; Van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J. Circulating Activin A Predicts Survival in Cancer Patients. J. Cachexia Sarcopenia Muscle 2017, 8, 768–777. [Google Scholar] [CrossRef]
- Queiroz, A.L.; Dantas, E.; Ramsamooj, S.; Murthy, A.; Ahmed, M.; Zunica, E.R.M.; Liang, R.J.; Murphy, J.; Holman, C.D.; Bare, C.J.; et al. Blocking ActRIIB and Restoring Appetite Reverses Cachexia and Improves Survival in Mice with Lung Cancer. Nat. Commun. 2022, 13, 4633. [Google Scholar] [CrossRef] [PubMed]
- Puchert, M.; Adams, V.; Linke, A.; Engele, J. Evidence for the Involvement of the CXCL12 System in the Adaptation of Skeletal Muscles to Physical Exercise. Cell. Signal. 2016, 28, 1205–1215. [Google Scholar] [CrossRef]
- Emmons, R.; Niemiro, G.M.; Owolabi, O.; De Lisio, M. Acute Exercise Mobilizes Hematopoietic Stem and Progenitor Cells and Alters the Mesenchymal Stromal Cell Secretome. J. Appl. Physiol. 2016, 120, 624–632. [Google Scholar] [CrossRef]
- Yamada, M.; Hokazono, C.; Tokizawa, K.; Marui, S.; Iwata, M.; Lira, V.A.; Suzuki, K.; Miura, S.; Nagashima, K.; Okutsu, M. Muscle-Derived SDF-1α/CXCL12 Modulates Endothelial Cell Proliferation but Not Exercise Training-Induced Angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R770–R779. [Google Scholar] [CrossRef]
- Martinelli, G.B.; Olivari, D.; Re Cecconi, A.D.; Talamini, L.; Ottoboni, L.; Lecker, S.H.; Stretch, C.; Baracos, V.E.; Bathe, O.F.; Resovi, A.; et al. Activation of the SDF1/CXCR4 Pathway Retards Muscle Atrophy during Cancer Cachexia. Oncogene 2016, 35, 6212–6222. [Google Scholar] [CrossRef]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin Is an Activity-Stimulated Myokine That Enhances Physical Endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Re Cecconi, A.D.; Forti, M.; Chiappa, M.; Zhu, Z.; Zingman, L.V.; Cervo, L.; Beltrame, L.; Marchini, S.; Piccirillo, R. Musclin, A Myokine Induced by Aerobic Exercise, Retards Muscle Atrophy During Cancer Cachexia in Mice. Cancers 2019, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakano, S.-I.; Miyoshi, T.; Yamanouchi, K.; Nishihara, M. Loss of Sparc in Mouse Skeletal Muscle Causes Myofiber Atrophy: SPARC Knockdown in Muscle. Muscle Nerve 2013, 48, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Kanzleiter, T.; Rath, M.; Görgens, S.W.; Jensen, J.; Tangen, D.S.; Kolnes, A.J.; Kolnes, K.J.; Lee, S.; Eckel, J.; Schürmann, A.; et al. The Myokine Decorin Is Regulated by Contraction and Involved in Muscle Hypertrophy. Biochem. Biophys. Res. Commun. 2014, 450, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Daou, H.N. Exercise as an Anti-Inflammatory Therapy for Cancer Cachexia: A Focus on Interleukin-6 Regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R296–R310. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin Is a Pro-Myogenic Factor That Induces Skeletal Muscle Hypertrophy and Rescues Denervation-Induced Atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Kodera, K.; Tsukihara, S.; Takahashi, S.; Kobayashi, Y.; Koyama, M.; Kanno, H.; Ishiyama, S.; Hanyu, N.; Eto, K. Prognostic Significance of Osteosarcopenia in Older Adults with Colorectal Cancer. Ann. Gastroenterol. Surg. 2023, 7, 637–644. [Google Scholar] [CrossRef]
- Takeda, T.; Sasaki, T.; Okamoto, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Mie, T.; Furukawa, T.; Kasuga, A.; Matsuyama, M.; et al. Prognostic Impact of Osteosarcopenia in Patients with Advanced Pancreatic Cancer Receiving Gemcitabine plus Nab-Paclitaxel. Pancreatology 2023, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Loyola, W.A.; Morselli, J.; Quintanilla, F.; Castro Teixeira, D.; Álvarez-Bustos, A.; Molari, M.; Probst, V.; Valenzuela, J. Clinical Impact of Osteosarcopenia on Mortality, Physical Function and Chronic Inflammation: A 9-Year Follow up Cohort Study. Nutr. Clin. Diet. Hosp. 2023, 43, 133–140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopetraki, A.; Giannopoulos, A.; Maridaki, M.; Zagouri, F.; Droufakou, S.; Koutsilieris, M.; Philippou, A. The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity. Cancers 2023, 15, 5856. https://doi.org/10.3390/cancers15245856
Papadopetraki A, Giannopoulos A, Maridaki M, Zagouri F, Droufakou S, Koutsilieris M, Philippou A. The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity. Cancers. 2023; 15(24):5856. https://doi.org/10.3390/cancers15245856
Chicago/Turabian StylePapadopetraki, Argyro, Antonios Giannopoulos, Maria Maridaki, Flora Zagouri, Stavroula Droufakou, Michael Koutsilieris, and Anastassios Philippou. 2023. "The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity" Cancers 15, no. 24: 5856. https://doi.org/10.3390/cancers15245856
APA StylePapadopetraki, A., Giannopoulos, A., Maridaki, M., Zagouri, F., Droufakou, S., Koutsilieris, M., & Philippou, A. (2023). The Role of Exercise in Cancer-Related Sarcopenia and Sarcopenic Obesity. Cancers, 15(24), 5856. https://doi.org/10.3390/cancers15245856








